Abstract
Tremendous progress has been made during the last few years in identification of novel tumor-associated microRNAs and experimental validation of their cancer relevant gene targets. Indeed, these small non-coding RNAs are now known to modulate many biological pathways related to cancer progression, metastasis and therapy-resistance. Therefore, modulating miRNA functions may provide novel therapeutic opportunities for cancer treatment. This article reviews recent literature on the role of miRNAs in cancer with an emphasis on their potential as cancer therapeutics.
Keywords: MicroRNAs, Small RNAs, Cancer, Therapeutics
Introduction
MicroRNAs (miRNAs or miRs) have received significant attention as a new class of noncoding-RNAs engaged in the regulation of gene expression. miRNAs are short 19–25nt stretches of RNA that bind to the target mRNAs and suppress their translation or initiate decay (1). More than 500 different miRNAs have been identified in humans and their total number is predicted to exceed 1000 (1;2). Despite this large number of identified miRNAs, the target mRNAs and the biological function have been ascribed to only a few of them. With increasing interest and research investigations, it is now becoming evident that miRNAs exhibit differential expression in several human diseases, including cancer and play important roles in the disease processes (3). Some of the miRNAs have clearly been shown to influence the development and progression of cancer (3). Therefore, identification of novel differentially-expressed miRNAs, establishing their cancer-associated functions and developing ways to modulate their activities may yield powerful therapeutic strategies against cancer. This review summarizes some of the recent and significant findings on role of miRNAs in cancer development with a focus on their future in cancer therapeutics.
MicroRNAs
Biogenesis and mechanism of gene silencing
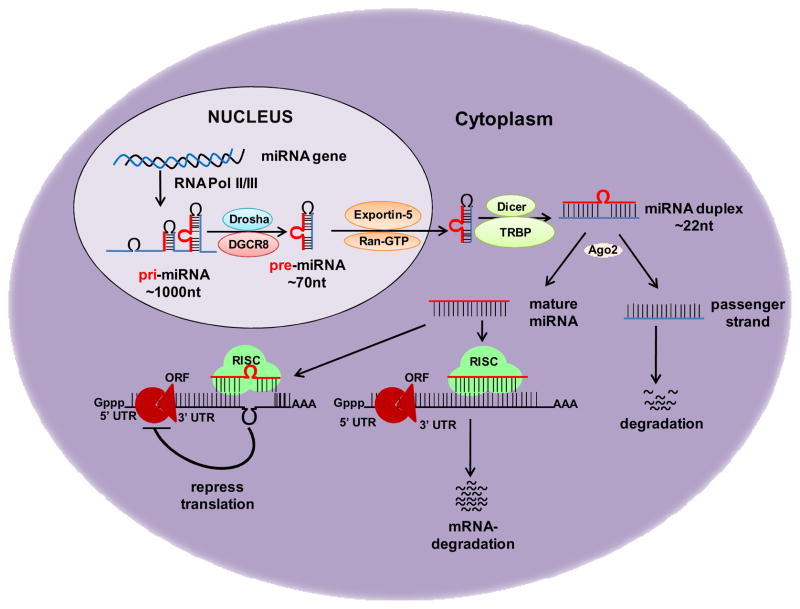
Biogenesis of miRNAs begins with transcription by RNA polymerase II or RNA polymerase III, producing primary miRNA (pri-miRNA) (4). Pri-miRNAs contain a single or multiple (miRNA cluster) folded hairpin stem structure(s) with flanking single-stranded upstream and downstream regions (Figure 1). Following synthesis, pri-miRNA is first processed inside the nucleus by RNase III (Drosha) and the DGCR8 (DiGeorge critical region 8) to yield a ~70 nucleotides long precursor miRNA (pre-miRNA). Pre-miRNA is transported to the cytoplasm by the nuclear export factor Exportin-5/Ran-GTP, where it is processed through a series of cuts by RNase III endonuclease, Dicer/TRBP and Ago2 to generate a mature 17–25 bp miRNA duplex (4). The miRNA duplex is unwound into a mature single-stranded miRNA (one of the strand) by a helicase, which then enters into the RNA-induced silencing complex (RISC) and directs the complex to target mRNA through a poorly defined mechanism (5) (Figure 1). It is commonly accepted that mature miRNAs regulate gene expression by binding to the 3’-untranslated region (3’-UTR) of target mRNA, causing degradation of mRNA or inhibition of their translation (4;5). The specificity in choosing target transcripts is mainly decided by sequence complementarity between mRNA target sites and the nucleotide sequence from position 2 to 8 at the 5′ end of miRNAs known as “Seed Region”. Although base pairing to the 3′ end of miRNA is thought to be less important in target recognition, it may contribute in target selection especially when sites have weaker miRNA seed matches (6).
