Abstract
Background: Anemia in school-age children is an important public health problem and available data of its prevalence and existing risk factors are essential for planning preventive strategies. The purpose of the current study was to assess the prevalence of and the risk factors associated with anemia among the school-age children 7-14 years years old in Serbia.
Methods: In the 2000 National Health Survey, a cross-sectional, multistage cluster survey, performed in 1688 private and refugee campuses households across the territory of Serbia a total of 525 cases were recruited. Socioeconomic, nutritional, physical activities and lifestyle data have been collected and hemoglobin levels were determined.
Results: The overall prevalence of anemia was 18% (94/525) [95% CI 15-21]. Age of 12-14 yrs (odds ratio 3.56 [95% CI 2.17-5.85], p=0.000), male gender (3.22 [1.92-5.42], p=0.000), refugee campuses residence (1.98 [1.22- 3.23], p=0.000), lunch skipping (3.43 [1.40-8.33], p=0.007), defective poultry intake (1.65 [1.01-2.62], p=0.047), lack of fish consumption (1.84 [1.07-3.18], p=0.028), disagreement that sport contributes protecting health (3.80 [2.02-6.95], p=0.000), absence of learning (1.80 [1.12-2.90], p=0.016) and defective book reading in free time (2.18 [1.03-4.61], p=0.04), were independent risk factors of anemia. The frequency of anemia was highest in schoolaged of male gender adolescent males 12-14 years old (46/105, 44%); in 12-14 years aged participants living in refugee campuses' households (22/63, 35%); in refugees of 7-14 yrs old male gender (32/101, 32%); in subjects with defective fish and poultry intake (35/118, 30%) and in participants who escaped reading and learning as lifestyle practices in free time (53/204, 26%).
Conclusions: Socioeconomic, nutritional, physical and lifestyle risk factors could be considered by introducing preventive strategies of anemia in school-age children in Serbia.
Keywords: anemia, school-age children, socioeconomic factors, nutritive factors, physical activities, lifestyle
Anemia among school-age children (SAC) is known to be a significant global problem affecting 305 million SAC1. In developing countries in population group of SAC for school-age children and adolescents prevalence rates from 29.2%2 to 79.6%3, respectively, have been reported. This may reflect inadequate nutritional iron intake3, generalized malnutrition2 or low iron bioavailability of the diet4. Furthermore, it is well confirmed that increased risk of iron deficiency in SAC of 12-14 yrs oldadolescents is associated with incremented iron demands because of the rapid growth5–7. The consequences of iron deficiency are many and seriously affect not only individuals' health but also the development of societies and countries. Therefore, preventive and control measures need to be performed when the prevalence of anemia in the population varies between 5% and 19.9%1. Nutritive deficiency causes 50% of all types of anemia8 and is the most common cause of isolated chronic anemia9 occurring more often in SAC originating of low income families10–12. Cross-sectional study conducted to investigate nutritive and socioeconomic risk factors for iron-deficiency anemia among 548 girls, 11-16 yrs old, has shown that girls' fish dietary deficiency and parents' occupancy were independent predictors of decreased hemoglobin (Hb) values13. Likewise, socio-demographic and physical activity underlying factors, as mothers formal education14, race15, nutritional practices knowledge16 and decreased physical activities17, respectively, were additional risk factors associated with lower Hb levels. Even in some countries the existence of culturally defined, gender based proscriptions for child feeding causes iron deficiency 2.4 times as likely in school-aged girls, compared to the boys the same age18. More recently, randomised interventional study performed to educate anaemic female 12-17.9 yrs old in necessity of increasing dietary iron consumption, resulted in changing nutritive habits and preventing anemia worsening, in treatment, compared to control study arm19. There is strong evidences in favour that households incomes increasing preceded to the higher meat, fish and poultry consumption in the population20. Very recent cross-sectional surveydesign also revealed that frequency of meat and dairy consumption determines Hb values in SAC21. Particular vulnerability to iron-deficiency is reported in living in SAC refugee campuses22. The measurement of Hb is the most common method of assessing anemia23 and the American Academy of Family Physician, strongly recommends ordering screening tests for Hb in neonates24 and the Centres for Disease Control and Prevention and American Academy of Paediatrics postpone screening to the high risk preschool children subpopulation23,25,26. Most children with anemia are asymptomatic and an abnormal Hb levels are discovered on routine screening27. Although World Health Organization (WHO) reports country estimates of anemia prevalence in Serbian preschool-age children (PreSAC) of 29.5% (95% Confidence Interval [CI] 23.4-36.5%)1, nevertheless thus far, there are no studies conductedare no studies conducted in Serbia evaluating prevalence of anemia in SAC. It is worth noting that, there were also, no available data of anemia for SAC since the early 1990s when worsening of economic conditions for many families became reality associated with a great refugee movements directed to Serbia. In the same time the concern raised because of missing data of the nutritive status of SAC as an indicator for further preventive strategies planning. We therefore, accomplished cross-sectional survey to investigate the frequency of anemia and the associated risk factors in SAC in Serbia including the private and households within refugee campuses alongside the whole territory of the country.
