Abstract
Fabry disease is a lysosomal storage disorder caused by an α-galactosidase A (α-Gal A) deficiency and resulting in the accumulation of glycosphingolipids, predominantly globotriaosylceramide (Gb3). A transgenic mouse expressing the human α-Gal A R301Q mutant in an α-Gal A-knockout background (TgM/KO) should be useful for studying active-site-specific chaperone (ASSC) therapy for Fabry disease. However, the Gb3 content in the heart tissue of this mouse was too low to detect an ASSC-induced effect. To increase the Gb3 levels in mouse organs, we created transgenic mice (TgG3S) expressing human α1,4-galactosyltransferase (Gb3 synthase). High levels of Gb3 were observed in all major organs of the TgG3S mouse. A TgG3S (+/−)M(+/−)/KO mouse was prepared by cross-breeding the TgG3S and TgM/KO mice and the Gb3 content in the heart of the TgG3S(+/−)M(+/−)/KO mouse was 1.4 µg/mg protein, higher than in the TgM(+/−)/KO (<0.1 µg/mg protein). Treatment with an ASSC, 1-deoxygalactonojirimycin, caused a marked induction of α-Gal A activity and a concomitant reduction of the Gb3 content in the TgG3S(+/−) M(+/−)/KO mouse organs. These data indicated that the TgG3S(+/−) M(+/−)/KO mouse was suitable for studying ASSC therapy for Fabry disease, and that the TgG3S mouse would be useful for studying the effect of high Gb3 levels in mouse organs.
Keywords: active-site-specific chaperone therapy, Fabry disease, globotriaosylceramide, mouse model
Fabry disease is an X-linked recessive disease caused by insufficient activity of lysosomal α-galactosidase A (α-Gal A, EC 3.2.1.22), an enzyme responsible for the catabolism of glycosphingolipids, predominantly globotriaosylceramide (Gb3) (1). Patients with Fabry disease show diverse clinical manifestations caused by generalized vasculopathy—pain in the extremities, hypohidrosis, angiokeratoma, corneal opacity, ischaemic heart disease, progressive nephropathy and cerebrovascular disease (2). Approximately 60% of the mutations reported in patients with Fabry disease are missense mutations in the α-Gal A gene. Although many products encoded by these missense mutations have normal catalytic properties, they also have low thermostability and degrade rapidly after their synthesis in the endoplasmic reticulum (ER) (3–5). A novel therapeutic strategy for the treatment of Fabry disease, which uses competitive inhibitors of α-Gal A as active-site-specific chaperones (ASSCs), has been suggested (6–8). The normal folding of those missense mutant enzymes can be restored by ASSC treatment, thus preventing excessive ER-associated degradation and improving the transport of the mutant enzyme to the lysosomes (4). Cultivation of patients’ cells with an ASSC of α-Gal A [e.g. 1-deoxygalactonojirimycin (DGJ)] at low concentrations resulted in a substantial increase in both residual enzyme activity and the amount of intracellular enzyme protein (4, 6, 9).
A mouse line deficient in α-Gal A (GLAko mice) was established in 1997 by disruption of the murine α-Gal A gene (10). Despite the accumulation of Gb3 in Fabry disease-relevant organs of the GLAko mice, the mice were clinically normal and had a typical life span. These mice have served as an excellent model for studies on enzyme replacement therapy (11), gene therapy (12–14) and substrate reduction therapy (15), wherein the increased α-Gal A activity and reduced Gb3 accumulation were primary objectives. However, this mouse model is not suitable for studying ASSC therapy, which requires the expression of a human mutant enzyme. In a previous report (16), we established a homozygous transgenic mouse in a murine α-Gal A knockout background (TgM/KO mouse) by crossbreeding a TgM mouse expressing the human α-Gal A R301Q mutant (17) in GLAko mice. Like the GLAko mouse, the TgM/KO mouse does not exhibit a disease phenotype, however these mice do express human mutant α-Gal A and serve as an excellent biochemical model for studying ASSC (18). Heterozygous TgM/KO mice show a lower α-Gal A activity than the homozygotes, and Gb3 accumulates in the kidney of the heterozygous mouse, but not in the heart, which is affected in Fabry disease (16).
The purpose of this study was to establish a transgenic mouse that expressed high levels of Gb3 as well as the human mutant α-Gal A enzyme in heart tissue, which would permit us to assess the effect of ASSC (enhancement of α-Gal A activity and decrease in Gb3 accumulation) in the heart. Here, in order to increase cardiac Gb3 production, we simply overexpressed human α1,4-galactosyltransferase [A4GalT, Gb3 synthase (G3S, EC 2.4.1.228)] to obtain the TgG3S mouse line. We confirmed that these mice expressed high levels of Gb3 in their major organs, and then bred them with the TgM/KO mice to obtain TgG3S(+/−)M(+/−)/KO mice. Here we report the characterization and potential usefulness of this new mouse line.
Materials and Methods
Establishment of a stable transformant expressing A4GalT in COS-7 cells and A4GalT-overexpressing transgenic mice (TgG3S)
The human Gb3 synthase (G3S) cDNA containing the full-length coding sequence and an EcoRI site at both ends was prepared by polymerase chain reaction (PCR) using a PhusionTM High-Fidelity PCR Kit [New England BioLabs (NEB), Ipswich, MA, USA]. The cDNA for α1,4Gal-TpVTR1 (19) was the template, and the primer sequences were 5′-TGGGAATTCCATGTCCAAGCC-3′ and 5′-GGGGAATTCACAAGTACATTTTCATGGC-3′. The 1.1-kb PCR product was purified with a PCR Purification Kit (QIAGEN K.K., Japan) and digested with EcoRI (NEB). The digested cDNA was subcloned into the EcoRI site of expression vector pCXN2 (20), and the product was designated as pCXN2-G3S. For the preparation of the stably transformed G3S/COS-7 cells, pCXN2-G3S was linearized with HindIII (NEB) and then transfected into COS-7 cells with the FuGENETM6 transfection reagent (Roche Molecular Biochemicals, Basel, Switzerland), according to the manufacturer’s protocol. Stably transformed G3S/COS-7 cells were selected by growing them in culture medium containing 400 µg/ml of G418 (Sigma, St Louis, MO, USA) for 4 weeks.