Figure 1. MicroRNA biogenesis and mechanisms of gene silencing.
MicroRNA (miRNA) genes are generally transcribed by RNA polymerase II/III in nucleus to form large primary transcript (pri-miRNA). These pri-miRNA transcripts are processed to release the ~70-nucleotide hairpin RNA known as precursor-miRNA (pre-miRNA), which is transported to the cytoplasm and undergoes another processing to yield a transient ~22- nucleotide RNA duplex. RNA duplex is unwound into a mature single-stranded miRNA, and loaded into RNA-induced silencing complex (RISC). miRNA then binds to complementary sites in the 3’-untranslated region (3’-UTR) of target mRNA and regulate its expression either by causing degradation of mRNA or repression of their translation, depending on the degree of complementarity between the miRNA and its target.
MicroRNAs in cancer
Croce and colleagues, for the first time, reported a link between miRNAs and cancer by mapping the genomic locus of miR15 and miR16 to chromosome 13q14, a region deleted in majority of B cell chronic lymphocytic leukemias (B-CLL) (7). Since then, studies in a variety of human tumors have shown that miRNAs are frequently associated with sites of chromosomal instability or amplification (8). Furthermore, many recent experimental and clinical studies have revealed that the aberrant expression of miRNAs is associated with the stage, progression, and metastasis of cancers (8, 9). It has been shown that miRNAs can function as tumor promoters (oncomirs) or tumor suppressors (anti-oncomirs) (3, 10). Moreover, miRNAs are also shown to be implicated in cancer stem cells (CSCs) and epithelial–mesenchymal transition (EMT), which are critically associated with cancer metastasis and drug resistance (8, 11).
Oncogenic microRNAs (Oncomirs)
The “oncomirs” promote tumor development by negatively inhibiting tumor suppressor genes and/or genes that control cell differentiation or apoptosis. Oncomirs are significantly overexpressed in various tumors because of gene amplification, epigenetic mechanisms or transcriptional dysregulation (10). miR-17-92 cluster is a typical example, which is located at chromosome 13q31, a region amplified in lung and other malignancies (12). Myc-induced up-regulation of miR-17-92 cluster has been shown to enhance tumorigenesis and angiogenesis (13). Recent studies also revealed that miR-17-92 was upregulated in sonic hedgehog-driven medulloblastomas and induced by N-myc in sonic hedgehog-treated cerebellar neural precursors (14, 15). Among others oncogenic miRNAs, miR-21 has been shown to promote apoptosis through activation of caspases in human glioblastoma cells (16). In breast cancer cells, silencing of miR-21 inhibited cell growth in vitro and in vivo by causing downregulation of Bcl-2 and induction of apoptosis (17). Among the experimentally validated targets of miR-21 are Pdcd4, bone morphogenetic protein receptor II (BMPRII) and LRRFIP1 (an inhibitor of NF-κB signaling) (18–20). miRNA expression profiling have reported increased expression of miR-155 in various cancers. Its expression was significantly correlated with poor survival in pancreatic cancer patients (21). In another study, transfection of anti-miR-155 oligonucleotides into pancreatic cancer induced the expression of tumor suppressor protein 53-induced nuclear protein 1 (TP53INP1) and enhanced apoptosis (22). miR-372 and miR-373 are two additional examples of oncogenic miRNAs that are shown to promote cell proliferation and tumor development by neutralizing p53-mediated CDK inhibition, possibly through direct targeting of the tumor suppressor gene LATS2 (23). Further research is likely to add many more miRNAs to the growing list of oncomirs.
