Abstract
Early in T-cell development, cells proceed through stages that are critically dependent on signaling through the Notch receptor. As cells mature, thymocytes transition from being Notch dependent to being Notch independent, but the stage of development during which this transition occurs is unknown. We used an in vitro differentiation system in which thymocytes can be cultured in the presence or absence of a Notch ligand to identify the stage of development in which thymocytes transition from being Notch responsive to Notch non-responsive. We identified the immature single-positive (ISP) CD8+ stage of T-cell development as being this transition point. ISP thymocytes were responsive to Notch, but ISP cells responded to Notch ligation in a manner that was distinct from the response by double-negative (DN) thymocytes. Fewer ISP thymocytes proliferated and more ISP cells died in culture than DN thymocytes. Further, fewer double-positive (DP) thymocytes generated by culturing ISP thymocytes were in the S, G2 or M phase of the cell cycle as compared with DP thymocytes derived from DN thymocytes. These data indicate that the DP population created varied depending on the input population. In summary, the data presented here indicate that ISP thymocytes responded to Notch differently than DN thymocytes and ISP thymocytes represent the transition stage from Notch-dependent survival and proliferation to Notch-independent survival and proliferation.
Keywords: signaling pathways, thymocytes
Introduction
The earliest identifiable T-cell precursors within the thymus lack expression of CD4 and CD8 and are therefore named CD4−CD8− double-negative (DN) thymocytes. DN thymocytes pass through a series of at least five developmental stages that are defined by the expression of CD44 and CD25: DN1 (CD44+CD25−), DN2 (CD44+CD25+), DN3E (CD44−CD25hi), DN3L (CD44−CD25lo) and DN4 (CD44−CD25−) (1, 2). T-cell precursors entering the thymus become committed to the T-cell lineage and then proliferate before beginning the genomic rearrangements necessary for TCRβ expression. TCRβ protein can first be detected within the DN3E population, where it pairs with an invariant protein, pre-Tα, to form the pre-TCR (3). Expression of the pre-TCR complex promotes the survival, proliferation and differentiation of DN thymocytes (3–5). After the DN stages, thymocytes express CD8 and become immature single-positive (ISP) CD8+ thymocytes before expressing CD4 and becoming CD4+CD8+ double-positive (DP) thymocytes.
The signaling pathways required for the survival, proliferation and differentiation of TCRβ-expressing DN thymocytes are not clearly defined. While TCRβ is required, it is not sufficient as culturing cells without stroma results in cell death (6). Signaling pathways that have emerged as being important for early T-cell development are activated via the Notch receptor (7, 8). T-cell precursors entering the thymus require Notch for commitment to the T-cell lineage (9–16). In addition, Notch is necessary for TCRβ rearrangement and commitment to the αβ T-cell lineage (14, 17).
After TCRβ is expressed in DN3 thymocytes, the role of Notch is less clear. Several studies showed that Notch is required for the survival and differentiation of DN3 thymocytes (16, 18–20), but the interpretation of these data is complicated because the DN3 population is heterogenous; only 20–25% of DN3 thymocytes express TCRβ, a few cells express TCRγδ and the majority of DN3 cells do not express either TCRβ or TCRγδ. Notch does not appear to be required for the survival, proliferation or differentiation of TCRγδ+ DN3 thymocytes (19, 21, 22). Taghon et al. (21) demonstrated that TCRβ+ DN3 thymocytes survived in the presence of a Notch ligand, while TCRβ− DN thymocytes could not survive or differentiate. These data indicate that Notch is required early after TCRβ expression.
During the differentiation of TCRβ+ DN thymocytes into DP thymocytes, Notch1 receptor expression declines such that DP thymocytes express little Notch1 protein on their surface (23, 24). This observation suggests that Notch may not be required for the survival of DP thymocytes. In support of a limited role for Notch signaling following TCRβ expression, mRNA levels of the Notch-dependent genes Deltex-1, Hes-1 and pre-Tα decline after TCRβ is expressed (21, 23, 25–27). Further, deleting Notch1 expression or function late in T-cell development had no detectable consequences on the size of the DP population (28, 29). These observations establish a window between TCRβ+ DN3E thymocytes and DP thymocytes in which cells transition from being Notch dependent to Notch independent. However, the precise stage of development during which this transition occurs is unknown.
In this study, we defined the stage of development during which the transition from Notch-dependent survival to Notch-independent survival occurs. We used an in vitro differentiation system (30) to characterize the survival, proliferation and differentiation of DN and ISP thymocytes in the presence or absence of Notch ligands. By comparing the in vitro-created DP thymocytes that were derived from each subset, we were able to determine the stage of development in which thymocytes become Notch dependent.
Methods
Mice
Wild-type C57BL/6 mice were housed under specific pathogen-free conditions. Mice were used between the ages of 3 and 5 weeks and all experiments were performed in compliance with the University of Kansas Medical Center Institutional Care and Use Committee.
Antibodies
Anti-CD4-FITC, anti-TER119-FITC, anti-CD24-PE, anti-CD44-PE-Cy7, anti-CD44-PECy5.5, anti-CD25-APCCy7 and anti-TCRβ-PECy5.5 were purchased from eBioscience (San Diego, CA, USA). Anti-CD45.2-PE was purchased from BioLegend (San Diego, CA, USA). Anti-CD8-FITC, anti-CD8-Alexa Fluor 647, anti-CD4-V450 and anti-CD4-Pacific Blue were purchased from BD Biosciences (San Jose, CA, USA).
FACS purification
For DN and ISP thymocyte subsets, total thymocytes were depleted using anti-mouse CD4 magnetic particles-dodecyl maltoside (BD Biosciences). Remaining cells were surface labeled with anti-CD4, anti-TER119, anti-CD8, anti-CD24, anti-CD25 and anti-CD44. Then, cells were gated on TER119−CD4−CD8− thymocytes and TER119−CD8+ thymocytes and DN3E, DN3L, DN4 and ISP subsets were Fluorescence activated cell sorting (FACS) purified. For DP thymocytes, total thymocytes were surface labeled with anti-CD4 and anti-CD8, and DP thymocytes were FACS purified.
For experiments in which cultured thymocytes were repurified, FACS-purified DN3E thymocytes were cultured with OP9-DL1 cells for 3 days, harvested and surface labeled with anti-CD4 and anti-CD8. DN, ISP and DP subsets were re-FACS purified.
For cycling and non-cycling DP subsets, fresh total thymocytes were surface labeled with anti-CD4, anti-CD24, anti-CD8 and anti-TCRβ. Singlet thymocytes were gated according to the forward scatter (FSC) Area versus width dot plots. DP singlets were then divided into CD24hiTCRβlo and CD24intTCRβlo subsets and were analyzed for size using FSC. FACS purification was performed using a BD FACSAria (BD Biosciences).
