Abstract
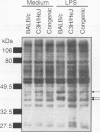
Development of a congenic BALB/c mouse strain that contains a segment of chromosome 4 including the Lpsd allele of the lipopolysaccharide (LPS)-hyporesponsive C3H/HeJ strain is presented. On the basis of LPS-induced spleen cell mitogenesis, macrophage tumor necrosis factor secretion, and tyrosine phosphorylation in vitro and lethality in galactosamine-sensitized mice in vivo, the C.C3H-Lpsd strain provides a model of LPS hyporesponsiveness that is comparable to that of the parental C3H/HeJ strain. Analysis of markers in this region indicates that length of the donor fragment is approximately 5.5 centimorgans. Thus, the C.C3H-Lpsd strain provides an important genetic tool for analysis of markers in this region and for examining functional effects of Lpsd expression on the BALB/c background.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott C. M., Blank R., Eppig J. T., Fiedorek F. T., Jr, Frankel W., Friedman J. M., Huppi K. E., Jackson I., Steel K., Mock B. A. Encyclopedia of the mouse genome III. October 1993. Mouse chromosome 4. Mamm Genome. 1993;4(Spec No):S58–S71. doi: 10.1007/BF00360830. [DOI] [PubMed] [Google Scholar]
- Bahary N., McGraw D. E., Shilling R., Friedman J. M. Microdissection and microcloning of mid-chromosome 4: genetic mapping of 41 microdissection clones. Genomics. 1993 Apr;16(1):113–122. doi: 10.1006/geno.1993.1148. [DOI] [PubMed] [Google Scholar]
- Begley C. G., Visvader J., Green A. R., Aplan P. D., Metcalf D., Kirsch I. R., Gough N. M. Molecular cloning and chromosomal localization of the murine homolog of the human helix-loop-helix gene SCL. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):869–873. doi: 10.1073/pnas.88.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd L. G., McDonald A. H., Gold L. G., Potter M. Specific pathogen-free BALB/cAn mice are refractory to plasmacytoma induction by pristane. J Immunol. 1991 Nov 15;147(10):3632–3637. [PubMed] [Google Scholar]
- Copeland N. G., Jenkins N. A., Gilbert D. J., Eppig J. T., Maltais L. J., Miller J. C., Dietrich W. F., Weaver A., Lincoln S. E., Steen R. G. A genetic linkage map of the mouse: current applications and future prospects. Science. 1993 Oct 1;262(5130):57–66. doi: 10.1126/science.8211130. [DOI] [PubMed] [Google Scholar]
- De Maeyer E., Dandoy F. Linkage analysis of the murine interferon alpha locus (Ifa) on chromosome 4. J Hered. 1987 May-Jun;78(3):143–146. doi: 10.1093/oxfordjournals.jhered.a110346. [DOI] [PubMed] [Google Scholar]
- Dietrich W., Katz H., Lincoln S. E., Shin H. S., Friedman J., Dracopoli N. C., Lander E. S. A genetic map of the mouse suitable for typing intraspecific crosses. Genetics. 1992 Jun;131(2):423–447. doi: 10.1093/genetics/131.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson J. S., Hudson D. M., Armstrong B. L. Location of the Mup-a locus on mouse linkage group 8. Genet Res. 1969 Dec;14(3):329–331. doi: 10.1017/s0016672300002159. [DOI] [PubMed] [Google Scholar]
- Fultz M. J., Vogel S. N. The physical separation of Lps and Ifa loci in BXH recombinant inbred mice. J Immunol. 1989 Nov 1;143(9):3001–3006. [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A., Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan M. M., Vogel S. N. Production of tumor necrosis factor by rIFN-gamma-primed C3H/HeJ (Lpsd) macrophages requires the presence of lipid A-associated proteins. J Immunol. 1988 Dec 15;141(12):4196–4202. [PubMed] [Google Scholar]
- Hudson D. M., Finlayson J. S., Potter M. Linkage of one component of the major urinary protein complex of mice to the brown coat color locus. Genet Res. 1967 Oct;10(2):195–198. doi: 10.1017/s0016672300010922. [DOI] [PubMed] [Google Scholar]