Methods
Subjects and settings
One thousand six hundred and eighty eight (n=1688) private and refugee campuses' households across the territory of Serbia were surveyed.
Five hundred and twenty five (525) school-age children were recruited in the survey from July 1, 2000 until July 31, 2000.
Study design
Two stages stratified sample of clusters has been performed. In the first stage according to the probability approach 300 municipalities on the level of Serbia have been selected (units of the first stage). In the second stage, in each municipality one cluster has been selected. Each cluster consisted of 15 nearby households (units of the second stage). A structured questionnaire was used for collecting information about the socioeconomic, nutritional, physical, lifestyle factors and underlying diseases as following: age; gender; menarche; residence; immigration status; number of children; separate room owner; week's pocket money; scholar status; regular: meal, breakfast, collation, lunch and dinner consumption; intake of milk, dairy products, potato, potato/rice, poultry, fish, pork, beef, meat products, fresh vegetables and fruits, eggs, sweets; sweet beverages; healthy food lifestyle; attendance of physical exercises and school sport classes regularity; agreement or disagreement with: sport/recreation is fun to be occupied with, contributes to meet friends, protects health, makes to be popular, contributes to good looking, good physical condition and relaxes; training sport induced by parent's request; sport as future activity; watching television; learning; helping at home; drawing and writing; book or magazine and drawing novel reading and music listening; presence of hypertension, chronic bronchitis, emphysema, asthma, diabetes mellitus, rheumatic diseases, back pain in children; psychiatric and neurological disorders. For each subject values of blood pressure, height and weight were measured and blood samples were collected. Anemia was defined as decreased concentration of Hb compared with the age-matched controls according the criteria described elsewhere28 (e.g., for children 7-11 yrs, both genderes Hb values <11.5g/dL and for females and males 12-14 yrs old, <12g/dL and <13g/dL, respectively). Capillary samples were analyzed with a HemoCue photometer (Hemo-Cue, Angelholm, Sweden)29,30.
Statistical analysis
Results are reported as mean (SD) or as proportions. Statistical tests used included Kolmogorov-Smirnov and Shapiro-Wilk tests, Mann Whitney U test, χ2 test, binomial test and multiple logistic regression analysis. Stringent entry criteria (p<0.05 in univariate analysis) and separate analyses of groups of risk factors were performed in order to reduce the number of introduced variables and to avoid over fitting. Interactions were analysed pair wise by entering interaction term in the logistic regression model and results are reported separately when interaction was found (p<0.05). Unadjusted and adjusted odds ratios (OR) are reported with 95% CI. Statistical analyses were performed by using SPSS software (version 15.0, SPSS Inc, Chicago).