In vitro differentiation
The in vitro differentiation system was used as described previously (2, 30). Briefly, 5 × 104 FACS-purified thymocytes were cultured with OP9-delta-like (DL) 1 cells, OP9-DL4 cells or OP9-green fluorescent protein (GFP) cells [generous gifts of Dr Juan-Carlos Zúñiga-Pflücker (30, 31)] in MEM alpha medium (Invitrogen, Carlsbad, CA, USA) supplemented with 20% FBS, penicillin, streptomycin, 5 ng ml−1 IL-7 and 5 ng ml−1 Flt3L (PeproTech, Rocky Hill, NJ, USA). For some experiments, FACS-purified thymocytes were labeled with 5 μM 5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester (CFSE) before culturing.
Cell labeling for flow cytometry
Surface staining was performed in staining buffer [PBS containing 2% alpha calf fraction (Hyclone, Waltham, MA, USA)] and fixed in 1% paraformaldehyde before analysis.
For ethidium monoazide (EMA) labeling, 0.5 μg ml−1 EMA (Invitrogen) was added to thymocytes during surface labeling and EMA was bound to DNA by exposing cells to a 60-W light bulb for 15 min. CountBright™ absolute counting beads (Invitrogen) were also added to each sample.
For cell cycle analysis, thymocytes were surface labeled and fixed in 4% paraformaldehyde. After washing, cells were incubated with 1 μg ml−1 4′,6-diamidino-2-phenylindole (DAPI) in staining buffer containing 0.2% Tween 20 and analyzed immediately.
Flow cytometry
Cells were analyzed using a BD LSR II (BD Biosciences). Data were analyzed using BD FACSDiva software (BD Biosciences).
Quantitative real-time PCR
Total RNA was isolated from the FACS-purified cells and converted to cDNA using the TaqMan® Gene Expression Cells-to-Ct™ kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. Eight microliter cDNA, 10 μl TaqMan® Gene Expression Master Mix (Applied Biosystems), 1 μl TaqMan® Gene Expression Assay of pre-Tα target gene or GAPDH housekeeping gene (both were purchased from Applied Biosystems) and 1 μl nuclease-free water (Promega, Madison, WI, USA) were added to each PCR. Relative quantification PCR amplification was performed using a 7500 Fast Real-Time PCR System (Applied Biosystems). Data were analyzed using 7500 Fast System Software in a relative quantification study. Relative expression levels of pre-Tα in each subset were calculated using the comparative Ct method (2−ΔΔCt).
To calculate each bar in the graphs, each sample was analyzed in triplicate and averaged. Replicates for each subset were acquired using three to four independent experiments. Statistical significance was determined by averaging the means of the independently acquired experiments.
Statistics
Statistics were performed using the two-tailed Student’s t-test (unless otherwise indicated) and significance was defined as P < 0.05.
Results
Notch is not required for the differentiation of TCRβ+ DN and ISP thymocytes into DP thymocytes
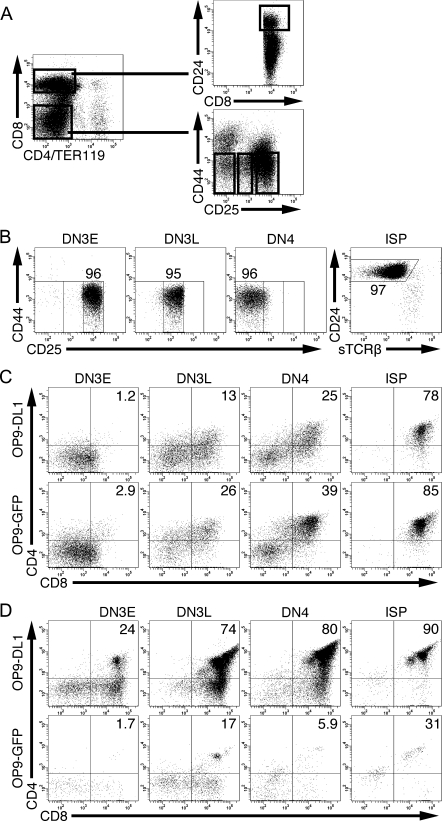
DN3E, DN3L, DN4 and ISP thymocyte subsets were FACS purified according to the strategy shown in Fig. 1A. The gating strategy for the DN subsets was based on our previous work showing that DN3E, DN3L and DN4 thymocytes are phenotypically and functionally distinct subsets (2). The purities of each subset were consistently >90% (Fig. 1B). DN3E, DN3L, DN4 and ISP thymocytes were cultured in the presence of OP9-DL1 cells or OP9-GFP cells. After 1 day, a greater percentage of recovered thymocytes were DP thymocytes when cells were cultured with OP9-GFP cells than with OP9-DL1 cells (Fig. 1C), consistent with observations using human CD34+ cells (32). However, after 5 days of culture, few thymocytes remained in the cultures containing OP9-GFP cells and the percentage of cells in the DP gate had declined (Fig. 1D). These data are consistent with previous observations (6, 33, 34) and suggest that Notch may not be required for differentiation, although the signal received through Notch in vivo may be sufficient to enable differentiation. Further, Notch may either impair differentiation or may be required for survival or proliferation of DN and ISP thymocytes.
Fig. 1.
Notch is not required for the differentiation of TCRβ+ DN and ISP thymocytes into DP thymocytes. (A) Gating strategy for the FACS purification of DN and ISP thymocytes. (B) A representative figure illustrating the post-sort purity of DN3E, DN3L, DN4 and ISP thymocytes. The post-sort purity of each subset is shown in each subset. DN3E, DN3L, DN4 and ISP thymocytes were cultured with either OP9-DL1 cells or OP9-GFP cells for 1 (C) or 5 days (D). The percentages of recovered CD45.2+ cells that were DP thymocytes are shown. Representative of seven independent experiments.
Notch is required for TCRβ+ DN thymocytes to increase in cell number
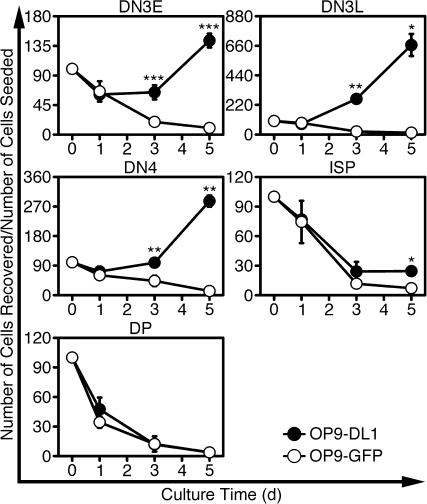
To determine whether Notch could be required for survival of TCRβ+ DN thymocytes, we cultured DN3E, DN3L, DN4 or ISP thymocytes with either OP9-DL1 or OP9-GFP cells and calculated the number of viable thymocytes recovered over time (Fig. 2). When DN3E, DN3L and DN4 thymocytes were cultured with OP9-DL1 cells for 5 days, more thymocytes were recovered than were seeded. This trend continued as DN thymocytes cultured for up to 10 days continued to expand in number (data not shown). By contrast, when DN thymocytes were cultured with OP9-GFP cells, the number of cells recovered from wells declined over time, suggesting that Notch is required for survival of TCRβ+ DN thymocytes.