- Johnson D. R. Polysyndactyly, a new mutant gene in the mouse. J Embryol Exp Morphol. 1969 Apr;21(2):285–294. [PubMed] [Google Scholar]
- Krall M., Ruff N., Zimmerman K., Aggarwal A., Dosik J., Reeves R., Mock B. A. Isolation and mapping of four new DNA markers from mouse chromosome 4. Mamm Genome. 1992;3(11):653–655. doi: 10.1007/BF00352484. [DOI] [PubMed] [Google Scholar]
- Kuhn N. J., Woodworth-Gutai M., Gross K. W., Held W. A. Subfamilies of the mouse major urinary protein (MUP) multi-gene family: sequence analysis of cDNA clones and differential regulation in the liver. Nucleic Acids Res. 1984 Aug 10;12(15):6073–6090. doi: 10.1093/nar/12.15.6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthey C. L., Qureshi N., Stütz P. L., Vogel S. N. Lipopolysaccharide antagonists block taxol-induced signaling in murine macrophages. J Exp Med. 1993 Aug 1;178(2):695–702. doi: 10.1084/jem.178.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam K. P., Ryan J. L. C57BL/10/CR mice: nonresponders to activation by the lipid a moiety of bacterial lipopolysaccharide. J Immunol. 1978 Jan;120(1):249–253. [PubMed] [Google Scholar]
- McIntire F. C., Sievert H. W., Barlow G. H., Finley R. A., Lee A. Y. Chemical, physical, biological properties of a lipopolysaccharide from Escherichia coli K-235. Biochemistry. 1967 Aug;6(8):2363–2372. doi: 10.1021/bi00860a011. [DOI] [PubMed] [Google Scholar]
- Mock B. A., Krall M. M., Dosik J. K. Genetic mapping of tumor susceptibility genes involved in mouse plasmacytomagenesis. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9499–9503. doi: 10.1073/pnas.90.20.9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter M., Wiener F. Plasmacytomagenesis in mice: model of neoplastic development dependent upon chromosomal translocations. Carcinogenesis. 1992 Oct;13(10):1681–1697. doi: 10.1093/carcin/13.10.1681. [DOI] [PubMed] [Google Scholar]
- Prowse K. R., Baumann H. Molecular characterization and acute phase expression of the multiple Mus caroli alpha 1-acid glycoprotein (AGP) genes. Differences in glucocorticoid stimulation and regulatory elements between the rat and mouse AGP genes. J Biol Chem. 1990 Jun 25;265(18):10201–10209. [PubMed] [Google Scholar]
- Rosenstreich D. L., Vogel S. N., Jacques A. R., Wahl L. M., Oppenheim J. J. Macrophage sensitivity to endotoxin: genetic control by a single codominant gene. J Immunol. 1978 Nov;121(5):1664–1670. [PubMed] [Google Scholar]
- Smith C. A., Gruss H. J., Davis T., Anderson D., Farrah T., Baker E., Sutherland G. R., Brannan C. I., Copeland N. G., Jenkins N. A. CD30 antigen, a marker for Hodgkin's lymphoma, is a receptor whose ligand defines an emerging family of cytokines with homology to TNF. Cell. 1993 Jul 2;73(7):1349–1360. doi: 10.1016/0092-8674(93)90361-s. [DOI] [PubMed] [Google Scholar]
- Vogel S. N., Hansen C. T., Rosenstreich D. L. Characterization of a congenitally LPS-resistant, athymic mouse strain. J Immunol. 1979 Feb;122(2):619–622. [PubMed] [Google Scholar]
- Watson J., Kelly K., Largen M., Taylor B. A. The genetic mapping of a defective LPS response gene in C3H/HeJ mice. J Immunol. 1978 Feb;120(2):422–424. [PubMed] [Google Scholar]
- Watson J., Largen M., McAdam K. P. Genetic control of endotoxic responses in mice. J Exp Med. 1978 Jan 1;147(1):39–49. doi: 10.1084/jem.147.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J., Riblet R., Taylor B. A. The response of recombinant inbred strains of mice to bacterial lipopolysaccharides. J Immunol. 1977 Jun;118(6):2088–2093. [PubMed] [Google Scholar]
- Weinstein S. L., Sanghera J. S., Lemke K., DeFranco A. L., Pelech S. L. Bacterial lipopolysaccharide induces tyrosine phosphorylation and activation of mitogen-activated protein kinases in macrophages. J Biol Chem. 1992 Jul 25;267(21):14955–14962. [PubMed] [Google Scholar]