Results
Study participants
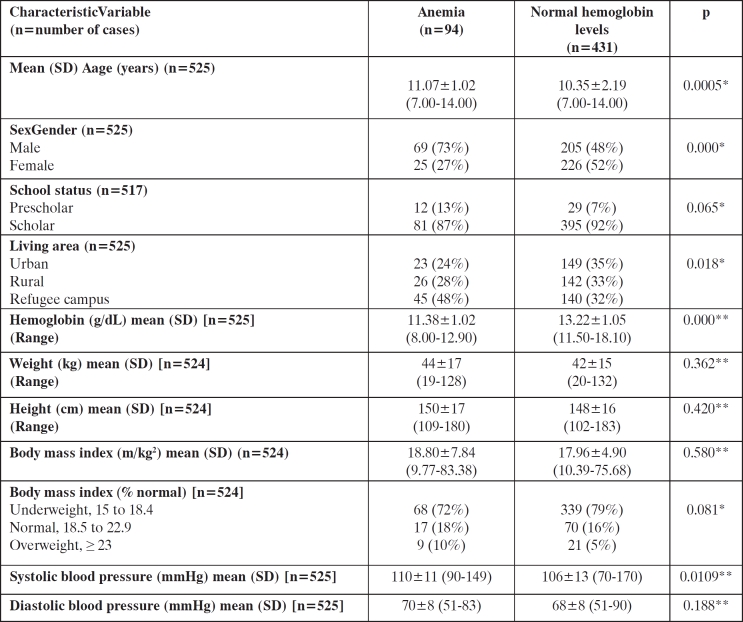
A total of 525 (274 male and 251 female SAC, mean age [SD] 10.4 yrs [2.2] and 10.6 years [2.3], respectively), completed the survey. In overall 94 out of 525 (18%) [95% CI 15-21] survey participants anemia has been diagnosed compared to 431/525 (82%) [95% CI 78-85], in whom normal Hb values were confirmed with Hb ranges between 8.00 to 12.90 mg/dL, in the former and 11.50 to 18.10, in the latter one. Table 1 compares the general data of the surveyed individuals grouped according to the Hb level. Study groups significantly differentiated in elderly, gender, living area and systolic blood pressure. There was a lack of statistically significant difference between anemic and non-anemic groups on menarche and existing underlying diseases.
Table 1. General data of participants with anemia and normal hemoglobin levels.
Values expressed as mean±SD (range). * t-test, ** Mann-Whitney U-test.
Socio-demographic risk factors of anemia
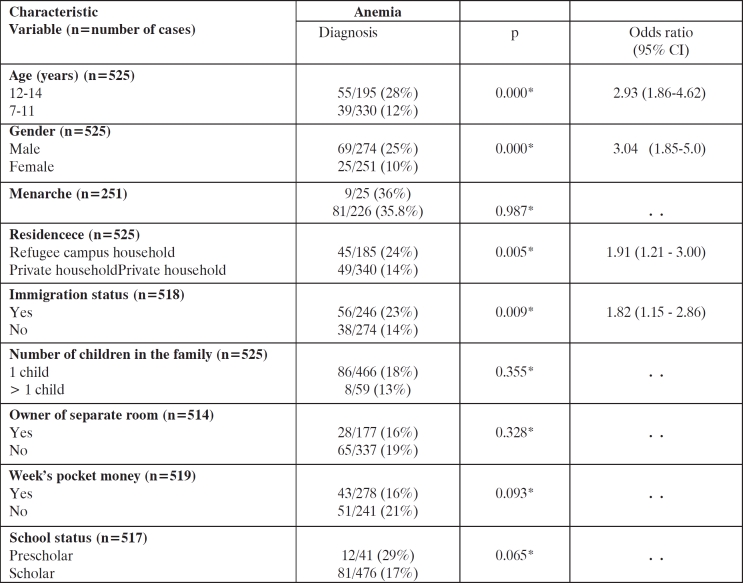
Anemia was associated in the univariate analysis with elderly (males 12-14 yrs of age), male gender, refugee campuses residence and immigration status (Table 2). Multivariate (logistic-regression) analysis revealed that age of 12-14 yrs (odds ratio 3.56 [95% CI 2.17-5.85], p=0.000), male sexgender (3.22 [1.92- 5.42], p=0.000) and refugee campuses residence (1.98 [1.22–3.23], p=0.000) are independent risk factors of anemia.
Table 2. Univariate variable statistics for socioeconomic risk factors of anemia.
Odds ratios with 95% CI are reported for all variables entered into multivariate analysis (p<0.05). * test.