Tumor suppressor miRNAs (Anti-oncomirs)
There are several miRNAs that are considered as tumor suppressors, viz. let-7, miR-17-5p, miR-29, miR-34, miR-127 etc (10, 24). Indeed, the earliest miRNAs identified to be tumor-associated (miR-15 and miR-16) were from this class (7), which are now experimentally shown to possess anti-oncogenic activity (25). miR-15/16 induce apoptosis by negatively regulating the expression of anti-apoptotic gene, BCL2 (26). In another study, miR-16 is shown to suppress the growth of prostate cancer cells by regulating the expression of CDK1 and CDK2, which are associated with cell cycle control and proliferation (27). It has also been demonstrated that miR-15a and miR-16-1 cluster in prostate cancer cells targets CCND1 (encoding cyclin D1) and WNT3A, and thus impact survival, proliferation and invasion (28). Among the most studied tumor suppressor miRNAs are the members of let-7 family. Expression of let-7 miRNAs is downregulated in various cancers and they are good candidates as diagnostic and prognostic biomarkers (10, 29). Expression of let-7 is frequently decreased in lung cancer and forced expression of let-7 in A549 lung adenocarcinoma cell line inhibited cancer cell growth (24). RAS and MYC are the direct targets of let-7 (29), indicating its pathogenic potential in many other RAS and MYC-driven cancers. miR-34a is another miRNA in this category that participates in p53 tumor suppressor network and have been shown to be directly transactivated by p53 (30, 31). Overexpression of miR-34a induces apoptosis and alters the expression of several genes related to cell cycle progression, apoptosis, DNA repair, and angiogenesis (30). In a recent study, it was also shown that miR-34a inhibited human pancreatic cancer tumor-initiating cells and restored the tumor-suppressor function of p53 in p53-deficient human pancreatic cancer cells (31).
MicroRNAs in cancer stem cells and epithelial to mesenchymal transition
Cancer stem cells (CSCs) are considered that rare subpopulation of cancer cells, which is responsible for tumor heterogeneity, maintenance and therapy-resistance (32). Therefore, efforts are now being made to understand the mechanisms that sustain the ‘stemness’ of CSCs. It was shown that induction of epithelial to mesenchymal transition (EMT) stimulated cultured breast cells to adopt the characteristics of stem cells (33). During EMT, cells attain a migratory phenotype by losing the cell-cell and cell-extracellular matrix interactions and literature suggests an important role of EMT in cancer progression and metastasis. Recent emerging evidence indicate a critical role of miRNAs in the maintenance of normal and cancer stem cells and acquisition of EMT phenotype (34, 35). Indeed, some miRNAs, such as let-7, miR 30, miR-34a, miR 155, miR 370 have been shown to be implicated in self-renewal, differentiation and survival of CSCs (31, 34, 36). Let-7 is reported to interact with MYC and LIN-28 to control stem cell properties (37). In another study, a drastically reduced expression of let-7 was reported in breast cancer stem cells, which increased as the cells differentiated (38). It was shown that let-7 regulated stem cell properties and tumorigenic behavior of cancer cells by targeting the expression of multiple genes, including H-RAS and HMGA-2 (38). In additional studies, certain miRNAs have been shown to act as the regulators of CSCs and EMT by regulating the expression of BMI1, E-cadherin and other proteins (35). In a recent study, it was reported that the induction of members of miR-200 and let-7 families by natural agents led to the reversal of EMT in pancreatic cancer cells (39). The expression of miR-200 and let-7 family miRNAs was downregulated in gemcitabine-resistant pancreatic cancer cell lines with more mesenchymal characteristics. Ectopic expression of miR-200a, miR-200b, and miR-200c pre-miRNAs resulted in the acquisition of epithelial characteristics and enhanced drug-sensitivity (39). Another study from the same group in prostate cancer cells reported that re-expression of miR-200b led to the reversal of EMT along with the downregulation of ZEB1, ZEB2 and Slug expression (40).