Fig. 2.
Notch increases the recovery of DN3E, DN3L, DN4 and ISP thymocytes but not DP thymocytes. FACS-purified DN3E, DN3L, DN4, ISP and DP thymocytes were cultured with OP9-DL1 or OP9-GFP cells. After 1, 3 and 5 days, DN- and ISP-derived cells were labeled with anti-CD45.2 and EMA, while DP-derived cells were labeled with EMA only. The absolute numbers of live cells were calculated using counting beads. The number of cells seeded in each well was normalized to 100% and the normalized numbers of live thymocytes recovered are shown (mean ± SD). *P < 0.05; **P < 0.005; ***P < 0.001, when comparing cells cultured with OP9-DL1 and OP9-GFP cells for each time point; n = 4 independent experiments.
Unlike DN thymocytes, ISP thymocytes did not expand in number during culture; only 24 ± 4.6% of the number of seeded ISP thymocytes were recovered after culturing with OP9-DL1 cells for 5 days (Fig. 2). However, only 7.1 ± 2.5% of the number of seeded ISP thymocytes were recovered from wells seeded with OP9-GFP cells (P = 0.01, n = 4). These data indicate that ISP thymocytes are responsive to Notch ligation, but ISP cells respond to Notch ligands differently than DN thymocytes.
In contrast to DN and ISP thymocytes, when freshly isolated DP thymocytes were cultured with OP9-DL1 or OP9-GFP cells, hardly any thymocytes could be recovered after 5 days (Fig. 2). This observation indicates that few, if any, DP thymocytes can survive in culture, regardless of the presence of a Notch ligand.
Collectively, these data indicate that Notch contributes to the survival of TCRβ+ DN and ISP thymocytes, but DP thymocytes could not respond to Notch ligation. Because each population analyzed differentiated into DP thymocytes in this culture, these data suggest that DP thymocytes responded differently in culture depending whether they were derived from DN thymocytes, derived from ISP thymocytes or freshly isolated from mice.
Notch is required for proliferation of TCRβ+ DN and ISP thymocytes
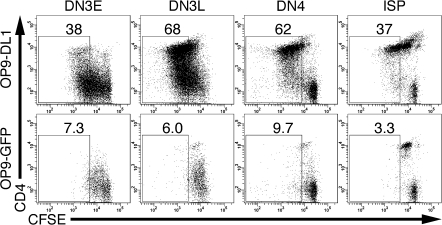
We next tested whether Notch was required for proliferation of TCRβ+ DN and ISP thymocytes by labeling FACS-purified DN3E, DN3L, DN4 and ISP thymocytes with CFSE and coculturing the cells with OP9-DL1 or OP9-GFP cells for 4 days (Fig. 3). Many cells in each population of thymocytes proliferated more than three times in the presence of OP9-DL1 cells, but most cells cultured with OP9-GFP cells only divided one to three times.
Fig. 3.
Notch is required for the proliferation of DN3E, DN3L, DN4 and ISP thymocytes. DN3E, DN3L, DN4 and ISP thymocytes were labeled with CFSE and cultured with either OP9-DL1 or OP9-GFP cells for 4 days. Thymocytes were stained with anti-CD45.2, anti-CD4 and analyzed by flow cytometry. The percentages of thymocytes that underwent more than three cell divisions are shown. Representative of three independent experiments.
Like the DN populations, ISP thymocytes also proliferated in a Notch-dependent manner (Fig. 3). However, the percentage of ISP thymocytes that divided more than three times was less than the percentage of DN3L and DN4 thymocytes that divided more than three times, consistent with a model in which ISP thymocytes respond to Notch ligation differently than TCRβ+ DN thymocytes.
Collectively, these data indicate that Notch is required for the survival and proliferation of TCRβ+ DN and ISP thymocytes in vitro. Although ISP cells were Notch responsive and proliferated, ISP cells did not survive and proliferate as readily as DN thymocytes and did not expand in number over time.
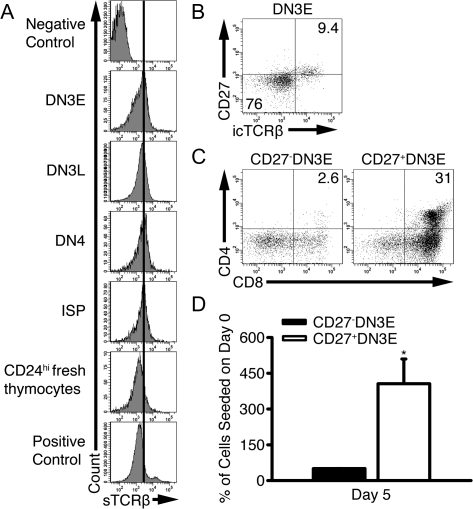
Fewer DN3E thymocytes underwent multiple rounds of cell division than DN3L and DN4 thymocytes. This difference is likely due to the fact that most DN3E thymocytes do not express TCRβ (2). We tested whether TCRβ− cells could survive or proliferate in the in vitro differentiation system by comparing surface TCRβ expression on freshly isolated CD24hi immature thymocytes with DN and ISP thymocytes that were cultured with OP9-DL1 cells for 4 days (Fig. 4A). At harvest, all viable thymocytes expressed levels of surface TCR that were slightly higher than freshly isolated immature thymocytes but less than TCRhi thymocytes. This slight increase in TCR expression is consistent with previous observations that CD4 ligation in vivo suppresses surface TCR expression (35, 36). These data indicate that few, if any, TCR− thymocytes persisted throughout the assay; TCR− cells either died or differentiated into TCR+ cells.
Fig. 4.
Notch does not promote survival and proliferation of TCRβ- DN thymocytes. (A) DN3E, DN3L, DN4 and ISP thymocytes were cultured with OP9-DL1 cells for 4 days and surface labeled with anti-TCRβ. The expression levels of surface TCRβ in each subset are shown. For comparison, TCRβ expression on freshly isolated CD24hi thymocytes is shown. As a positive control, TCRβ expression was analyzed on total thymocytes. Representative of four independent experiments. (B) Thymocytes were surface labeled with anti-CD4, anti-CD8, anti-CD44, anti-CD25 and anti-CD27 and intracellularly stained with anti-TCRβ. Cells were gated on DN3E thymocytes and analyzed for CD27 and TCRβ expression. (C) CD27− DN3E and CD27+ DN3E thymocytes were cultured with OP9-DL1 cells for 5 days. The percentages of recovered CD27− DN3E and CD27+ DN3E cells that were DP thymocytes are shown. (D) The numbers of recovered viable thymocytes were calculated as described in the legend to Fig. 2 (mean ± SD). *P < 0.05, n = 3 independent experiments.
