Abstract
Beta-blockers are a multiform group of drugs with multiple applications in the treatment of patients with cardiovascular disease. Their adverse actions are multiple and relate mainly to the β-adrenergic receptor blockade.
They are used to treat all forms of coronary disease, but especially in acute myocardial infarction and acute coronary syndromes. The administration to patients with coronary artery disease resulted in increased survival and improved QoL of these patients and therefore they are a key group of drugs for their management. However, because of side effects, they should be used with caution, especially in hemodynamically unstable patients. Therefore, the choice of the appropriate β-blocker for each patient will result in the best possible results with fewer side effects.
Keywords: β-blockers, coronary artery disease, stable angina, acute coronary syndromes, review
Beta-blockers, constitute a numerous, multiform, heterogeneous and continuously developing group of drugs which has offered much and continues to be very useful in patients with cardiovascular disease.
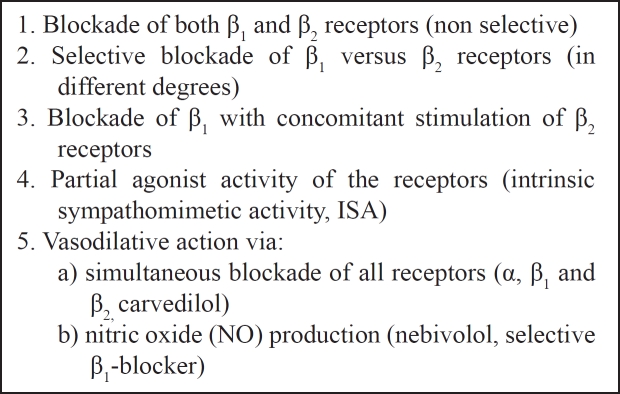
Mainly, their action is to blockade the -receptors of the sympathetic nervous system (SNS). Nevertheless, SNS disposes α-receptors (subdivided into α1- located postsynaptically on the vascular smooth-muscle wall and α2 - which are presynaptic located on the sympathetic neuron terminal) and -receptors (subdivided into β1- located in the heart, kidneys and eyes, stimulation of which results in positive inotropic and chronotropic effects, renin release and increase in aqueous humor production respectively and β2- located in the peripheral vascular smooth muscle cells, which when stimulated result in vasodilatation and relaxation of bronchial, uterine, and gastrointestinal smooth muscle1–2). Though, their actions differ, depending on their influence on these receptors (Table 1) and particularly they can present: a) blockade of both β1 and β2 receptors (non selective), b) selective blockade of β1 versus β2 receptors (in different degrees), c) blockade of β1 with concomitant stimulation of β2 receptors, d) partial agonist activity of the receptors (intrinsic sympathomimetic activity, ISA) and e) simultaneous blockade of all receptors (α, β1 and β2). The newer β-blockers have vasodilative action via either -blockade (carvedilol) or nitric oxide (NO) production (nebivolol). Also, some of them are hydrophilic and some are lipophilic, which penetrate the blood-brain barrier causing nightmares1.
Table 1: The different actions of β-blockers on adrenergic receptors.
They are used in a variety of cardiac (arterial hypertension, coronary artery disease, heart failure, cardiomyopathies and arrhythmias) and non-cardiac diseases (glaucoma, portal hypertension) and it is worth emphasizing that their use has improved the survival of the patients with cardiovascular diseases.
Their side effects are multiple, ranging from mild to severe and, in some cases life threatening, the majority of them arising from their cardiovascular action but they also have non-cardiovascular side effects (such as purpura, rise of nuclear antibodies with arthralgias and myalgias). The most common side effects are associated with their hypotensive, and negative chronotropic action, reduction of atrioventricular conduction and bronchoconstriction (which is less prominent with the cardioselective β-blockers). For this reason, they are contraindicated in patients with low blood pressure, low cardiac output, low heart rate and atrioventricular block and lung diseases, especially bronchial asthma and chronic obstructive pneumonopathy1–3.
Coronary artery disease (CAD)
Coronary artery disease (CAD) is a syndrome with many clinical entities. The classification of the different clinical entities consisting the CAD, alters depending on the onset and duration of symptoms, the changes of the biochemical indices, the influence on left ventricular function and with the better understanding of the underlying pathology.