A DNA fragment comprising a mammalian expression unit and the human Gb3 synthase cDNA was prepared by digesting the pCXN2-G3S with SalI and BamHI (NEB). A fragment that was ∼3.5 kb was isolated by agarose gel electrophoresis and purified by a Gel Extraction Kit (QIAGEN). A transgenic mouse (TgG3S) expressing human Gb3 synthase was generated by injecting the DNA fragments into the pronuclei of fertilized eggs taken from superovulated C57BL/6J Jms female mice, and the embryos were implanted into pseudopregnant Jcl:MCH mice, as described previously (21). Transgenic founder mice were identified by PCR with a primer set (5′-ATTGTTCTCAAGAACCTGCG-3′ and 5′-ATTTGTGAGCCAGGGCATTG-3′). A transgene fragment was confirmed as a single 548-bp band on agarose gel electrophoresis. To generate an animal model exclusively expressing both human G3S and human mutant α-Gal A in an endogenous GLA knockout background [TgG3S(+/−)M(+/−)/KO mice], we first prepared a mouse line overexpressing the human G3S transgene in an α-Gal A knockout background (G3Stg/GLAko) by crossbreeding male TgG3S mice and homozygous female GLAko mice (since GLA is located on the X chromosome). After the G3Stg/GLAko mouse line was established, TgM/KO and G3Stg/GLAko mice were crossbred to obtain TgG3S(+/−)M(+/−)/KO mice.
Determination of A4GalT mRNA expression in mouse tissues
The A4GalT mRNA expression of the transgene (human A4GalT) was determined by a reverse transcriptase (RT)-PCR using Takara RNA PCR Kit (AMV) Ver.3.0 (Takara Bio Inc., Shiga, Japan). The total RNA samples from tissues of wild-type and transgenic mice were prepared with RNAiso (Takara Bio Inc.), and the RNA concentrations were determined by absorbance at 260 nm. The RT reaction was performed at 30°C for 10 min, and then at 42°C for 15 min, followed by incubation at 99°C for 5 min. PCR amplification was performed using the following conditions: initial denaturation (94°C, 5 min) followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 58°C for 30 s, elongation at 72°C for 30 s and further elongation at 72°C for 2 min. The DNA fragment (409 bp), which contained a sequence that is highly conserved between the mouse and human A4GalT genes, was amplified with primers 5′-GGCATCTC(A/T)CTTCTGAGCTG-3′ and 5′-GGATGGAACACCACTTCTTG-3′. PCR products were run on a 2% agarose gel, stained with ethidium bromide and photographed. The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as an expression control gene as described previously (22).
Administration of DGJ to TgG3S(+/−)M(+/−)/KO mice
TgG3S(+/−)M(+/−)/KO mice were supplied with fresh tap water ad libitum and rodent pellets. DGJ (Toronto Research Chemicals, Toronto, Canada) was administrated to one group of female TgG3S(+/−)M(+/−)/KO mice in tap water as a DGJ aqueous solution without any other substances. After 4 weeks, the animals were sacrificed and the organs were quickly removed and rinsed with phosphate-buffered saline (PBS). Tissue homogenates were subjected to enzyme assays and lipid extraction.
Assay of enzyme activity and protein content
All samples were kept on ice and processed as rapidly as possible. Approximately 10% (w/v) tissue homogenates were prepared in water using a micro-homogenizer (Physcotron, Niti-on Inc., Chiba, Japan). The supernatant obtained from the homogenate after centrifugation at 10,000g for 5 min was used in the enzyme assays. The α-Gal A activity was assayed with 4-methylumbelliferyl α-d-galactoside (Sigma) as the substrate and N-acetyl-d-galactosamine (75 mM) as the inhibitor for α-N-acetylgalactosaminidase in 0.1 M sodium citrate buffer (pH 4.6) as described previously (16). The protein concentration was determined using a DC Protein Assay kit (Bio-Rad Laboratories, Hercules, CA, USA) with bovine serum albumin as the standard.
Detection of neutral and acidic glycosphingolipids by thin-layer chromatography analysis
Glycosphingolipids were extracted from cultured cells and mouse tissues and were analysed as described previously (14), with some modifications. Crude lipids were extracted from cultured cells (eq. to 1 mg of protein) and tissue homogenates (eq. to 5 mg of protein), using a mixture of chloroform : methanol (2 : 1, v/v). Glycosphingolipids were dried under a stream of nitrogen. The dried samples were dissolved in chloroform : methanol : water (30 : 60 : 8, v/v) and applied to a TOYOPEARL DEAE-650 column (Tosoh Corporation, Tokyo, Japan). The column was washed with chloroform : methanol : water (30 : 60 : 8, v/v). The pass-through fraction was pooled for the preparation of neutral glycosphingolipids. The acidic glycosphingolipids including gangliosides were eluted with chloroform : methanol : 0.8 M sodium acetate (30 : 60 : 8, v/v), and they were desalted with Sep-Pak C18 reverse-phase cartridge (Waters, Milford, MA, USA) and then analysed by thin-layer chromatography (TLC). The neutral glycosphingolipids fraction was dried, and then subjected to mild alkaline treatment with 1 ml of 0.1 N NaOH in methanol at 40°C for 2 h. After neutralizing the solution with glacial acetic acid, glycosphingolipids were further subjected to the Folch’s partition (choloroform : methanol : H2O, 8 : 4 : 3 in v/v/v), recovered in the lower phase, and then quantitatively applied to TLC plates. TLC analyses were performed with HPTLC-Silica gel 60 plates (Merck & Co., Inc., Whitehouse Station, NJ, USA) using a solvent system of chloroform : methanol : water (60 : 35 : 8, v/v/v) and chloroform : methanol : 0.2% CaCl2 (60 : 35 : 8, v/v/v) for the separation of neutral and acidic glycosphingolipids, respectively. Glycosphingolipids were detected by spraying the plate with orcinol-sulphuric acid reagent, and heating the plate at 110°C. The Gb3 from porcine erythrocytes was purchased from Nakalai Tesque (Kyoto, Japan), and other glycosphingolipid standards were purchased from Wako Pure Chemicals (Osaka, Japan). The ganglioside standards from bovine brain (GM1, GM2 and GM3) were purchased from Wako.