As an additional assay to determine whether TCR− thymocytes contribute to our survival and proliferation data, we used CD27 to separate TCRβ+ DN3E thymocytes from TCRβ− DN3E thymocytes and cultured CD27− DN3E and CD27+ DN3E thymocytes with OP9-DL1 cells for 5 days. As previously reported (21, 37), CD27 expression correlated with intracellular TCRβ expression in DN3E thymocytes (Fig. 4B). Few CD27− DN3E thymocytes differentiated into DP thymocytes (Fig. 4C), consistent with prior studies (21). Further, the number of thymocytes recovered from wells seeded with CD27− DN3E thymocytes was 11 ± 1.5% of the number of cells recovered from wells seeded with CD27+ DN3E cells (P = 0.008, n = 3) (Fig. 4D). These data indicate that the effects of Notch signaling on survival, proliferation and differentiation described above were likely caused by the direct effect of DL1 on TCRβ-expressing cells and not due to the effects of DL1 on TCRβ− cells.
In vitro-created DP thymocytes continue to proliferate in the presence of Notch ligands
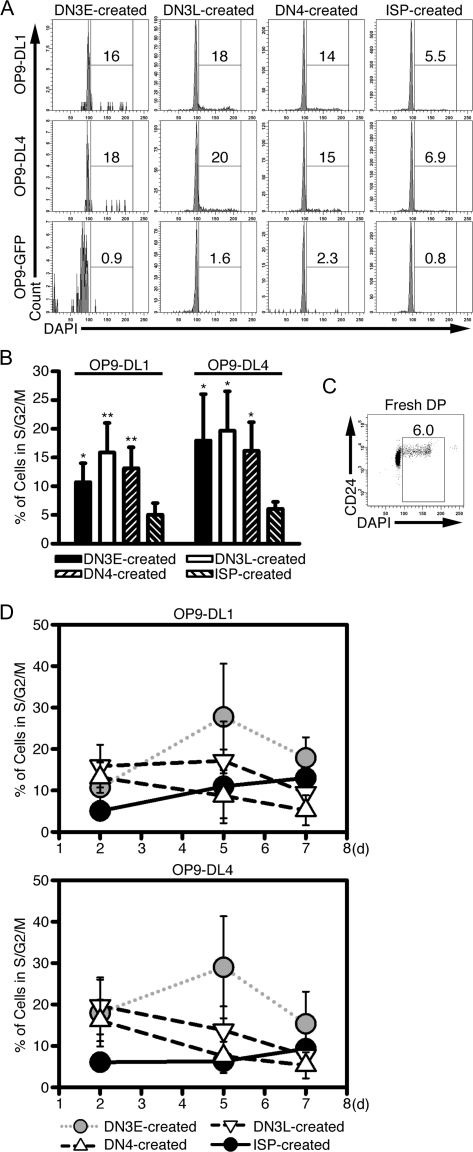
The CFSE data shown in Fig. 3 suggested that thymocytes cultured with OP9-DL1 cells continued to proliferate after the cells differentiated into DP thymocytes. To test whether in vitro-created DP thymocytes can proliferate, we measured the DNA content of DP thymocytes created after culturing DN3E, DN3L, DN4 and ISP thymocytes with OP9-DL1 cells or the control cell line OP9-GFP (Fig. 5A and B). Approximately 15% of the DP thymocytes created from DN thymocytes in the presence of OP9-DL1 cells were in the S, G2 or M phase of the cell cycle, while only 5.0 ± 2.0% of DP thymocytes created from ISP cells were in the S, G2 or M phase. For DN-derived DP thymocytes and ISP-derived DP thymocytes, the proliferation was Notch dependent as few thymocytes cultured with OP9-GFP cells were in the S, G2 or M phase of the cell cycle. This difference between DN and ISP thymocytes is consistent with our data showing that ISP thymocytes do not undergo as many rounds of cell division as DN thymocytes (Fig. 3). For comparison, 5.9 ± 1.4% of freshly isolated DP thymocytes were in the S, G2 or M phase of the cell cycle (Fig. 5C).
Fig. 5.
In vitro-created DP thymocytes proliferate in the presence of the Notch ligand. (A) DN3E, DN3L, DN4 and ISP thymocytes were cultured with OP9-DL1, OP9-DL4 or OP9-GFP cells for 2 days. Cells were labeled with anti-CD4, anti-CD8 and 4′,6-diamidino-2-phenylindole (DAPI) for cell cycle analysis. Cells were gated on DP thymocytes and analyzed for DNA content. The percentages of cells in the S, G2 or M phase of the cell cycle are shown. (B) A summary of five independent experiments as represented by (A) is shown. *P < 0.05; **P < 0.005, when comparing DN-created DP thymocytes and ISP-created DP thymocytes. (C) Freshly isolated thymocytes were labeled with anti-CD4, anti-CD8, anti-CD24 and DAPI and the percentage of DP thymocytes that were in S, G2 or M phase of the cell cycle is shown. (D) Thymocytes were cultured and analyzed as described in (A) except cells were harvested 2, 5 and 7 days after culture. Shown are the percentages of DP thymocytes derived from each subset that were in the S, G2 or M phase of the cell cycle (mean ± SD of five independent experiments).
Recent data indicate that DL4 is the Notch ligand that is most abundant in the thymus and most physiologically relevant for early T-cell development (38–40). In addition, DL4 can bind DN thymocytes more efficiently than DL1 (38), suggesting that DL4 could promote a signal of different intensity than DL1. Thus, it is possible that the cell cycle induction seen in our assay is due to non-physiologic Notch signaling and not reflective of the signals received in vivo. To examine this possibility, we compared the cell cycle status of DP thymocytes derived from DN3E, DN3L, DN4 and ISP thymocytes cultured with OP9-DL1 and OP9-DL4 cells (Figs 5A and B). The percentages of DP thymocytes that were in the S, G2 or M phase of the cell cycle were comparable between cells cultured with OP9-DL1 and OP9-DL4 cells. These data indicate that DL4 and DL1 were identical in their abilities to promote the proliferation of DN and ISP thymocytes.
Because ISP thymocytes differentiate into DP thymocytes more rapidly than the DN populations, it is possible that the reduced percentage of ISP-derived DP thymocytes in the S, G2 or M phase of the cell cycle could be caused by the fact that ISP-derived DP thymocytes were cultured longer than DN-derived DP thymocytes. To test this possibility, we analyzed the DNA content of in vitro-generated DP thymocytes after 5 and 7 days of culture. These extended times would account for the differences in the differentiation kinetics between DN3L, DN4 and ISP populations. The percentage of DP thymocytes derived from ISP thymocytes that were in the S, G2 or M phase of the cell cycle increased over time, particularly when cultured with OP9-DL1 cells; 13 ± 6.4% of ISP-derived DP thymocytes were in the S, G2 or M phase of the cell cycle after 7 days of culture with OP9-DL1 cells (P = 0.047 as compared with cells cultured for 2 days) (Fig. 5D). By contrast, the percentages of DP thymocytes derived from DN3L and DN4 thymocytes that were in the S, G2 or M phase of the cell cycle decreased over time. The percentages of DN3E-derived DP thymocytes increased over the first 5 days of culture, but the percentages trended downward between 5 and 7 days [P = 0.073 and 0.035 (one-tailed Student’s t-test) for OP9-DL1 and OP9-DL4 cells, respectively].