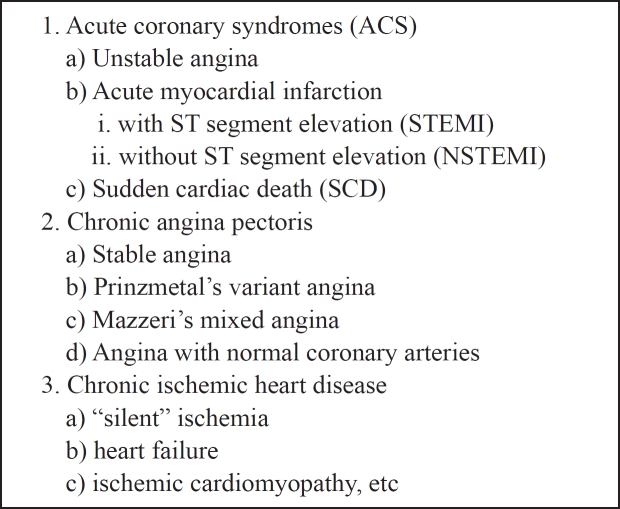
Unstable angina (UA), acute myocardial infarction (AMI, subdivided into ST segment elevation myocardial infarction, STEMI and non STEMI) and sudden cardiac death (SCD) comprise the acute coronary syndromes (ACS), which are the most studied manifestations of the CAD needing the most intensive and immediate treatment (Table 2). On the other hand, chronic angina pectoris (including stable angina, Prinzmetals variant angina, Mazzeri's mixed angina, and angina with normal coronary arteries) and chronic ischemic heart disease (including "silent" ischemia, heart failure, ischemic cardiomyopathy) are mild but not less significant manifestations that need chronic treatment.
Table 2: The clinical manifestations of coronary artery disease (CAD).
The principal goals of CAD management are the augmentation of coronary artery blood flow combined with the limitation of myocardial oxygen demands. But in the "modern" treatment of the CAD the substantial goals are: increase of survival, decrease of the atherosclerotic process in the coronary arteries, prevention of left ventricular (LV) dysfunction via minimizing of myocardial necrosis and LV remodeling, prevention of new onset of ACS and improving the quality-of-life (QoL).
Because of the significance of CAD resulting from its prevalence on the morbidity and mortality, especially among young people, the evolution of its management was amazing during the last decades of the twentieth century. Nitrates were the first drugs used in the treatment of angina in 1880. After about a century in 1965, β-blockers were used in the treatment of CAD and then coronary artery bypass graft surgery (1969), calcium channel blockers (CCBs, 1975), percutaneous coronary intervention (PCI, 1977) and finally in the 90's aspirin and the other antiplatelet agents and statins were used. All these pharmacological and interventional therapies led to significant decrease of cardiovascular mortality and morbidity, increase of survival and to achievement of the goals of CAD treatment in general.
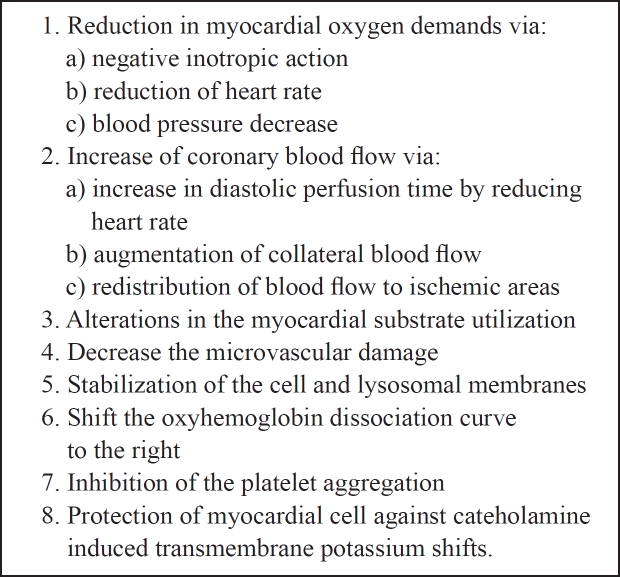
The proposed mechanisms by which β-blockers express their action and protect the ischemic myocardium are multiple (Table 3) and particularly, they reduce the myocardial oxygen demands (via their negative inotropic action, reduction of heart rate, and blood pressure decrease), increase coronary blood flow (via increase in diastolic perfusion time by reducing heart rate, augmentation of collateral blood flow and redistribution of blood flow to ischemic areas), alter the myocardial substrate utilization, decrease the microvascular damage, stabilize the cell and lysosomal membranes, shift the oxyhemoglobin dissociation curve to the right, inhibit the platelet aggregation and protect myocardial cell against cateholamine- induced transmembrane potassium shifts1.