Binding assay
The Stx1B binding assay was performed as described previously (23), with some modifications. After glycosphingolipids were separated by TLC as described above, a TLC plate was sunk in a 0.4% polyisobutylmethacrylate (GlycoTech, Rockville, MD, USA) solution (2.5% polyisobutylmethacrylate in chloroform was diluted to 0.4% with hexane) and then blocked with 1% bovine serum albumin in PBS (BSA-PBS). The plate was incubated with 2.5 µg/ml of Stx1B in BSA-PBS at room temperature for 20 min and washed with PBS. After incubation with 7.0 µg/ml of anti-Stx1B polyclonal antibody (produced in rabbits with purified Stx1B) for 20 min the plate was washed with PBS. After further incubation with horseradish peroxidase-conjugated anti-rabbit IgG (Pierce Chemical, Rockford, IL, USA) at room temperature for 20 min and the following final washing, Stx1B-binding was visualized with an enhanced chemiluminescent substrate (Pierce).
Immunoelectron microscopy
The G3S/COS-7 cells were cultured with or without 10 mM DGJ for 4 days, then fixed in 2% glutaraldehyde in 0.2 M phosphate buffer (pH 7.4) for 3 h; they were then further incubated in 1% osmium tetraoxide for 2 h, dehydrated in ethanol and embedded in Epok 812. Immunoelectron microscopy was performed as described previously (24). In summary, each thin section was briefly microwaved in Target Retrieval Solution, pH 10 (DAKO, Carpinteria, CA, USA) and then incubated for 30 min at room temperature with Stx1B (2.5 µg/ml). After being washed with Wash Buffer [50 mM Tris–HCl (pH 7.6) containing 0.8% NaCl and 0.1% BSA], the ultrathin section was incubated with anti-Stx1B antibody (7 µg/ml). After another wash, the section was incubated with gold-conjugated goat anti-rabbit IgG. The section was then washed again, stained with uranyl acetate and lead citrate, and examined using a transmission electron microscope (JEM-1200EXII, JEOL, Akishima, Tokyo, Japan).
Results
Establishment of TgG3S mice
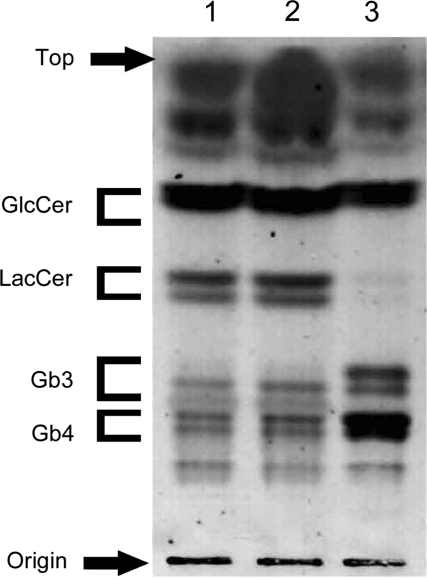
First, we prepared a stable transformant COS-7 cell line expressing Gb3 synthase (G3S/COS-7 cells) to confirm that the expression construct prepared in our present study could increase intracellular Gb3 content. We extracted neutral glycosphingolipids from the cells and quantitated them by high performance TLC (HPTLC) (Fig. 1). The Gb3 and globotetraosylceramide (Gb4) levels in the G3S/COS-7 cells were markedly higher than those in the parental COS-7 cells or in a control line transformed with only the empty vector. The content of lactosylceramide (LacCer) in the G3S/COS-7 cells was lower than it was in the COS-7 cells, indicating that the expression product from the A4GalT gene in our construct catalysed the construction of Gb3 from LacCer.
Fig. 1.
Neutral glycosphingolipids in stably transformed G3S/COS-7 cells. Neutral glycosphingolipids were extracted from cell homogenates (containing 1 mg protein), as described in ‘Materials and Methods’ section. Glycosphingolipids were visualized with orcinol-sulphuric acid reagent. Lane 1, intact COS-7 cells; lane 2, mock transfection; lane 3, stable transformant G3S/COS-7 cells.
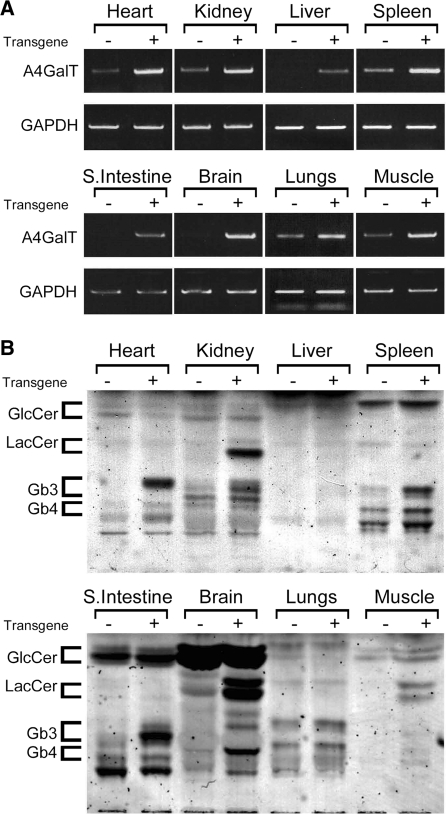
A mouse line expressing the A4GalT gene (TgG3S) was generated by injecting the DNA fragment into the pronuclei of fertilized mouse ova taken from C57BL/6J female mice. A marked increase in the expression of the A4GalT gene in heart, kidney, liver, spleen, small intestine, brain, lungs and muscle of the TgG3S mice compared with wild-type C57BL/6J mice was observed by RT-PCR (Fig. 2A). High levels of Gb3 synthase expression resulted in an increase in Gb3 levels of all organs examined in this study (Fig. 2B). A glycosphingolipid band (possibly galabiosylceramide) with an Rf value similar to that of LacCer by HPTLC was also increased in the kidney and brain of the TgG3S mouse. No change was observed in the content of acidic glycosphingolipids including gangliosides in organs of TgG3S mice (data not shown). The TgG3S mouse line did not show any clinical phenotype and had a typical life span.
Fig. 2.