MicroRNAs as novel cancer therapeutics
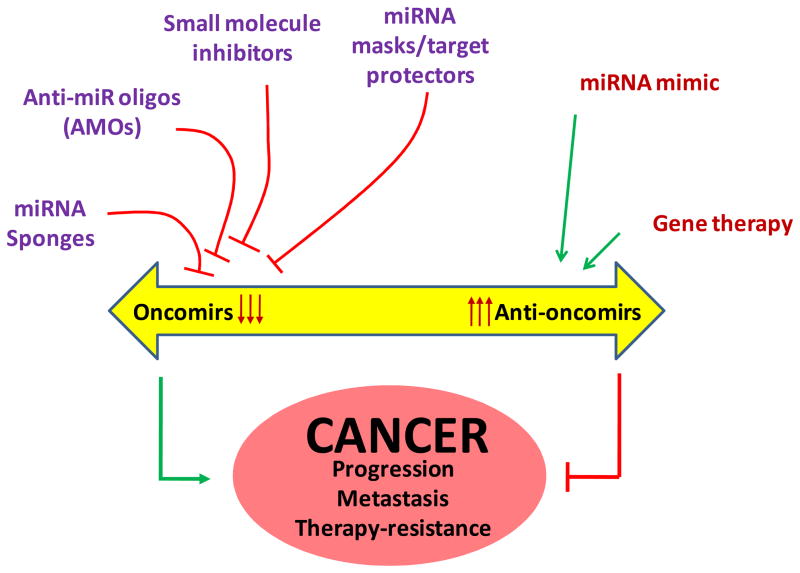
In consideration of the fact that miRNAs involve in tumor initiation, progression and metastasis, their targeting is expected to emerge as an effective therapeutic option for cancer treatment. Plausible approaches for therapy would include achieving “gain” or “loss” of miRNA functions in the cancer cells (Figure 2). Since many miRNAs have been identified to impart tumor suppressive effects, restoring their expression (endogenously or exogenously) may yield therapeutic effects. In an earlier study, ectopic overexpression of let-7 tumor suppressor miRNA was shown to inhibit the growth of lung, liver and pancreatic cancer cells (24, 29). Furthermore, systemic administration of miR-26a using adeno-associated virus (AAV) in an animal model of hepatocellular carcinoma (HCC) also inhibited tumor progression without toxicity (41). This study not only provided an important target miRNA with tumor suppressive activity, but also indicated that adenoviral vector-based delivery could be a clinically feasible approach. In other reports, synthetic miRNA mimics have also been used to restore miRNA function within the tumor cell (42). For oncogenic miRNAs, multiple approaches have been designed and tested to achieve their downregulation. These approaches include the use of anti-miRNA oligonucleotides (AMOs), small molecule inhibitors, miRNA sponges and miRNA masking. AMOs can block the interactions between miRNA and its target mRNAs through competitive inhibition of base-pairing (43). AMOs against miR-21 were shown to inhibit the growth of MCF-7 cells in vivo (17). Other study has shown that intravenous administration of AMOs against miR-16, miR-122, miR-192 and miR-194 in animals offer efficient and sustained silencing of corresponding miRNAs (44). The downregulation of miRNAs can also be achieved by targeting their biogenesis. In a recent study, several small organic molecules were screened for their potential to block miR-21 function and azobenzene was identified as an efficient inhibitor of miR-21 expression (45). Another strategy that has been tested to block miRNA function is the use of synthetic mRNA containing multiple pairing sites for endogenous miRNA. Such synthetic mRNAs, referred as miRNA sponges, function by sucking up the target miRNAs and thus blocking their regulatory function in the cell (46). Some recent studies have also employed an approach of using target protectors (miR-masks) (47, 48). miR-masks are like AMOs, however, they do not directly interact with its target miRNA but instead bind to the pairing site of miRNAs in the 3’ UTR of the target mRNA by fully complementary mechanism. In a rat model, miR-masks complementary to HCN2 and HCN4 genes masked the actions of their respective targeting miRNAs, miR-1 and miR-133 (48). In another study, protection of squint or lefty mRNAs from miR-430 was also reported (47). These studies suggest that silencing or restoration of miRNA function can serve as a promising therapeutic strategy for cancer treatment. Furthermore, as miRNAs are also associated with chemo- and radio-sensitization of cancer cells (19;49), a combination approach can also be developed for improved therapeutic outcome.
Figure 2. miRNA-based therapeutic strategies against cancer.
Inhibiting the function of oncomirs by use of anti-miR oligonucleotides (AMOs), small molecule inhibitors, miRNA sponges and miRNA masks/target protectors, and promoting the activity of anti-oncomirs through gene therapy or delivery of miRNA mimics can serve as novel therapeutic options against cancer.
Conclusion and perspectives
Emergence of miRNAs as a new class of gene regulators and their proven role in cancer progression has opened new avenues for therapeutic discovery. Interest in miRNAs is now more than ever and the literature is getting enriched rapidly with reports on novel miRNAs, their validated gene targets and the development of miRNA-based therapeutics. The realization of a miRNA-based therapeutic approach in clinics; however, may still be far from sight and several hindrances pertaining to the stability, specificity and delivery of short oligonucleotides need to be overcome. Nonetheless, the use of LNA (locked nucleic acid) technology has presented a breakthrough and a phase I clinical trial with LNA-antimiR-122 has already been initiated based on exciting preliminary data in non-human primates (50). Also, advances have been made in the delivery systems and the systemic administration of miRNA using adeno-associated virus (AAV) has been tested in pre-clinical model (41). Similarly, cationic liposomes or polymer-based nanoparticle formulations can be developed to achieve the delivery of miRNA mimics and tested for therapeutic efficacy. With increasing interest, research progress in miRNA functions and technological advancements, miRNA-based therapeutics may create a paradigm-shift in medicine and pharmaceutical industry.