Nutritional risk factors of anemia
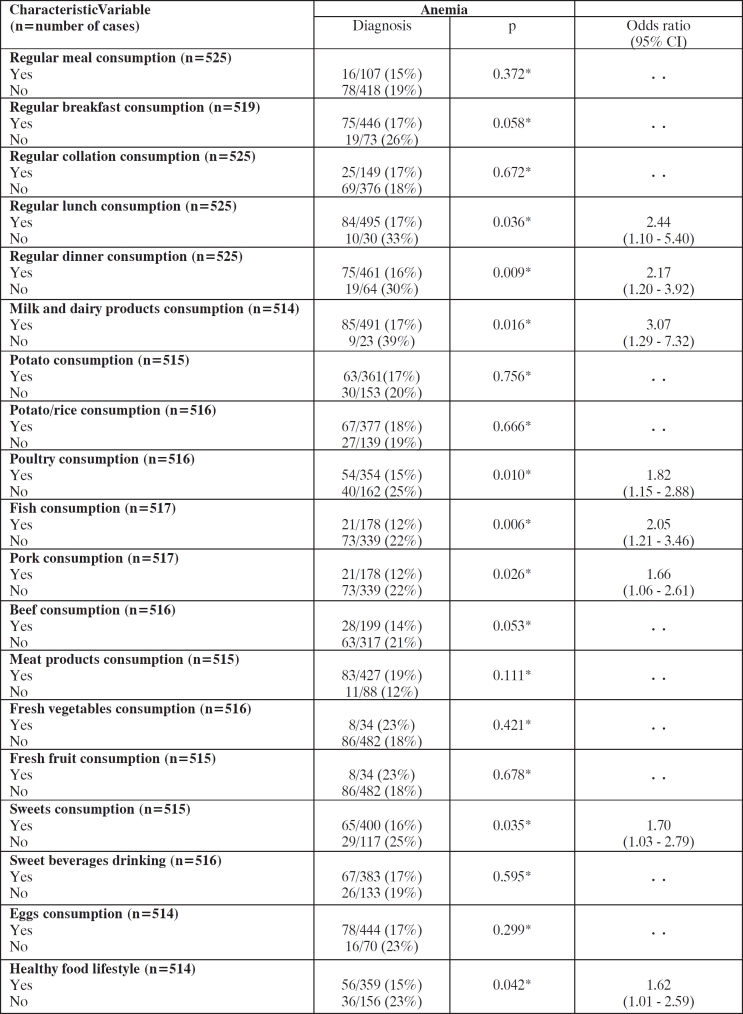
Anemia was significantly associated with eight nutritive risk factors. Those shared variables including lack of regular lunch and dinner skipping, as well as, defective poultry, milk and milk products, fish, pork and sweets consumption and disagreement to healthy food lifestyle (Table 3).
Table 3. Univariate variable statistics for nutritive risk factors of anemia.
Odds ratios with 95% CI are reported for all variables entered into multivariate analysis (p<0.05). * test.
Lunch skipping (3.43 [1.40–8.33], p=0.007), defective poultry (1.65 [1.01–2.62], p=0.047), and lack of fish consumption (1.84 [1.07–3.18], p=0.028), were independently associated with anemia.
Physical risk factors of anemia
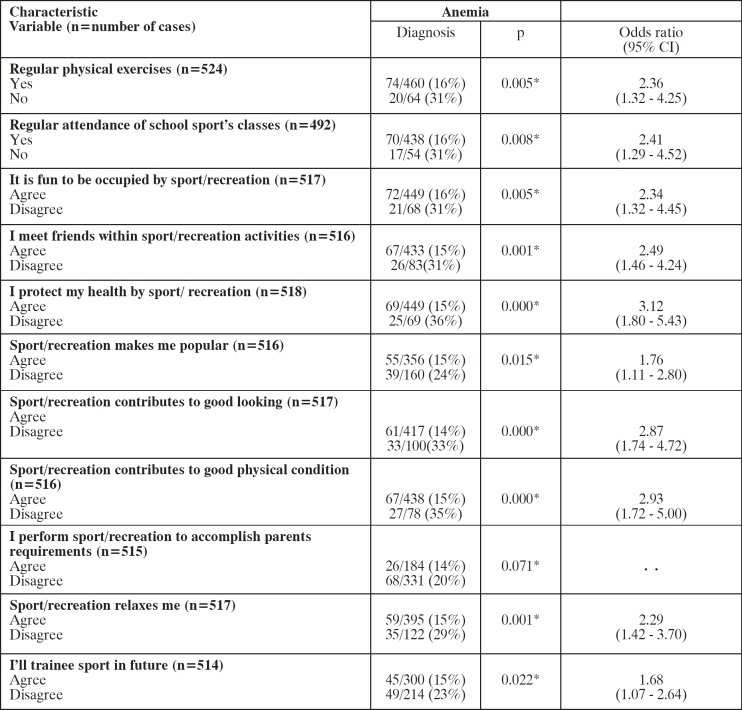
Relating to the physical risk factors, variables as, irregular physical exercises activity and irregular school sport classes attendance, as well as participants' disagreement with the opinions that: sport is fun to be occupied with, sport/recreation contributes to meet friends, to protect health, to be popular, to good looking and to good physical condition, to be relaxed and rejection future sport activity, were significantly related to anemia by the univariate analysis (Table 4).