Expression of A4GalT gene and glycosphingolipids in tissues of TgG3S mice. A human G3S-overexpressing mouse line was prepared as described in ‘Materials and Methods’ section. (A) The expression of A4GalT mRNA was determined by RT–PCR analysis with a primer set that can amplify both mouse and human genes. The glyceraldehyde phosphate dehydrogenase (GAPDH) gene was amplified as an internal control. (B) Neutral glycosphingolipids were extracted from tissue homogenates (containing 5 mg protein) prepared from age-matched TgG3S and wild-type mice and applied to HPTLC, as described in the legend to Fig. 1.
Preparation of TgG3S(+/−)M(+/−)/KO mouse line as a model mouse for ASSC therapy
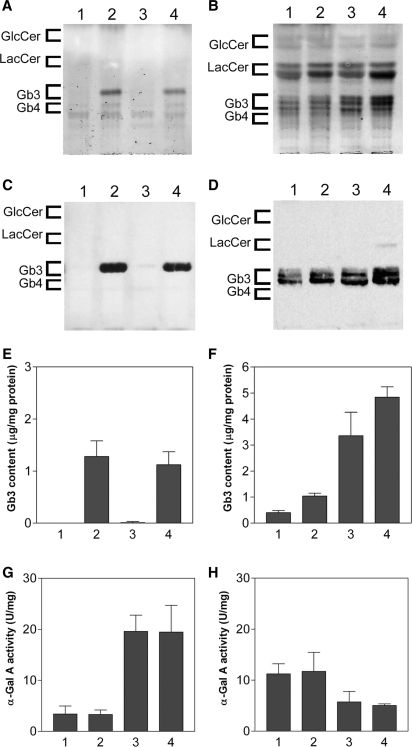
To study the effect of ASSC on Gb3 content in the heart, we tried to increase the cardiac Gb3 content of the TgM/KO mouse by introducing the G3S-overexpressing transgene. The TgG3S(+/−)M(+/−)/KO mouse was created by crossbreeding the G3Stg/GLAko mouse and the TgM/KO mouse. The Gb3 content in the TgG3S(+/−)M(+/−)/KO heart and kidney was determined and compared with that in wild-type C57BL/6J, TgG3S(+/−) and TgM(+/−)/KO mice (Fig. 3). A Gb3 level as high as that of the TgG3S mice (1.3 ± 0.3 µg/mg protein) was observed in the heart of the TgG3S(+/−)M(+/−)/KO mice (1.1 ± 0.3 µg/mg protein) (Fig. 3A). Furthermore, when we probed the HPTLC plate with Shiga toxin 1 B-subunit (Stx1B), which selectively binds Gb3, we did not detect Gb3 in the heart tissue of mouse lines that did not express the A4GalT transgene (i.e. wild-type and TgM(+/−)/KO mice) (Fig. 3C). In contrast, Gb3 was detectable in kidney tissue of all the mouse lines, and a slight increase was observed in the lines expressing the A4GalT transgene (Fig. 3B, D and F). The A4GalT transgene did not change the α-Gal A activity in either the heart or kidney (Fig. 3G and H). Marked increase in Gb3 content was detected in the heart and kidney by the expression of Gb3 synthase, however no abnormality was observed in the histological examination of both organs from TgG3S(+/−)M(+/−)/KO mouse (data not shown).
Fig. 3.
Characterization of TgG3S(+/−)M(+/−)/KO mouse line. Gb3 content and α-Gal A activity in heart (A, C, E, and G) and kidney (B, D, F, and H) tissue from 10-week-old TgG3S(+/−)M(+/−)/KO mice were compared to those from age-matched mice from other lines. In (A–H), the lanes and columns show results from the following genotypes: 1, wild-type; 2, TgG3S(+/−); 3, TgM(+/−)/KO; 4, TgG3S(+/−)M(+/−)/KO. The neutral glycosphingolipids were extracted and visualized with orcinol-sulphuric acid reagent (A and B) or by Stx1B-binding (C and D). The Gb3 content (E and F) and α-Gal A activity (G and H) were determined as described in ‘Materials and Methods’ section.
Age-related increase in the kidney Gb3 content in TgG3S(+/−)M(+/−)/KO mice
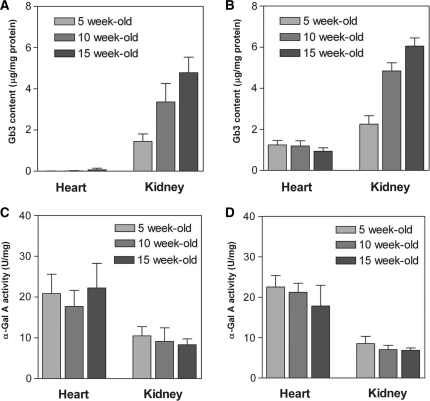
The Gb3 content in the kidney of both the TgM(+/−)/KO and the TgG3S(+/−)M(+/−)/KO mice increased ∼3-fold between 5 and 10 weeks of age (Fig. 4A and B). In contrast, neither the Gb3 content in the heart nor the α-Gal A activity in the heart or kidney changed during this time (Fig. 4B–D). These data showed that we needed to use age-matched TgG3S(+/−)M(+/−)/KO mice to determine the effect of DGJ on Gb3 content in the kidney. Because the change in the Gb3 content in the kidney changed relatively little between 10 and 15 weeks of age, we used mice in this age range to study the effects of DGJ treatment.
Fig. 4.
Developmental changes in Gb3 content and α-Gal A activity in mouse heart and kidney. The Gb3 content and α-Gal A activity in the heart and kidney of TgM(+/−)/KO (A and C) and TgG3S(+/−)M(+/−)/KO mice (B and D) were determined at the indicated ages, as described in ‘Materials and Methods’ section. Each bar represents the means ± SD of data from three or four mice.