These data indicate that the reduced percentages of the ISP-derived DP thymocytes in the S, G2 or M phase of the cell cycle seen on day 2 was not related to the length of time since the differentiation of the cells into the DP stage. Rather, the ISP-derived thymocytes responded to the Notch ligands differently than DN3L-derived and DN4-derived thymocytes. Because DN3E thymocytes differentiated with much slower kinetics than DN3L, DN4 and ISP thymocytes, DN3E-derived DP thymocytes may be a mixture of recently differentiated cells and cells that had been DP thymocytes since the first day of the assay. This mixture likely accounts for the initial increase in cell cycle progression. The decrease in the percentage of DN3E thymocytes observed at later time points mimicked the decrease seen with DN3L-derived and DN4-derived thymocytes. In addition, these observations suggest that the in vitro-created DP thymocytes might represent a small population of proliferating DP thymocytes.
In vitro-created DP thymocytes resemble proliferating DP thymocytes in vivo
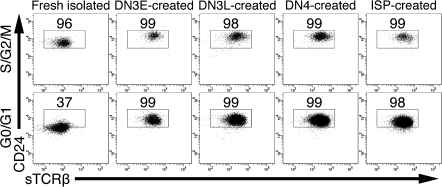
To test whether freshly isolated cycling DP thymocytes were distinct from non-cycling DP thymocytes, we analyzed CD24 and TCRβ expression on each population (Fig. 6). We found that 95 ± 1.9% of freshly isolated cycling DP thymocytes were CD24hiTCRlo, whereas only 39 ± 7.2% of non-cycling freshly isolated DP thymocytes were CD24hi (P = 0.00033, n = 4). To ascertain which population of freshly isolated DP thymocytes in vitro-created DP thymocytes more closely resembled, we analyzed CD24 and TCRβ expression and found that almost all of the in vitro-created DP thymocytes were CD24hiTCRβlo (Fig. 6).
Fig. 6.
In vitro-created DP thymocytes are phenotypically similar to proliferating DP thymocytes in vivo. Freshly isolated thymocytes and DN3E, DN3L, DN4 and ISP thymocytes cultured for 4 days were surface labeled with anti-CD4, anti-CD8, anti-CD24 and anti-TCRβ. Cells were then permeabilized and stained with 4′,6-diamidino-2-phenylindole. Cells were gated on the DP population and then gated according to their cell cycle status. Cycling and non-cycling cells were analyzed for CD24 and TCRβ expression. The percentages of cells in each subset that were CD24hiTCRβlo are shown. Representative of four independent experiments.
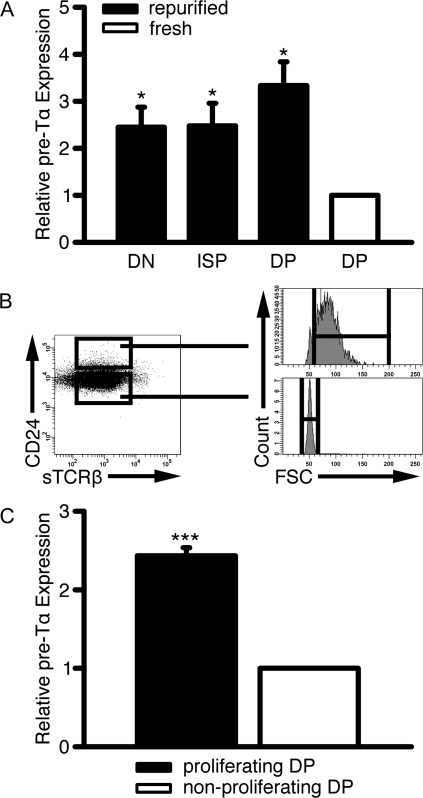
We also measured pre-Tα mRNA levels in cultured thymocytes, freshly isolated cycling DP thymocytes and freshly isolated non-cycling DP thymocytes. Pre-Tα is a Notch-dependent gene (27) and is expressed during stages prior to TCRα expression. DN3E thymocytes were FACS purified and cultured with OP9-DL1 cells. After 3 days, the DN, ISP and DP thymocytes from the culture were isolated and pre-Tα mRNA levels were analyzed. The pre-Tα mRNA levels in cultured DN, ISP and DP cells were 2.5 ± 0.4, 2.5 ± 0.5 and 3.3 ± 0.5-fold higher than freshly isolated total DP thymocytes, respectively (Fig. 7A).
Fig. 7.
Cycling DP thymocytes produced in vivo or in vitro express pre-Tα mRNA. (A) DN3E thymocytes were cultured with OP9-DL1 cells for 3 days and stained with anti-CD4 and anti-CD8. The resulting DN, ISP and DP thymocyte populations were re-FACS purified. For comparison, freshly isolated DP cells were also analyzed. The ratios of pre-Tα mRNA to GAPDH mRNA were calculated in each subset by real-time PCR. These ratios were divided by the pre-Tα mRNA levels in DP thymocytes. Shown is mean ± SD *P < 0.05, n = 3 independent experiments. (B) Freshly isolated large CD24hiTCRβlo and small CD24intTCRβlo DP thymocytes were FACS purified. The gating strategy is shown. (C) Pre-Tα mRNA levels in large CD24hiTCRβlo and small CD24intTCRβlo DP thymocytes were compared using real-time PCR. Shown is mean ± SD ***P < 0.001, n = 4 independent experiments.
Next, we compared pre-Tα mRNA levels in freshly isolated cycling DP thymocytes, defined as CD24hiTCRβloFSChi, and non-cycling DP thymocytes, defined as CD24intTCRβloFSClo (Fig. 7B). Pre-Tα mRNA levels in cycling DP thymocytes were 2.4 ± 0.1-fold higher than in non-cycling DP thymocytes (Fig. 7C).
Collectively, these data suggest that in vitro-created DP thymocytes phenotypically resemble freshly isolated cycling DP thymocytes. These cells are immature as indicated by their high level of CD24 and pre-Tα expression and low level of surface TCRβ.
Discussion
In this paper, we defined the ISP CD8+ thymocyte population as the stage of T-cell development in which cells transition from being Notch dependent to Notch independent. ISP thymocytes responded to the Notch ligand in a manner that led to less survival and proliferation than DN thymocytes. However, ISP thymocytes survived and proliferated better in the presence of DL1 than in the absence of the Notch ligand. This level of Notch-dependent survival and proliferation seen in ISP thymocytes was in contrast to DP thymocytes, which could not survive the culture conditions.
Several studies, including (16, 18–20), showed that Notch is required for the survival, proliferation and differentiation of DN3 cells. Further, previous studies demonstrated that Notch ligation was required for the survival of TCRβ+ DN3 thymocytes (21). However, it is unclear if the requirement for Notch persists as cells proceed through the DN4 and ISP developmental stages. Deleting Notch1 expression or function after the CD4 promoter becomes activated had no consequence on the size of the DP population (28, 29), suggesting that DP thymocytes use mechanisms other than Notch1 for their survival.