Table 3: Possible mechanisms by which β-blockers protect the ischemic myocardium.
Beta-blockers are used in all coronary artery disease type management, but they have proved to be especially useful in the treatment of AMI and ACS in general, and this was found early after their application in the treatment of AMI4. The administration of β-blockers in patients with STEMI begins from the first hours of the symptoms' onset, but if they are given orally their action starts from the second day5,6. The 2004 STEMI Guidelines recommendations were based on studies that showed a reduced incidence of subsequent reinfarction and recurrent ischemia in patients receiving both fibrinolytic therapy and intravenous (IV) β-blockers7. However, uncertainty about the use of IV beta blockers in the setting of fibrinolytic therapy has increased following two later randomised trials of IV beta blockade8,9, a post-hoc analysis of the use of atenolol in the GUSTO-I trial10, and a review of early beta-blocker therapy in myocardial infarction (MI)11 that did not find significant reductions in mortality12. On the other hand, IV administration of β-blockers was associated with side effects such as hypotension, shock and bradycardia in about 30 per cent of the patients especially the hemodynamically unstable. Therefore, during the early phase of STEMI, careful β-blockers IV administration is recommended. However, Lopez-Sendon J, et al13 suggest that, from day two, when beneficial effects on reinfarction and ventricular fibrillation are seen, oral administration of β-blockers appears to be safe in hemodynamically stable patients with STEMI who are free of contraindications. It is prudent to initiate a low dose of β-blockers orally, transitioning to the maximum tolerated dose. It should be noted that long-term use of oral β-blockers is strongly recommended (Class I, Level of Evidence: A) for secondary prevention in patients at highest risk, such as those with low ejection fraction, heart failure, or postshock, once they have stabilized, with gradual dose titration7,13,14.
Persons after myocardial infarction should have their modifiable coronary artery risk factors intensively treated. Aspirin or clopidogrel, β-blockers, and ACE inhibitors should be given indefinitely unless contraindications exist to the use of these drugs. Long-acting nitrates are effective antianginal and antiischemic drugs15,16. Postinfarction patients at very high risk for sudden cardiac death should have an implantable cardioverter-defibrillator. The two indications for coronary revascularization are prolongation of life and relief of unacceptable symptoms despite optimal medical management17.
The optimal pharmacological treatment of the ACS includes antiplatelet agents, antithrombotic therapy, β-blockers, angiotensin converting enzyme inhibitors (ACEI) and statins with excellent results18.
One of the goals of the pharmacological therapy in the management of the patients, who experienced an AMI, is to increase survival. This is achieved with the thrombolytic therapy, aspirin and β-blockers administration. Beta-blockers were the first group of drugs that led to increase of the survival in these patients4. Nevertheless, a significant percentage of patients with AMI present heart failure (HF) during the acute phase or later and the administration of β-blockers in this case was contraindicated in the past but not in the recent management, except when unstable hemodynamic conditions are present. Coexistence of left ventricular (LV) dysfunction constitutes a significant risk factor in patients with AMI and it is associated with increased mortality. Improvement of LV function, in patients experienced an AMI, was achieved by the administration of ACEI, and this was accompanied with a decrease of heart failure appearance and increase of survival. Recently, improvement of LV function was also found with β-blockers administration19,20.
The goals of pharmacological treatment of stable angina pectoris are to improve QoL by reducing the severity and/or frequency of symptoms and to improve the prognosis of the patient. In the management of patients with chronic stable angina, β-blockers administration is usually necessary. The ideal patients for β-blockers administration are those with arterial hypertension, arrhythmias, after an AMI, when angina is directly associated with the physical activity and when anxiety is the underlying mechanism. Contraindications for β-blockers administration are: hypotension (systolic blood pressure < 100 mmHg), bradycardia (heart rate < 50 bpm), phenomenon Raynaud, severe pneumonopathy (especially chronic obstructive and bronchial asthma), and severe renal insufficiency. Administration of β-blockers in patients with diabetes mellitus is relatively contraindicated particularly because it may cover the symptoms of hypoglycemia, while it is not contraindicated in patients with LV dysfunction and congestive heart failure, even severe, except for hemodynamically unstable.