Effect of 0.05 mM DGJ treatment on Gb3 content in heart and kidney of TgG3S(+/−)M(+/−)/KO mice
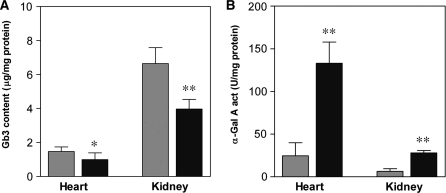
DGJ was administrated to TgG3S(+/−)M(+/−)/KO mice in their water as a 0.05 mM solution, available ad libitum, for 4 weeks. Based on the daily water consumption, the DGJ dosage was calculated to be ∼3 mg/kg body weight/day. In the heart tissues of TgG3S(+/−)M(+/−)/KO mice after treatment with DGJ, a 0.69-fold decrease in Gb3 content (1.44 ± 0.38 and 0.99 ± 0.40 µg/mg protein in control and DGJ-treated mice, respectively) was observed, along with a 5.4-fold increase in α-Gal A activity (24.7 ± 7.6 and 133.0 ± 24.9 unit/mg protein in control and DGJ-treated mice, respectively) (Fig. 5). Likewise, the Gb3 content of the kidney tissue decreased 0.61-fold (6.56 ± 1.33 and 3.97 ± 0.57 µg/mg protein in control and DGJ-treated mice, respectively), and the α-Gal A activity in the kidney increased 4.4-fold (6.4 ± 3.1 and 28.0 ± 2.8 unit/mg protein in control and DGJ-treated mice, respectively) after treatment. These data indicate that the enhancement of the mutant α-Gal A activity by treatment with DGJ reduced the Gb3 content of the heart as well as the kidney, and that the TgG3S(+/−)M(+/−)/KO mouse line is a useful mouse model for the study of ASSC treatment in Fabry disease-relevant organs.
Fig. 5.
Effect of DGJ treatment on Gb3 content and α-Gal A activity in TgG3S(+/−)M(+/−)/KO mice. DGJ (0.05 mM) was administrated to 10-week-old TgG3S(+/−)M(+/−)/KO mice in their drinking water for 4 weeks. The dosage of DGJ was estimated to be 3 mg/kg/day. Six female mice each were used in the DGJ-treated (dark-coloured bar) and control (light-coloured bar) groups. The Gb3 content (A) and α-Gal A activity (B) in the heart and kidney were determined as described in ‘Materials and Methods’ section. The statistical significance of the difference was determined by Student’s t test. *P < 0.05, **P < 0.01.
Inhibitory effect of high-concentration DGJ on α-Gal A activity causes Gb3 accumulation in G3S/COS-7 cells
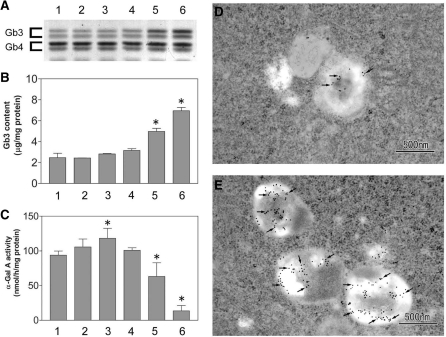
To determine the critical concentration of DGJ required to reduce the Gb3 accumulation of mammalian cells, G3S/COS-7 cells were cultured in DGJ (0–10 mM)-supplemented medium for 4 days. Significant Gb3 accumulation was observed in cases where DGJ dosage was >1 mM (Fig. 6A and B). As an explanation, we found that DGJ decreased the intracellular α-Gal A activity at these higher concentrations, although it slightly increased the α-Gal A activity at 10 µM DGJ, by the ASSC effect (Fig. 6C).
Fig. 6.
Treatment of G3S/COS-7 cells with high-concentration DGJ. G3S/COS-7 cells were cultured for 4 days in high-glucose DMEM medium containing 10% foetal bovine serum with different concentrations of DGJ. After cells were rinsed with PBS, cells were harvested with PBS by a plastic scraper, and collected by centrifugation (3000g, 5 min). The cell pellet was homogenized with water and used for the determination of Gb3 content and α-Gal A activity. In (A), HPTLC analysis of Gb3 and Gb4 was visualized by orcinol-sulphuric acid reagent. The values of Gb3 content (B) and α-Gal A activity (C) are the means ± SD from three cultures. The statistical significance of the difference was determined by Student’s t test; *P < 0.05 versus DGJ-free culture. In (A, B and C), lanes and columns are the same, and 1, DGJ-free control culture; 2, 1 µM DGJ; 3, 10 µM DGJ; 4, 100 µM DGJ; 5, 1 mM DGJ; 6, 10 mM DGJ. The accumulation of Gb3 in lysosomes was determined by Stx1B-binding immunoelectron microscopic study in DGJ-free (D) and 10 mM DGJ-treated G3S/COS-7 cells (E). The ultrathin sections were incubated with Stx1B, then with anti-Stx1B polyclonal antibody, followed by immunogold labeling. Typical gold particles are pointed out by arrows.
To confirm that the Gb3 accumulated in the lysosomes of the G3S/COS-7 cells, an immunoelectron microscopic study was conducted using the Stx1B and anti-Stx1B polyclonal antibodies to label the Gb3 (Fig. 6D and E). The number of gold particles in each lysosome was markedly increased in the samples treated with 10-mM DGJ, indicating that the Gb3 had accumulated in them.
Administration of DGJ at high concentrations to TgG3S(+/−)M(+/−)/KO mice
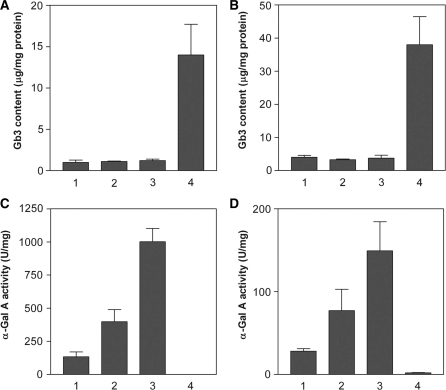
To elucidate whether high concentrations of DGJ would also cause Gb3 accumulation in mouse tissues, TgG3S(+/−)M(+/−)/KO mice (three 10-week-old female mice for each group) were treated with DGJ at 5-fold (0.25 mM) and 25-fold (1.25 mM) higher concentrations than in the original experiment, for 4 weeks. No significant increase in Gb3 content either in heart or kidney tissue was observed after treatment with DGJ at high concentrations, and the low level of Gb3 in the TgG3S(+/−)M(+/−)/KO mouse tissues (∼1.0 and 3.7 µg/mg protein in heart and kidney, respectively) was maintained (Fig. 7A and B), compared to that of age-matched G3Stg/GLAko mice (14 and 38 µg/mg protein in heart and kidney, respectively), which have no α-Gal A activity in their organs. Likewise, DGJ increased α-Gal A activity dose-dependently in the heart and kidney of the TgG3S(+/−)M(+/−)/KO mice (Fig. 7C and D). These data indicated that at least a 25-fold higher DGJ concentration (75 mg/kg body weight/day) may be safe for the treatment of patients with Fabry disease.