We directly tested whether DN, ISP and DP thymocyte subsets could respond to Notch ligands. As reported previously (21), only thymocytes expressing TCRβ could readily survive, proliferate and differentiate in the in vitro differentiation system (Fig. 4). All surviving thymocytes expressed a level of surface TCR that was comparable to immature thymocytes, indicating that TCR− cells failed to survive. In addition, few thymocytes were recovered from the culture when wells seeded with CD27− thymocytes, whereas CD27+ thymocytes expanded in number during the experiment. Freshly isolated DP thymocytes failed to survive in the culture (Fig. 2), consistent with the model that DP thymocytes are no longer sensitive to Notch ligation. These observations support the model describing that as cells differentiate from TCRβ+ DN3E to DP thymocytes, the cells lose their responsiveness to Notch signals. However, the exact stage of T-cell development in which the transition from being Notch responsive to Notch non-responsive occurs was unknown.
We narrowed the search for the transition between Notch responsive and Notch non-responsive by isolating each thymocyte subset between DN3E and DP. For each subset, expression of CD4 and CD8 was independent of Notch ligation (Fig. 1). This could indicate that the pre-TCR triggers signaling pathways that drive differentiation. Alternatively, TCRβ+ DN thymocytes and ISP cells could have received a signal in vivo that drove the expression of CD4 and CD8. Cells clearly received stimulation prior to harvesting from the mouse, as seen by the limited proliferation of each subset in culture (Fig. 3). The DN3E, DN3L, DN4 and ISP thymocytes underwent one to three rounds of cell division despite the absence of a Notch ligand. The stimulus that led to this proliferation may have triggered the differentiation.
We consistently recovered fewer thymocytes from wells seeded with ISP thymocytes than any of the DN populations (Fig. 2). Even with the decline in the number of thymocytes recovered from wells seeded with ISP thymocytes, the number of ISP thymocytes recovered was greater when cells were cultured in the presence of OP9-DL1 cells than OP9-GFP cells. Further, the number of thymocytes recovered when ISP thymocytes were cultured with OP9-DL1 cells for 3 days was comparable to the number of cells recovered after 5 days. This implies that homeostasis was achieved within the culture system; the amount of proliferation was balanced by the amount of cell death. Indeed, ISP thymocytes proliferated in culture, as seen by the dilution of CFSE (Fig. 3). The proliferation was offset by the fact that the percentage of ISP thymocytes that survived 5 days of culture was lower than the percentage of DN thymocytes that survived.
The number of thymocytes recovered from wells seeded with DN3E thymocytes initially declined but increased at later time points (Fig. 2). This observation was in contrast to the DN3L and DN4 populations, which expanded in number beginning early in the culture period. This initial loss of cell numbers could likely be explained by the fact that only 20–30% of DN3E thymocytes express TCRβ as compared with 70–80% of DN3L and DN4 thymocytes (2). When we purified CD27− DN3E thymocytes, which represents the TCRβ− cells (21, 37), we found that the number of TCRβ− DN3E thymocytes that could be recovered after culturing with OP9-DL1 cells was ∼10% of the number of CD27+ cells recovered (Fig. 4C). This observation suggests that the TCRβ− cells fail to survive, proliferate or both in response to Notch ligation. The decline in cell number suggests that TCRβ− cells do not survive as well as TCRβ+ cells. This may not be reflected in our survival assay because TCRβ+ DN thymocytes expanded in cell number and became the predominant population.
We consistently recovered more thymocytes from wells seeded with DN3L thymocytes than DN4 thymocytes (Fig. 2), even though both populations initially contained approximately the same percentage of cells that express TCRβ. These data are consistent with our previous observations that a greater percentage of freshly isolated TCRβ+ DN3L cells are in the S, G2 or M phase of the cell cycle than TCRβ+ DN4 cells and TCRβ+ DN4 thymocytes express less Bcl-2 than TCRβ+ DN3L cells (2, 41). Thus, DN3L thymocytes may proliferate more robustly and may survive better than TCRβ+ DN4 thymocytes.
The comparison of DP thymocytes created after culturing DN3E, DN3L, DN4 and ISP thymocytes provided additional support that ISP thymocytes represent the transition between Notch dependence and Notch independence. A smaller percentage of DP thymocytes derived from ISP thymocytes were in the S, G2 or M phase of the cell cycle than DP thymocytes derived from DN thymocytes (Fig. 5). Also, the percentage of ISP-derived DP thymocytes in the S, G2 or M phase of the cell cycle increased with the duration of the culture, while the percentages of DN-derived DP thymocytes decreased over time. These data indicated that the DP cells created from ISP thymocytes were different than the DP cells created from DN thymocytes. Extending this conclusion, these data strongly suggest that ISP thymocytes are different than DN thymocytes. Furthermore, because the cell cycle status of the DP thymocytes varied depending on the founding population, it is likely that the difference between DN and ISP thymocytes is not intrinsic to the stage of T-cell development. Rather, thymocytes are likely exposed to stimuli in vivo that change their responsiveness to Notch ligation as they progress from the DN stages to the ISP stage of development. These stimuli are not duplicated in the in vitro differentiation system.
Our proliferation assays suggested that thymocytes in the culture continued to proliferate after they became DP thymocytes. When we gated on the DP thymocytes created in vitro, we observed that the DP thymocytes were cycling (Fig. 5). We compared the surface phenotypes and pre-Tα mRNA levels of created DP thymocytes, cycling freshly isolated DP thymocytes and non-cycling freshly isolated DP thymocytes (Figs 6 and 7). Cycling freshly isolated DP thymocytes expressed higher levels of CD24 protein and pre-Tα mRNA than non-cycling cells, indicating that cycling cells were less mature than non-cycling cells. The DP thymocytes created in the in vitro differentiation assay had levels of CD24 protein and pre-Tα mRNA that were comparable to the cycling freshly isolated DP thymocytes, suggesting that the DP thymocytes created in vitro may represent a small population of cycling DP thymocytes found in vivo. In addition, because pre-Tα is a Notch-dependent gene (27), the higher expression of pre-Tα in cycling DP thymocytes may indicate that cycling DP thymocytes have been recently stimulated through Notch. The mechanism by which these DP cells proliferate may be dependent on the pre-TCR or may be via Notch-dependent c-myc expression (42) as c-myc is required for the proliferation of DN thymocytes (43).
The surface levels of CD24 and TCRβ and the continued cell cycle progression of in vitro-created DP thymocytes were maintained in experiments in which cells were cultured for 2 weeks (data not shown). Even as cells down-regulated CD8 to become SP CD4+ cells, the thymocytes retained high levels of CD24 expression, low levels of surface TCR and continued to proliferate. The cells also failed to express CD69 (data not shown). These data suggest that the trigger for down-regulating CD8 expression is unrelated to positive selection and maturation.