Acknowledgments
The authors are supported by grants from the NIH/NCI (CA137513), Department of Defense/US Army (W81XWH-09-1-0137) and USAMCI.
Footnotes
Conflicts of Interest
No potential conflicts of interest to disclose.
References
- 1.Bentwich I, Avniel A, Karov Y, et al. Identification of hundreds of conserved and nonconserved human microRNAs. Nat Genet. 2005;37:766–70. doi: 10.1038/ng1590. [DOI] [PubMed] [Google Scholar]
- 2.Griffiths-Jones S, Saini HK, van DS, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res. 2008;36:154–158. doi: 10.1093/nar/gkm952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–66. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 4.Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11:228–34. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 6.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calin GA, Dumitru CD, Shimizu M, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2002;99:15524–9. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–8. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 9.Shimono Y, Zabala M, Cho RW, et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138:592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 11.Wellner U, Schubert J, Burk UC, et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11:1487–95. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- 12.Hayashita Y, Osada H, Tatematsu Y, et al. A polycistronic microRNA cluster, miR-17–92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res. 2005;65:9628–32. doi: 10.1158/0008-5472.CAN-05-2352. [DOI] [PubMed] [Google Scholar]
- 13.Dews M, Homayouni A, Yu D, et al. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat Genet. 2006;38:1060–5. doi: 10.1038/ng1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Northcott PA, Fernandez L, Hagan JP, et al. The miR-17/92 polycistron is up-regulated in sonic hedgehog-driven medulloblastomas and induced by N-myc in sonic hedgehog-treated cerebellar neural precursors. Cancer Res. 2009;69:3249–55. doi: 10.1158/0008-5472.CAN-08-4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uziel T, Karginov FV, Xie S, et al. The miR-17~92 cluster collaborates with the Sonic Hedgehog pathway in medulloblastoma. Proc Natl Acad Sci U S A. 2009;106:2812–7. doi: 10.1073/pnas.0809579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–33. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 17.Si ML, Zhu S, Wu H, et al. miR-21-mediated tumor growth. Oncogene. 2007;26:2799–803. doi: 10.1038/sj.onc.1210083. [DOI] [PubMed] [Google Scholar]
- 18.Asangani IA, Rasheed SA, Nikolova DA, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–36. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Li W, Yang Y, et al. MicroRNA-21 targets LRRFIP1 and contributes to VM-26 resistance in glioblastoma multiforme. Brain Res. 2009;1286:13–8. doi: 10.1016/j.brainres.2009.06.053. [DOI] [PubMed] [Google Scholar]
- 20.Qin W, Zhao B, Shi Y, et al. BMPRII is a direct target of miR-21. Acta Biochim Biophys Sin (Shanghai) 2009;41:618–23. doi: 10.1093/abbs/gmp049. [DOI] [PubMed] [Google Scholar]
- 21.Greither T, Grochola LF, Udelnow A, et al. Elevated expression of microRNAs 155, 203, 210 and 222 in pancreatic tumors is associated with poorer survival. Int J Cancer. 2010;126:73–80. doi: 10.1002/ijc.24687. [DOI] [PubMed] [Google Scholar]
- 22.Gironella M, Seux M, Xie MJ, et al. Tumor protein 53-induced nuclear protein 1 expression is repressed by miR-155, and its restoration inhibits pancreatic tumor development. Proc Natl Acad Sci U S A. 2007;104:16170–5. doi: 10.1073/pnas.0703942104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voorhoeve PM, le SC, Schrier M, et al. A genetic screen implicates miRNA-372 and miRNA-373 as oncogenes in testicular germ cell tumors. Cell. 2006;124:1169–81. doi: 10.1016/j.cell.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 24.Takamizawa J, Konishi H, Yanagisawa K, et al. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004;64:3753–6. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- 25.Calin GA, Cimmino A, Fabbri M, et al. MiR-15a and miR-16-1 cluster functions in human leukemia. Proc Natl Acad Sci U S A. 2008;105:5166–71. doi: 10.1073/pnas.0800121105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cimmino A, Calin GA, Fabbri M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A. 2005;102:13944–9. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeshita F, Patrawala L, Osaki M, et al. Systemic delivery of synthetic microRNA-16 inhibits the growth of metastatic prostate tumors via downregulation of multiple cell-cycle genes. Mol Ther. 2010;18:181–7. doi: 10.1038/mt.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonci D, Coppola V, Musumeci M, et al. The miR-15a-miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat Med. 2008;14:1271–7. doi: 10.1038/nm.1880. [DOI] [PubMed] [Google Scholar]