Table 4. Univariate variable statistics for physical risk factors of anemia.
Odds ratios with 95% CI are reported for all variables entered into multivariate analysis (p<0.05). * x2 test.
The logistic-regression analysis showed only disagreement with the opinion that sport contributes to protect health (3.80 [2.02–6.95], p=0.000) as an independent factor associated with anemia.
Lifestyle risk factors of anemia
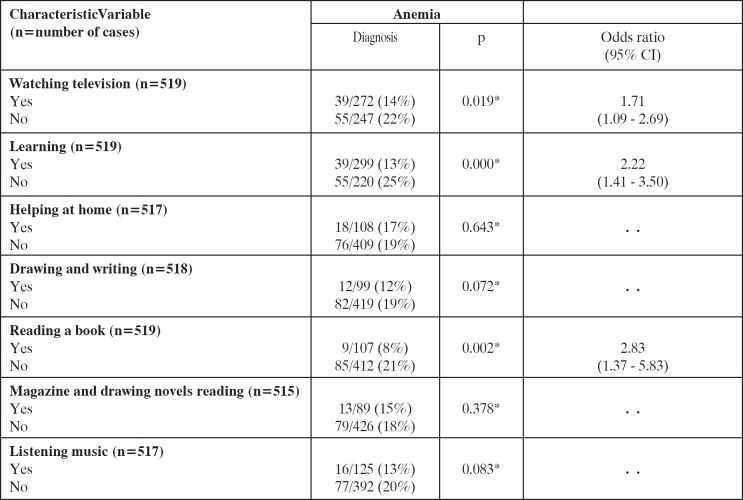
Absence of behavioural (lifestyle) risk factors as watching television, learning and reading books was significantly related to anemia (Table 5).
Table 5. Univariate variable statistics for lifestyle risk factors of anemia.
Odds ratios with 95% CI are reported for all variables entered into multivariate analysis (p<0.05). * test.
Furthermore, multivariate analysis revealed that absence of learning (1.80 [1.12–2.90], p=0.016) and defective book reading (2.18 [1.03–4.61], p=0.04), were independently associated with decreased Hb levels.
Analysis of interactions
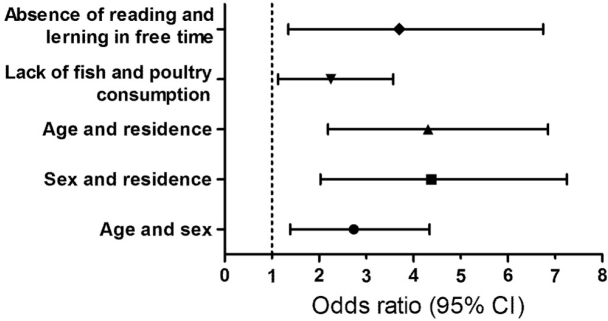
Elderly 12-14 yrs and male gender showed a significant interaction in the analysis of anemia (adjusted odds ratio for the interaction term 2.47 [1.39-4.38], p=0.002) [Figure 1]. The frequency of anemia was highest in male adolescents (males and elderly of 12-14yrs 46/105, 44%; males and elderly of 7-11yrs was 23/169, 14%; females and elderly of 12-14yrs, 9/90, 10%; females and elderly of 7-11yrs, 16/161, 10%). Residence in refugee campuses and males showed a significant interaction in the analysis of the anemia [Adj. OR for the interaction term 3.83 (2.03-7.25), p=0.000] (Figure 1). The frequency of anemia was highest in males residenced in refugee campuses (campus residence and male gender 32/101, 32%; campus residence and females was 13/84, 15%; households residence and males 37/172, 21%; household residence and females 12/167, 7%). Residence in refugee campuses and elderly 12-14 yrs showed a significant interaction in the analysis of the anemia [Adj. OR for the interaction term 3.88 (2.19-6.85), p=0.000] (Figure 1). The frequency of anemia was highest in elderly of 12-14 yrs old living in refugee campuses (campus residence and age 12-14 yrs 22/63, 35%; campus residence and age 7-11 yrs 23/122, 19%; households residence and elderly 12-13 yrs 33/130, 25%; household residence and elderly 7-11 yrs 16/210, 8%). Defective fish consumption and lack of poultry intake showed a significant interaction in the analysis of anemia [Adj. OR for the interaction term 2.00 (2.13-3.57), p=0.018] (Figure 1). The frequency of anemia was highest when children did not consume neither fish nor poultry (fish intake deficiency and defective poultry consumption 35/118, 30%; defective fish consumption and poultry intake 38/221, 17%; fish consumption and defective poultry intake 5/38, 13%; fish consumption and poultry consumption 16/140, 11%). Defective book reading and lack of learning activities in free time showed a significant interaction in the analysis of anemia [Adj. OR for the interaction term 3.02 (1.35-6.76), p=0.007] (Figure 1). Defective book reading and lack of learning capabilities were most often seen in anemic SAC (Defective book reading and lack of learning capabactivities 53/204, 26%; defective learning but book reading 2/16, 12%; learning but defective reading 32/209, 15%; learning and book reading capabilities maintained 7/90, 8%).