Disagreement exists in the literature in relation to the choice of the group of drugs initially given for the treatment of patients in whom chronic angina is the first manifestation of CAD, particularly between β-blockers and CCBs. Because of their different mechanisms of action there are relative advantages of them on selective groups of patients. Both groups of drugs are effective to relieve the symptoms of ischemia21. It has been extrapolated from the post-MI trials that β-blockers may be cardioprotective also in patients with stable coronary disease and as a rational consequence, β-blockers are preferred in these patients as initial treatment versus CCBs. However, this has not been proven in a placebo-controlled trial. On the other hand, short acting CCBs, especially nifedipin, were associated with increased incidence of AMI (although not proven). Beta-blockers without ISA have a negative biochemical profile causing an increase of triglycerides and decrease of HDL-cholesterol with unknown results. Long-term CCBs administration is not associated with increase of survival, although administration of diltiazem was suggested to prevent severe angina and reinfarction after non STEMI, verapamil minimizes the appearance of reinfarction and nifedipine prevents new myocardial damage in patients with CAD. However, large β-blockers studies in stable angina, the ASPIS22 and TIBET23 studies did not show a significant difference in outcome between patients treated with β-blockers and CCBs, either nifedipine or verapamil. In spite of this, in the recommendations of the European Society of Cardiology guidelines to improve prognosis in patients with stable angina, administration of β-blockers is class I, level of evidence A24.
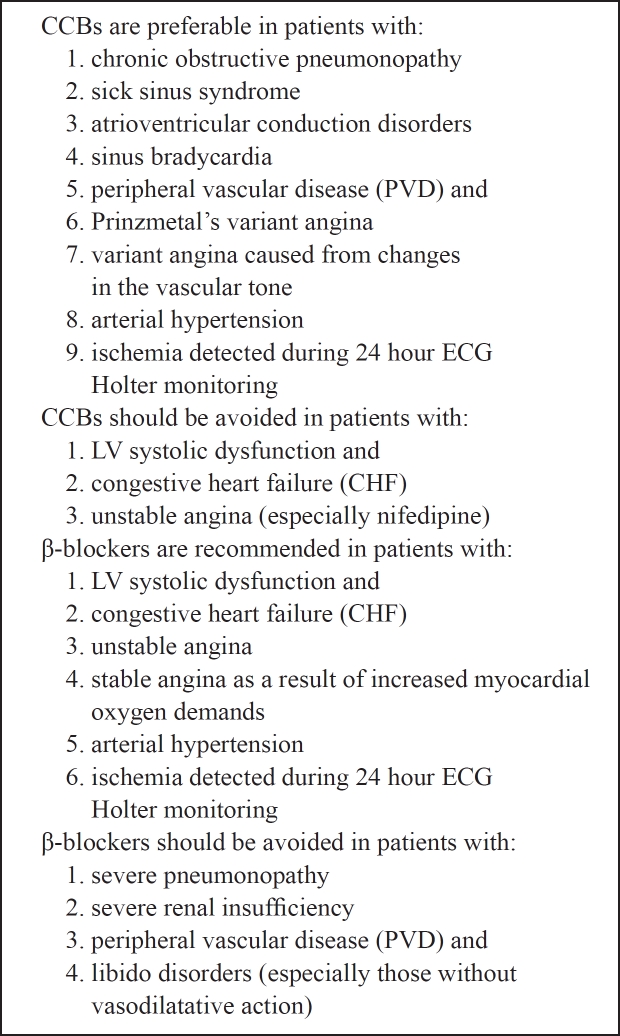
Therefore, the choice of the appropriate group of drugs for the initial therapy of chronic CAD depends on the clinical characteristics of each patient (Table 4) and particularly: CCBs are preferable in patients with chronic obstructive pneumonopathy, second generation nifedipin is preferable in patients with sick sinus syndrome, atrioventricular conduction disorders and sinus bradycardia. Also, patients with peripheral vascular disease (PVD) and Prinzmetal's variant angina should be preferably treated with CCBs. Beta-blockers should be avoided in patients with severe pneumonopathy, severe renal insufficiency, PVD and libido disorders (especially those without vasodilatative action)25. In patients with LV systolic dysfunction and congestive heart failure (CHF), CCBs should be avoided as well as nifedipin in patients with unstable angina in whom, immediate administration of nitrates and β-blockers is recommended. In patients with arterial hypertension both groups are effective26,27 as well as when myocardial ischemia (with or without symptoms) is detected during 24 hour ECG Holter monitoring, especially in combination. Stable angina as a result of increased myocardial oxygen demands should be treated with β-blockers, while in patients with variant angina caused from changes in the vascular tone administration of CCBs is recommended.