These data also indicate that a signal is lacking in the in vitro differentiation system that is required for thymocytes to cease proliferating, down-regulate pTα and continue the maturation process, which highlights a limitation in using this system to examine the signals required for DN thymocytes to differentiate into DP thymocytes. Thymocytes cultured with OP9-DL1 cells in vitro are subjected to supraphysiologic levels of Notch stimulation. Recent data show that DL4 is the more physiologic ligand for the Notch isoform expressed on thymocytes (38–40). However, switching to OP9-DL4 cells did not significantly alter the proliferation and differentiation of DN and ISP thymocytes (Fig. 5) indicating that the in vitro system still lacks a stimulus needed to fully mature DN thymocytes.
A likely function for this missing signal is to terminate Notch signaling. The mechanism by which the Notch signal is terminated is unknown. A candidate for this missing signal is the pre-TCR itself as pre-TCR-mediated signaling can lead to the down-regulation of Notch1 (44). While pTα is expressed at high levels in DP thymocytes derived in vitro (Fig. 7A), it is not clear whether all pre-TCR-mediated signaling pathways are active in this system because Notch can substitute for TCRβ rearrangement (45). Alternatively, there may be another signal that regulates Notch1 expression (23, 24) or expression of a transcriptional regulator, such as Ikaros (46, 47).
In conclusion, our data indicate that the transition between Notch-dependent and Notch-independent survival can be found at the ISP stage of development. Identifying this transitional population will advance our understanding of how Notch is regulated by providing a target population in which we can analyse changes in the expression or function of Notch regulatory proteins. Further, the DP thymocytes created using the in vitro differentiation system resemble a small population of DP thymocytes found in vivo that are immature and proliferate, potentially revealing the existence of a regulatory mechanism during T-cell development that is not found in the in vitro system.
Funding
National Institutes of Health Centers of Biomedical Research Excellence Program of the National Center for Research Resource (P20 RR016443); American Cancer Society Research Scholar (08-182-LIB).
Acknowledgments
The authors would like to thank Drs Stephen Benedict and Marcia Chan and members of the Benedict laboratory for helpful discussions. The authors would also like to thank Brooks Parker for technical assistance.
References
- 1.Godfrey DI, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3-CD4-CD8- triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J. Immunol. 1993;150:4244. [PubMed] [Google Scholar]
- 2.Zeng L, Dalheimer SL, Yankee TM. Gads-/- mice reveal functionally distinct subsets of TCRbeta+ CD4-CD8- double-negative thymocytes. J. Immunol. 2007;179:1013. doi: 10.4049/jimmunol.179.2.1013. [DOI] [PubMed] [Google Scholar]
- 3.Michie AM, Zuniga-Pflucker JC. Regulation of thymocyte differentiation: pre-TCR signals and beta-selection. Semin. Immunol. 2002;14:311. doi: 10.1016/s1044-5323(02)00064-7. [DOI] [PubMed] [Google Scholar]
- 4.Fehling HJ, Krotkova A, Saint-Ruf C, von Boehmer H. Crucial role of the pre-T-cell receptor alpha gene in development of alpha beta but not gamma delta T cells. Nature. 1995;375:795. doi: 10.1038/375795a0. [DOI] [PubMed] [Google Scholar]
- 5.Aifantis I, Gounari F, Scorrano L, Borowski C, von Boehmer H. Constitutive pre-TCR signaling promotes differentiation through Ca2+ mobilization and activation of NF-kappaB and NFAT. Nat. Immunol. 2001;2:403. doi: 10.1038/87704. [DOI] [PubMed] [Google Scholar]
- 6.Petrie HT, Hugo P, Scollay R, Shortman K. Lineage relationships and developmental kinetics of immature thymocytes: CD3, CD4, and CD8 acquisition in vivo and in vitro. J. Exp. Med. 1990;172:1583. doi: 10.1084/jem.172.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radtke F, Wilson A, Mancini SJ, MacDonald HR. Notch regulation of lymphocyte development and function. Nat. Immunol. 2004;5:247. doi: 10.1038/ni1045. [DOI] [PubMed] [Google Scholar]
- 8.Robey EA, Bluestone JA. Notch signaling in lymphocyte development and function. Curr. Opin. Immunol. 2004;16:360. doi: 10.1016/j.coi.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Pui JC, Allman D, Xu L, et al. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity. 1999;11:299. doi: 10.1016/s1074-7613(00)80105-3. [DOI] [PubMed] [Google Scholar]
- 10.Radtke F, Wilson A, Stark G, et al. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- 11.Koch U, Lacombe TA, Holland D, et al. Subversion of the T/B lineage decision in the thymus by lunatic fringe-mediated inhibition of Notch-1. Immunity. 2001;15:225. doi: 10.1016/s1074-7613(01)00189-3. [DOI] [PubMed] [Google Scholar]
- 12.Wilson A, MacDonald HR, Radtke F. Notch 1-deficient common lymphoid precursors adopt a B cell fate in the thymus. J. Exp. Med. 2001;194:1003. doi: 10.1084/jem.194.7.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izon DJ, Aster JC, He Y, et al. Deltex1 redirects lymphoid progenitors to the B cell lineage by antagonizing Notch1. Immunity. 2002;16:231. doi: 10.1016/s1074-7613(02)00271-6. [DOI] [PubMed] [Google Scholar]
- 14.Wolfer A, Wilson A, Nemir M, MacDonald HR, Radtke F. Inactivation of Notch1 impairs VDJbeta rearrangement and allows pre-TCR-independent survival of early alpha beta lineage thymocytes. Immunity. 2002;16:869. doi: 10.1016/s1074-7613(02)00330-8. [DOI] [PubMed] [Google Scholar]
- 15.Harman BC, Jenkinson EJ, Anderson G. Entry into the thymic microenvironment triggers Notch activation in the earliest migrant T cell progenitors. J. Immunol. 2003;170:1299. doi: 10.4049/jimmunol.170.3.1299. [DOI] [PubMed] [Google Scholar]
- 16.Ciofani M, Schmitt TM, Ciofani A, et al. Obligatory role for cooperative signaling by pre-TCR and Notch during thymocyte differentiation. J. Immunol. 2004;172:5230. doi: 10.4049/jimmunol.172.9.5230. [DOI] [PubMed] [Google Scholar]
- 17.Washburn T, Schweighoffer E, Gridley T, et al. Notch activity influences the alphabeta versus gammadelta T cell lineage decision. Cell. 1997;88:833. doi: 10.1016/s0092-8674(00)81929-7. [DOI] [PubMed] [Google Scholar]
- 18.Ciofani M, Zuniga-Pflucker JC. Notch promotes survival of pre-T cells at the beta-selection checkpoint by regulating cellular metabolism. Nat. Immunol. 2005;6:881. doi: 10.1038/ni1234. [DOI] [PubMed] [Google Scholar]