- 29.Johnson SM, Grosshans H, Shingara J, et al. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–47. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Chang TC, Wentzel EA, Kent OA, et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26:745–52. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ji Q, Hao X, Zhang M, et al. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS One. 2009;4:e6816. doi: 10.1371/journal.pone.0006816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alison MR, Lim SM, Nicholson LJ. Cancer stem cells: problems for therapy? J Pathol. 2011;223:148–62. doi: 10.1002/path.2793. [DOI] [PubMed] [Google Scholar]
- 33.Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gangaraju VK, Lin H. MicroRNAs: key regulators of stem cells. Nat Rev Mol Cell Biol. 2009;10:116–25. doi: 10.1038/nrm2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gregory PA, Bracken CP, Bert AG, Goodall GJ. MicroRNAs as regulators of epithelial-mesenchymal transition. Cell Cycle. 2008;7:3112–8. doi: 10.4161/cc.7.20.6851. [DOI] [PubMed] [Google Scholar]
- 36.Castilla MA, Moreno-Bueno G, Romero-Perez L, et al. Micro-RNA signature of the epithelial-mesenchymal transition in endometrial carcinosarcoma. J Pathol. 2011;223:72–80. doi: 10.1002/path.2802. [DOI] [PubMed] [Google Scholar]
- 37.Wang YC, Chen YL, Yuan RH, et al. Lin-28B expression promotes transformation and invasion in human hepatocellular carcinoma. Carcinogenesis. 2010;31:1516–22. doi: 10.1093/carcin/bgq107. [DOI] [PubMed] [Google Scholar]
- 38.Yu F, Yao H, Zhu P, et al. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–23. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, VandenBoom TG, Kong D, et al. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009;69:6704–12. doi: 10.1158/0008-5472.CAN-09-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kong D, Li Y, Wang Z, et al. miR-200 regulates PDGF-D-mediated epithelial-mesenchymal transition, adhesion, and invasion of prostate cancer cells. Stem Cells. 2009;27:1712–21. doi: 10.1002/stem.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kota J, Chivukula RR, O’Donnell KA, et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–17. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsuda N, Ishiyama S, Li Y, et al. Synthetic microRNA designed to target glioma-associated antigen 1 transcription factor inhibits division and induces late apoptosis in pancreatic tumor cells. Clin Cancer Res. 2006;12:6557–64. doi: 10.1158/1078-0432.CCR-06-0588. [DOI] [PubMed] [Google Scholar]
- 43.Weiler J, Hunziker J, Hall J. Anti-miRNA oligonucleotides (AMOs): ammunition to target miRNAs implicated in human disease? Gene Ther. 2006;13:496–502. doi: 10.1038/sj.gt.3302654. [DOI] [PubMed] [Google Scholar]
- 44.Krutzfeldt J, Rajewsky N, Braich R, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438:685–9. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 45.Gumireddy K, Young DD, Xiong X, et al. Small-molecule inhibitors of microrna miR-21 function. Angew Chem Int Ed Engl. 2008;47:7482–4. doi: 10.1002/anie.200801555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ebert MS, Neilson JR, Sharp PA. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods. 2007;4:721–6. doi: 10.1038/nmeth1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi WY, Giraldez AJ, Schier AF. Target protectors reveal dampening and balancing of Nodal agonist and antagonist by miR-430. Science. 2007;318:271–4. doi: 10.1126/science.1147535. [DOI] [PubMed] [Google Scholar]
- 48.Xiao J, Yang B, Lin H, et al. Novel approaches for gene-specific interference via manipulating actions of microRNAs: examination on the pacemaker channel genes HCN2 and HCN4. J Cell Physiol. 2007;212:285–92. doi: 10.1002/jcp.21062. [DOI] [PubMed] [Google Scholar]
- 49.Jeong SH, Wu HG, Park WY. LIN28B confers radio-resistance through the posttranscriptional control of KRAS. Exp Mol Med. 2009;41:912–8. doi: 10.3858/emm.2009.41.12.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elmen J, Lindow M, Schutz S, et al. LNA-mediated microRNA silencing in non-human primates. Nature. 2008;452:896–9. doi: 10.1038/nature06783. [DOI] [PubMed] [Google Scholar]