Table 3: Indications and contraindications of β-blockers and calcium channel blockers (CCBs) administration, according to the comorbidities in patients with coronary artery disease (CAD).
In conclusion, β-blockers are a particularly useful group of drugs in the treatment of patients with coronary artery disease and the appropriate use has led to increased survival and improved QoL of these patients.
References
- 1.Frishman WH. Clinical pharmacology of the β-adrenoceptor blocking drugs. ed. 2. New York: Appleton-Century Crofts; 1984. [Google Scholar]
- 2.Manrique C, Giles TD, Ferdinand KC, Sowers JR. Realities of newer beta-blockers for the management of hypertension. J Clin Hypertens (Greenwich) 2009;11:369–375. doi: 10.1111/j.1751-7176.2009.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobusiak-Prokopowicz M, Jołda-Mydłowska B, Zubkiewicz A, Szymczak M, Mysiak A, Skalik R. Impact of nebivolol on levels of serum nitric oxide, plasma von Willebrand factor and exercise stress testing parameters in hypertensive and ischemic heart disease patients. Cardiol J. 2008;15:162–168. [PubMed] [Google Scholar]
- 4.The Norwegian Multicenter Study Group Timolol induced reduction in mortality and reinfarction in patients surviving acute myocardial infarction. N Engl J Med. 1981;304:801–807. doi: 10.1056/NEJM198104023041401. [DOI] [PubMed] [Google Scholar]
- 5.Brandler E, Paladino L, Sinert R. Does the early administration of beta-blockers improve the in-hospital mortality rate of patients admitted with acute coronary syndrome? Acad Emerg Med. 2010;17:1–10. doi: 10.1111/j.1553-2712.2009.00625.x. [DOI] [PubMed] [Google Scholar]
- 6.Cuculi F, Radovanovic D, Pedrazzini G, Regli M, Urban P, Stauffer JC, et al. Is pretreatment with Beta-blockers beneficial in patients with acute coronary syndrome? Cardiology. 2010;115:91–97. doi: 10.1159/000256384. [DOI] [PubMed] [Google Scholar]
- 7.Antman EM, Hand M, Armstrong PW, Bates ER, Green LA, et al. 2007 Focused Update of the ACC/AHA 2004 Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction. Circulation. 2008;117:296. Erratum in: Circulation. 2008 Feb 12;117:e162. [Google Scholar]
- 8.Roberts R, Rogers WJ, Mueller HS, Lambrew CT, Diver DJ, Smith HC, et al. Immediate versus deferred beta-blockade following thrombolytic therapy in patients with acute myocardial infarction. Results of the Thrombolysis in Myocardial Infarction (TIMI) II-B Study. Circulation. 1991;83:422–437. doi: 10.1161/01.cir.83.2.422. [DOI] [PubMed] [Google Scholar]
- 9.van de Werf F, Janssens L, Brzostek T, Mortelmans L, Wackers FJ, Willems GM, et al. Short-term effects of early intravenous treatment with a beta-adrenergic blocking agent or a specific bradycardiac agent in patients with acute myocardial infarction receiving thrombolytic therapy. J Am Coll Cardiol. 1993;22:407–416. doi: 10.1016/0735-1097(93)90044-2. [DOI] [PubMed] [Google Scholar]
- 10.Pfisterer M, Cox JL, Granger CB, Brener SJ, Naylor CD, Califf RM, et al. Atenolol use and clinical outcomes after thrombolysis for acute myocardial infarction: the GUSTOI experience. Global Utilization of Streptokinase and TPA alteplase) for Occluded Coronary Arteries. J Am Coll Cardiol. 1998;32:634–640. doi: 10.1016/s0735-1097(98)00279-4. [DOI] [PubMed] [Google Scholar]
- 11.Freemantle N, Cleland J, Young P, Mason J, Harrison J. Beta Blockade after myocardial infarction: systematic review and meta regression analysis. BMJ. 1999;318:1730–1737. doi: 10.1136/bmj.318.7200.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of patients with acute myocardial infarction) J Am Coll Cardiol. 2004;44:e1–e211. doi: 10.1016/j.jacc.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Sendon J, Swedberg K, McMurray J, Tamargo J, Maggioni AP, Dargie H, et al. Expert consensus document on beta-adrenergic receptor blockers. Eur Heart J. 2004;25:1341–1362. doi: 10.1016/j.ehj.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Hall SL, Lorenc T. Secondary prevention of coronary artery disease. Am Fam Physician. 2010;8:289–296. [PubMed] [Google Scholar]