- 19.Garbe AI, Krueger A, Gounari F, Zuniga-Pflucker JC, von Boehmer H. Differential synergy of Notch and T cell receptor signaling determines alphabeta versus gammadelta lineage fate. J. Exp. Med. 2006;203:1579. doi: 10.1084/jem.20060474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maillard I, Tu L, Sambandam A, et al. The requirement for Notch signaling at the beta-selection checkpoint in vivo is absolute and independent of the pre-T cell receptor. J. Exp. Med. 2006;203:2239. doi: 10.1084/jem.20061020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taghon T, Yui MA, Pant R, Diamond RA, Rothenberg EV. Developmental and molecular characterization of emerging beta- and gammadelta-selected pre-T cells in the adult mouse thymus. Immunity. 2006;24:53. doi: 10.1016/j.immuni.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Ciofani M, Knowles GC, Wiest DL, von Boehmer H, Zuniga-Pflucker JC. Stage-specific and differential notch dependency at the alphabeta and gammadelta T lineage bifurcation. Immunity. 2006;25:105. doi: 10.1016/j.immuni.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Huang EY, Gallegos AM, Richards SM, Lehar SM, Bevan MJ. Surface expression of Notch1 on thymocytes: correlation with the double-negative to double-positive transition. J. Immunol. 2003;171:2296. doi: 10.4049/jimmunol.171.5.2296. [DOI] [PubMed] [Google Scholar]
- 24.Fiorini E, Merck E, Wilson A, et al. Dynamic regulation of notch 1 and notch 2 surface expression during T cell development and activation revealed by novel monoclonal antibodies. J. Immunol. 2009;183:7212. doi: 10.4049/jimmunol.0902432. [DOI] [PubMed] [Google Scholar]
- 25.Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R, Israel A. Signalling downstream of activated mammalian Notch. Nature. 1995;377:355. doi: 10.1038/377355a0. [DOI] [PubMed] [Google Scholar]
- 26.Deftos ML, He YW, Ojala EW, Bevan MJ. Correlating notch signaling with thymocyte maturation. Immunity. 1998;9:777. doi: 10.1016/s1074-7613(00)80643-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reizis B, Leder P. Direct induction of T lymphocyte-specific gene expression by the mammalian Notch signaling pathway. Genes Dev. 2002;16:295. doi: 10.1101/gad.960702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolfer A, Bakker T, Wilson A, et al. Inactivation of Notch 1 in immature thymocytes does not perturb CD4 or CD8T cell development. Nat. Immunol. 2001;2:235. doi: 10.1038/85294. [DOI] [PubMed] [Google Scholar]
- 29.Tanigaki K, Tsuji M, Yamamoto N, et al. Regulation of alphabeta/gammadelta T cell lineage commitment and peripheral T cell responses by Notch/RBP-J signaling. Immunity. 2004;20:611. doi: 10.1016/s1074-7613(04)00109-8. [DOI] [PubMed] [Google Scholar]
- 30.Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 31.Schmitt TM, Zuniga-Pflucker JC. T-cell development, doing it in a dish. Immunol. Rev. 2006;209:95. doi: 10.1111/j.0105-2896.2006.00353.x. [DOI] [PubMed] [Google Scholar]
- 32.Taghon T, Van de Walle I, De Smet G, et al. Notch signaling is required for proliferation but not for differentiation at a well-defined beta-selection checkpoint during human T-cell development. Blood. 2009;113:3254. doi: 10.1182/blood-2008-07-168906. [DOI] [PubMed] [Google Scholar]
- 33.MacDonald HR, Budd RC, Howe RC. A CD3- subset of CD4-8+ thymocytes: a rapidly cycling intermediate in the generation of CD4+8+ cells. Eur. J. Immunol. 1988;18:519. doi: 10.1002/eji.1830180405. [DOI] [PubMed] [Google Scholar]
- 34.Wilson A, Petrie HT, Scollay R, Shortman K. The acquisition of CD4 and CD8 during the differentiation of early thymocytes in short-term culture. Int. Immunol. 1989;1:605. doi: 10.1093/intimm/1.6.605. [DOI] [PubMed] [Google Scholar]
- 35.Nakayama T, June CH, Munitz TI, et al. Inhibition of T cell receptor expression and function in immature CD4+CD8+ cells by CD4. Science. 1990;249:1558. doi: 10.1126/science.2120773. [DOI] [PubMed] [Google Scholar]
- 36.McCarthy SA, Kruisbeek AM, Uppenkamp IK, Sharrow SO, Singer A. Engagement of the CD4 molecule influences cell surface expression of the T-cell receptor on thymocytes. Nature. 1988;336:76. doi: 10.1038/336076a0. [DOI] [PubMed] [Google Scholar]
- 37.Gravestein LA, van Ewijk W, Ossendorp F, Borst J. CD27 cooperates with the pre-T cell receptor in the regulation of murine T cell development. J. Exp. Med. 1996;184:675. doi: 10.1084/jem.184.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Besseyrias V, Fiorini E, Strobl LJ, et al. Hierarchy of Notch-Delta interactions promoting T cell lineage commitment and maturation. J. Exp. Med. 2007;204:331. doi: 10.1084/jem.20061442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hozumi K, Mailhos C, Negishi N, et al. Delta-like 4 is indispensable in thymic environment specific for T cell development. J. Exp. Med. 2008;205:2507. doi: 10.1084/jem.20080134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koch U, Fiorini E, Benedito R, et al. Delta-like 4 is the essential, nonredundant ligand for Notch1 during thymic T cell lineage commitment. J. Exp. Med. 2008;205:2515. doi: 10.1084/jem.20080829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mandal M, Borowski C, Palomero T, et al. The BCL2A1 gene as a pre-T cell receptor-induced regulator of thymocyte survival. J. Exp. Med. 2005;201:603. doi: 10.1084/jem.20041924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palomero T, Lim WK, Odom DT, et al. NOTCH1 directly regulates c-MYC and activates a feed-forward-loop transcriptional network promoting leukemic cell growth. Proc. Natl Acad. Sci. U S A. 2006;103:18261. doi: 10.1073/pnas.0606108103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dose M, Khan I, Guo Z, et al. c-Myc mediates pre-TCR-induced proliferation but not developmental progression. Blood. 2006;108:2669. doi: 10.1182/blood-2006-02-005900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yashiro-Ohtani Y, He Y, Ohtani T, et al. Pre-TCR signaling inactivates Notch1 transcription by antagonizing E2A. Genes Dev. 2009;23:1665. doi: 10.1101/gad.1793709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michie AM, Chan AC, Ciofani M, et al. Constitutive Notch signalling promotes CD4 CD8 thymocyte differentiation in the absence of the pre-TCR complex, by mimicking pre-TCR signals. Int. Immunol. 2007;19:1421. doi: 10.1093/intimm/dxm113. [DOI] [PubMed] [Google Scholar]
- 46.Chari S, Winandy S. Ikaros regulates Notch target gene expression in developing thymocytes. J. Immunol. 2008;181:6265. doi: 10.4049/jimmunol.181.9.6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kleinmann E, Geimer Le Lay AS, Sellars M, Kastner P, Chan S. Ikaros represses the transcriptional response to Notch signaling in T-cell development. Mol. Cell. Biol. 2008;28:7465. doi: 10.1128/MCB.00715-08. [DOI] [PMC free article] [PubMed] [Google Scholar]