Fig. 7.
Administration of high concentrations of DGJ to TgG3S(+/−)M(+/−)/KO mice. High concentrations of DGJ were administered to TgG3S(+/−)M(+/−)/KO mice (three or four 11-week-old female mice in each group) for 4 weeks. The Gb3 content and α-Gal A activity in the heart (A and C, respectively) and in the kidney (B and D, respectively) were determined as described in ‘Materials and Methods’ section. Lanes in A–D were the same, and lane 1, 0.05 mM DGJ treated; lane 2, 0.25 mM DGJ treated; and lane 3, 1.25 mM DGJ treated TgG3S(+/−)M(+/−)/KO mice, and lane 4, age-matched TgG3S(+/−)/KO mice.
Discussion
To increase the Gb3 content in mouse tissues, we tried overexpressing human Gb3 synthase in them. Since high Gb3 content was observed in tissues of the TgG3S mouse compared with wild-type mouse organs (Fig. 2), Gb3 synthase may be a critical enzyme for the synthesis of Gb3 in mouse organs, particularly in the heart and small intestine, where the increase was greatest. The increase in the tissue Gb3 level was determined by the binding of Stx1B (Fig. 3), which selectively binds Gb3 (25, 26). The effect of G3S-overexpression on Gb3 content was greater in the heart than in the kidney, indicating that Gb3 synthase is expressed at low levels in wild-type mouse heart. The absence of Gb3 in the TgM/KO mouse heart was caused not only by its low expression of the Gb3 synthase gene, but also by the high activity of the mutant α-Gal A. However, the G3S-stimulated Gb3 production outstripped Gb3’s decomposition by α-Gal A activity in the heart of the TgG3S(+/−)M(+/−)/KO mouse, leading to the accumulation of Gb3 (even though the Gb3 content was still lower than it was in the kidney).
We did not identify the neutral glycolipid, which has a similar Rf value to that of LacCer on HPTLC and which increased in the kidney and brain tissues in response to G3S-overexpression. However, it may be galabiosylceramide (Galα1,4Gal-Cer), since this glycolipid can be made by Gb3 synthase (19), and galactosylceramide (Gal-Cer) is produced in the kidney as well as in the brain (27).
The kidney Gb3 level increased with age in both TgM(+/−)/KO and TgG3S(+/−)M(+/−)/KO mice (Fig. 4). However, the α-Gal A activity was not changed in kidney tissue by the additional expression of the Gb3 synthase gene, and an increase in the kidney Gb3 level with age was also reported in GLAko mice (28), indicating that the age-related Gb3 accumulation in the kidney is not caused by a decrease in the catabolism of Gb3. Although the mechanism by which kidney Gb3 levels increase with age is unknown, these findings meant that we had to use age-matched mice to determine the ASSC effect on the kidney Gb3 level.
After successfully increasing the heart Gb3 level in mice, we were able to examine the ASSC effect of DGJ on Gb3 content in the heart tissue of TgG3S(+/−)M(+/−)/KO mice. A significant reduction of Gb3 in the heart was observed following treatment with 0.05 mM DGJ for 4 weeks, indicating that TgG3S(+/−)M(+/−)/KO mice are a useful animal model for determining the effect of ASSC therapy. A recent report on ASSC by Khanna et al. (29) using model mice also demonstrated that treatment with DGJ decreases the Gb3 level in the mouse heart. Although they used the same human mutant α-Gal A (R301Q) cDNA for the preparation of a model mouse, their strategy was very different from ours. Their mouse model, which shows Gb3 accumulation in heart tissues, was prepared by reducing the expression level of the mutant α-Gal A gene by using the human GLA promoter—which is a very weak promoter— instead of the stronger CAG (cytomegalovirus immediate-early enhancer/chicken β-actin hybrid) promoter. In contrast, we prepared a mouse model overexpressing both Gb3 synthase and the mutant α-Gal A genes. Our study confirmed the work of Khanna et al. (29) by reproducing their findings on the effect of DGJ in the heart, in a different mouse model. Although the effect of ASSC on Gb3 content can be determined in our mice and mice reported by Khanna et al. (29), the benefit of our mice is to be able to determine the localization and content of mutant enzyme in mice tissue with its antibody (16, 18) as well as the localization of Gb3. Our mice will be useful for the elucidation of the mechanism of ASSC effect in detail by the detection of both mutant enzyme and Gb3 at the same time.
Although treatment with DGJ at a low concentration (<100 µM) can increase mutant α-Gal A activity, at high concentrations (>1 mM) DGJ significantly inhibits α-Gal A (30), leading to a corresponding increase in Gb3 levels in G3S/COS-7 cells (Fig. 6). Here, the increase in Gb3 content correlated with a decrease in the intracellular α-Gal A activity, and we used immunoelectron microscopy to show that Gb3 accumulated in the lysosomes of G3S/COS-7 cells treated with 10 mM DGJ. These data demonstrate the importance of determining a safe and effective concentration of DGJ for use in clinical therapy. In our present study, we used a 25-fold higher concentration (∼75 mg/kg/day treatment) of DGJ than the usual dose (3 mg/kg/day treatment), which did not inhibit α-Gal A activity nor increase the tissue Gb3 level (Fig. 7), indicating that the tissue concentration of DGJ is below the inhibitory level at oral dosage. From no further reduction of Gb3 content either in heart or kidney tissue was observed by the treatment with DGJ at higher concentrations, the usual dose (3 mg/kg/day treatment) may be a suitable dose for the ASSC effect in our mouse model.
In conclusion, the Gb3-synthase-overexpressing mouse (TgG3S) shows an increased Gb3 level in its organs, and was useful in generating a mouse line (TgG3S(+/−)M(+/−)/KO) that can be used as an animal model for studying the efficacy of ASSC therapy for Fabry disease.
Funding
Grant-in-Aid for Scientific Research (C) [KAKENHI (20590300)] from Japan Society for the Promotion of Science.
Conflict of interest
None declared.