- 15.Pajak A, Jankowski P, Kawecka-Jaszcz K, Surowiec S, Wolfshaut R, Loster M, et al. Changes in secondary prevention of coronary artery disease in the post-discharge period over the decade 1997-2007. Results of the Cracovian Program for Secondary Prevention of Ischaemic Heart Disease and Polish parts of the EUROASPIRE II and III surveys. Kardiol Pol. 2009;67:1353–1359. [PubMed] [Google Scholar]
- 16.Fornasini M, Yarzebski J, Chiriboga D, Lessard D, Spencer FA, Aurigemma P, et al. Contemporary trends in evidence-based treatment for acute myocardial infarction. Am J Med. 2010;123:166–172. doi: 10.1016/j.amjmed.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aronow WS. Treatment after myocardial infarction. Compr Ther. 2007;33:39–47. doi: 10.1007/s12019-007-0002-4. [DOI] [PubMed] [Google Scholar]
- 18.Yan AT, Yan RT, Tan M, Huynh T, Soghrati K, Brunner LJ, et al. Canadian ACS Registries Investigators. Optimal medical therapy at discharge in patients with acute coronary syndromes: temporal changes, characteristics, and 1-year outcome. Am Heart J. 2007;154:1108–1115. doi: 10.1016/j.ahj.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 19.Neumayr RH, Hauptman PJ. beta-Adrenergic receptor blockers and heart failure risk after myocardial infarction: a critical review. Curr Heart Fail Rep. 2009;6:220–228. doi: 10.1007/s11897-009-0031-7. [DOI] [PubMed] [Google Scholar]
- 20.Duncker DJ, Boontje NM, Merkus D, Versteilen A, Krysiak J, Mearini G, et al. Prevention of myofilament dysfunction by beta-blocker therapy in postinfarct remodeling. Circ Heart Fail. 2009;2:233–242. doi: 10.1161/CIRCHEARTFAILURE.108.806125. [DOI] [PubMed] [Google Scholar]
- 21.Ardissino D, Savonitto S, Egstrup K, Rasmussen K, Bae EA, Omland T, et al. Selection of medical treatment in stable angina pectoris: results of the International Multicenter Exercise Angina (IMAGE) Study. J Am Coll Cardiol. 1995;25:1516–1521. doi: 10.1016/0735-1097(95)00042-3. [DOI] [PubMed] [Google Scholar]
- 22.Rehnqvist N, Hjemdahl P, Billing E, Björkander I, Eriksson SV, Forslund L, et al. Effects of metoporlol vs verapamil in patients with stable angina pectoris. The Angina Prognosis Study in Stockholm (ASPIS) Eur Heart J. 1996;17:76–81. doi: 10.1093/oxfordjournals.eurheartj.a014695. [DOI] [PubMed] [Google Scholar]
- 23.Dargie HJ, Ford I, Fox KM. Total Ischaemic Barden European Trial (TIBET). Effects of ischaemia and treatment with atenolol nifedipin SR and their combination on outcome in patients with chronic angina. The TIBET Study Group. Eur Heart J. 1996;17:104–112. doi: 10.1093/oxfordjournals.eurheartj.a014668. [DOI] [PubMed] [Google Scholar]
- 24.Fox K, Garcia MA, Ardissino D, Buszman P, Camici PG, Crea F, et al. Guidelines on the management of stable angina pectoris: executive summary: The Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur Heart J. 2006;27:1341–1381. doi: 10.1093/eurheartj/ehl001. [DOI] [PubMed] [Google Scholar]
- 25.Vergoulas G. Antihypertensive agents and renal transplantation. Hippokratia. 2007;11:3–12. [PMC free article] [PubMed] [Google Scholar]
- 26.Bavry AA, Anderson RD, Gong Y, et al. Outcomes Among hypertensive patients with concomitant peripheral and coronary artery disease: findings from the INternational VErapamil-SR/ Trandolapril STudy. Hypertension. 2010;55:48–53. doi: 10.1161/HYPERTENSIONAHA.109.142240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antonakoudis G. Blood pressure control and cardiovascular risk reduction. Hippokratia. 2007;11:114–119. [PMC free article] [PubMed] [Google Scholar]