Glossary
Abbreviations
- A4GalT
α1,4-galactosyltransferase
- ASSC
active-site-specific chaperone
- α-Gal A
α-galactosidase A
- DGJ
1-deoxygalactonojirimycin
- Gb3
globotriaosylceramide (Galα1,4Galβ1, 4Glc-Ceramide)
- GLAko mouse
α-Gal A knockout mouse
- Gb4
globotetraosylceramide
- G3Stg/GLAko mouse
transgenic mouse expressing human Gb3 synthase in α-Gal A knockout background
- G3S/COS-7 cells
COS-7 cells expressing human Gb3 synthase
- HPTLC
high-performance thin-layer chromatography
- LacCer
lactosylceramide
- PBS
phosphate-buffered saline
- Stx1B
Shiga toxin 1 B-subunit
- TgM/KO mouse
transgenic mouse expressing human mutant R301Qα-Gal A in α-Gal A knockout background
- TgG3S mouse
transgenic mouse expressing human Gb3 synthase
- TgG3S (+/−)M(+/−)/KO mouse
transgenic mouse expressing human Gb3 synthase and mutant R301Qα-Gal A in α-Gal A knockout background
References
- 1.Brady O.R., Gal A.E., Bradley R.M., Martensson E., Warshaw A.L., Laster L. Enzymatic defect in Fabry's disease: ceramidetrihexosidase deficiency. N. Engl. J. Med. 1967;276:1163–1167. doi: 10.1056/NEJM196705252762101. [DOI] [PubMed] [Google Scholar]
- 2.Desnick R.J., Ioannou Y.A., Eng C.M. In: The Metabolic and Molecular Bases of Inherited Disease. Scriver C.R., Beaudet A.L., Sly W.S., Valle D., editors. New York: McGraw-Hill; 2001. pp. 3733–3774. [Google Scholar]
- 3.Ishii S., Kase R., Sakuraba H., Suzuki Y. Characterization of a mutant α-galactosidase gene product for the late-onset cardiac form of Fabry disease. Biochem. Biophys. Res. Comm. 1993;197:1585–1589. doi: 10.1006/bbrc.1993.2659. [DOI] [PubMed] [Google Scholar]
- 4.Ishii S., Chang H.-H., Kawasaki K., Yasuda K., Wu H.-L., Garman S.C., Fan J.-Q. Mutant α-galactosidase A enzymes identified in Fabry patients with residual enzyme activity: biochemical characterization and restoration of normal intracellular processing by 1-deoxygalactonojirimycin. Biochem. J. 2007;406:285–295. doi: 10.1042/BJ20070479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamanaka R., Shinohara T., Yano S., Nakamura M., Yasuda A., Yokoyama S., Fan J.-Q., Kawasaki K., Watanabe M., Ishii S. Rescue of mutant a-galactosidase A in the endoplasmic reticulum by 1-deoxygalactonojirimycin leads to trafficking to lysosomes. Biochim. Biophys, Acta. 2008;1782:408–413. doi: 10.1016/j.bbadis.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Fan J.-Q., Ishii S., Asano N., Suzuki Y. Accelerated transport and maturation of lysosomal α-galactosidase A in Fabry lymphoblasts by an enzyme inhibitor. Nat. Med. 1999;5:112–115. doi: 10.1038/4801. [DOI] [PubMed] [Google Scholar]
- 7.Fan J.-Q., Ishii S. Cell-based screening of active site specific chaperone for the treatment of Fabry disease. Methods Enzymol. 2003;363:412–420. doi: 10.1016/S0076-6879(03)01069-3. [DOI] [PubMed] [Google Scholar]
- 8.Fan J.-Q., Ishii S. Active-site-specific chaperone therapy for Fabry disease: Yin and Yang of enzyme inhibitors. FEBS J. 2007;274:4962–4971. doi: 10.1111/j.1742-4658.2007.06041.x. [DOI] [PubMed] [Google Scholar]
- 9.Benjamin E.R., Flanagan J.J., Schilling A., Chang H.H., Agarwal L., Katz E., Wu X., Pine C., Wustman B., Desnick R.J., Lockhart D.J., Valenzano K.J. The pharmacological chaperone 1-deoxygalactonojirimycin increases alpha-galactosidase A levels in Fabry patient cell lines. J. Inherit. Metab. Dis. 2009;32:424–440. doi: 10.1007/s10545-009-1077-0. [DOI] [PubMed] [Google Scholar]
- 10.Ohshima T., Murray G.J., Swaim W.D., Longenecker G., Quirk J.M., Cardarelli C.O., Sugimoto Y., Pastan I., Gottesman M.M., Brady R.O., Kulkarni A.B. α-Galactosidase A deficient mice: a model of Fabry disease. Proc. Natl Acad. Sci. USA. 1997;94:2540–2544. doi: 10.1073/pnas.94.6.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ioannou Y.A., Zeidner K.M., Gordon R.E., Desnick R.J. Fabry disease: preclinical studies demonstrate the effectiveness of α-galactosidase A replacement in enzyme-deficient mice. Am. J. Hum. Genet. 2001;68:14–25. doi: 10.1086/316953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ziegler R.J., Yew N.S., Li C., Cherry M., Berthlette P., Romanczuk H., Ioannou Y.A., Zeidner K.M., Desnick R.J., Cheng S.H. Correction of enzymatic and lysosomal storage defects in Fabry mice by adenovirus-mediated gene transfer. Hum. Gene Ther. 1999;10:1667–1682. doi: 10.1089/10430349950017671. [DOI] [PubMed] [Google Scholar]
- 13.Takenaka T., Murray G.J., Qin G., Quirk J.M., Ohshima T., Qasba P., Clark K., Kurkarni A.B., Brady R.O., Medin J.A. Long-term enzyme correction and lipid reduction in multiple organs of primary and secondary transplanted Fabry mice receiving transduced bone marrow cells. Proc. Natl Acad. Sci. USA. 2000;97:7515–7520. doi: 10.1073/pnas.120177997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura G., Maruyama H., Ishii S., Shimotori M., Kameda S., Kono T., Miyazaki J., Kulkarni A.B., Gejyo F. Naked plasmid DNA-based alpha-galactosidase A gene transfer partially reduces systemic accumulation of globotriaosylceramide in Fabry mice. Mol. Biotechnol. 2008;38:109–119. doi: 10.1007/s12033-007-9008-5. [DOI] [PubMed] [Google Scholar]
- 15.Abe A., Gregoory S., Lee L., Killen P.D., Brady R.O., Kulkarni A., Shayman J.A. Reduction of globotriaosylceramide in Fabry disease mice by substrate deprivation. J. Clin. Invest. 2000;105:1563–1571. doi: 10.1172/JCI9711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishii S., Yoshioka H., Mannen K., Kulkarni A.B., Fan J.-Q. Transgenic mouse expressing human mutant α-galactosidase A in an endogenous enzyme deficient background: a biochemical animal model for studying active-site specific chaperone therapy for Fabry disease. Biochim. Biophys. Acta. 2004;1690:250–257. doi: 10.1016/j.bbadis.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Shimmoto M., Kase R., Itoh K., Utsumi K., Ishii S., Taya C., Yonekawa H., Sakuraba H. Generation and characterization of transgenic mice expressing a human mutant α-galactosidase with an R301Q substitution causing a variant form of Fabry disease. FEBS Lett. 1997;417:89–91. doi: 10.1016/s0014-5793(97)01263-5. [DOI] [PubMed] [Google Scholar]
- 18.Ishii S., Chang H.-H., Yoshioka H., Shimada T., Mannen K., Higuchi Y., Taguchi A., Fan J.-Q. Preclinical efficacy and safety of 1-deoxygalactonojirimycin in mice for Fabry disease. J. Pharmacol. Exp. Ther. 2009;328:723–731. doi: 10.1124/jpet.108.149054. [DOI] [PubMed] [Google Scholar]
- 19.Kojima Y., Fukumoto S., Furukawa K., Okajima T., Wiels J., Yokoyama K., Suzuki Y., Urano T., Ohta M., Furukawa K. Molecular cloning of globotriaosylceramide/CD77 synthase, a glycosyltransferase that initiates the synthesis of globo series glycosphingolipids. J. Biol. Chem. 2000;275:15152–15156. doi: 10.1074/jbc.M909620199. [DOI] [PubMed] [Google Scholar]
- 20.Niwa H., Yamamura K., Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–200. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 21.Matsuda J., Suzuki O., Oshima A., Yamamoto Y., Noguchi A., Takimoto K., Itoh M., Matsuzaki Y., Yasuda Y., Ogawa S., Sakata Y., Nanba E., Higaki K., Ogawa Y., Tominaga L., Ohno K., Iwasaki H., Watanabe H., Brady R.O., Suzuki Y. Chemical chaperone therapy for brain pathology in G(M1)-gangliosidosis. Proc. Natl Acad. Sci. USA. 2003;100:15912–15917. doi: 10.1073/pnas.2536657100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishii S., Katsumura T., Shiozuka C., Ooyauchi K., Kawasaki K., Takigawa S., Fukushima T., Tokuji Y., Kinoshita M., Ohnishi M., Kawahara M., Ohba K. Anti-inflammatory effect of buckwheat sprout in lipopolysaccharide-activated human colon cancer cells and mice. Biosci. Biotechnol. Biochem. 2008;72:3148–3157. doi: 10.1271/bbb.80324. [DOI] [PubMed] [Google Scholar]
- 23.Shin I.-S., Nishikawa K., Maruyama H., Ishii S. Histidine-tagged Shiga toxin B subunit binding assay: simple and specific determination of Gb3 content in mammalian cells. Chem. Pharm. Bull. 2006;54:522–527. doi: 10.1248/cpb.54.522. [DOI] [PubMed] [Google Scholar]
- 24.Yano S., Kashima K., Daa T., Urabe S., Tsuji K., Nakayama I., Yokoyama S. An antigen retrieval method using an alkaline solution allows immunoelectron microscopic identification of secretory granules in conventional epoxy-embedded tissue sections. J. Histochem. Cytochem. 2003;51:199–204. doi: 10.1177/002215540305100208. [DOI] [PubMed] [Google Scholar]
- 25.Jacewicz M., Clausen H., Nudelman E., Donohue-Rolfe A., Keusch G.T. Pathogenesis of shigella diarrhea. XI. Isolation of a shigella toxin-binding glycolipid from rabbit jejunum and HeLa cells and its identification as globotriaosylceramide. J. Exp. Med. 1986;163:1391–1404. doi: 10.1084/jem.163.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lingwood C.A., Law H., Richardson S., Petric M., Brunton J.L., De Grandis S., Karmali M. Glycolipid binding of purified and recombinant Escherichia coli produced verotoxin in vitro. J. Biol. Chem. 1987;262:8834–8839. [PubMed] [Google Scholar]
- 27.McCluer R.H., Gross S.K. Biosynthesis of neutral glycosphingolipids in kidney slices from male and female mice. J. Lipid Res. 1985;26:593–599. [PubMed] [Google Scholar]
- 28.Ohshima T., Schiffmann R., Murray G.J., Kopp J., Quirk J.M., Stahl S., Chan C.C., Zerfas P., Tao-Cheng J.H., Ward J.M., Brady R.O., Kulkarni A.B. Aging accentuates and bone marrow transplantation ameliorates metabolic defects in Fabry disease mice. Proc. Natl Acad. Sci. USA. 1999;96:6423–6427. doi: 10.1073/pnas.96.11.6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khanna R., Soska R., Lun Y., Feng J., Franscella M., Young B., Brignol N., Pellegrino L., Sitaraman S., Desnick R.J., Benjamin E.R., Lockhart D.J., Valenzano K.J. The pharmacological chaperone 1-deoxygalactonojirimycin reduces tissue globotriaosylceramide levels in a mouse model of Fabry disease. Mol. Ther. 2010;18:23–33. doi: 10.1038/mt.2009.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asano N., Ishii S., Kizu H., Ikeda K., Yasuda K., Kato A., Martin O.R., Fan J.-Q. In vitro inhibition and intracellular enhancement of lysosomal α-galactosidase A activity in Fabry lymphoblasts by 1-deoxygalactonojirimycin and its derivatives. Eur. J. Biochem. 2000;267:4179–4186. doi: 10.1046/j.1432-1327.2000.01457.x. [DOI] [PubMed] [Google Scholar]