Figure 1. Odds ratios with 95% Confidence intervals for interaction terms of socioeconomic, nutritive and lifestyle risk factors of anemia in school-age children and adolescents in Serbia. Vertical dashed line on 1 designates no difference in haemoglobin values between participants with anemia and cases with normal hemoglobin levels.
Discussion
The current paper presents results of the first crosssectional survey performed to evaluate prevalence and risk factors associated with anemia in SAC in Serbia. The overall prevalence of anemia among participants in Serbia was 18%. The frequency of anemia in our survey is remarkably consistent with rate of 16% (p=0.000) reported in developing country31, significantly lower than global anemia prevalence of 25.4%1, (p=0.000), significantly higher than rate estimated in developed country 3.2%32 (p=0.000) and significantly different than the population specific threshold of ≤4.99%1 (p=0.000). Hence, our findings of high rate of anemia in SAC could be explained as continuation of anemia existing in Serbian Pre SAC that is reported by WHO1. Such ascertains are also confirmed in other studies33–35. Our data also clearly confirm that school-aged 12-14 yrs old and participants of male gender had 3.55 and 3.22 times, respectively, greater risk of anemia than SAC 7-11 years old and female gender. Furthermore, the odds of anemia are 98% higher for SAC living in refugee campuses households. This was further substantiated by other investigators36,37 who demonstrated age and male gender as independent predictors of anemia. Rapid growth in that age increases the need for iron to such an extent38 that a positive balance is difficult to maintain39 and makes schoolaged 12-14 yrs old males and SAC living in refugee campuses40 particularly vulnerable to anemia. We clearly supported such hypothesis by the analysis of interactions in the multivariate model discovering prevalence of anemia of 44% for SAC of male gender 12-14 yrs old, 35% for 12-14 yrs aged subjects living in refugee campuses' households and 32% for males living in such campuses.
Three additional risk factors for anemia confirmed by multivariate analysis in our study as lunch skipping, lack of fish and poultry consumption deserve attention. We observed more than 3-fold risk of anemia in participants who skipped lunch compared to those on regular midday meal intake. We also discovered that SAC in our study with defective poultry and fish consumption had increased odds of anemia for 65% and 85%, respectively, compared to SAC with regular consumption. These finding are also supported by similar studies indicating lack of fish and meat (beef, pork) consumption to be independent predictors of anemia in SAC 41,42 from one and association of lunch skeeping with decreased Hb levels (anemia occurence), from another side43. We revealed particularly high prevalence of anemia in participants having both factors including inappropriate poultry and fish intake. It should be noted that meal skipping, defective poultry and fish intake among our participants was due to decreased household incomes. High bioavailability reach foods which are usually expensive were seldom consumed in Serbia as a consequence of worsening of socioeconomic status (SES). This is further supported by other surveys showing increasing prevalence of anemia in SAC from lower SES11,13 and decreasing anemia rates in surveyed participans living of better SES44,45.
SES associated with educational level, physical and psychological health, determines also lifestyle practices and physical activities of SAC. Lifestyle practices and physical activities, as absence of learning, defective book reading and disagreement with the opinion that sport contributes to protect health in our study independently predicted anemia in study sample. Furthermore, presence of anemia increased almost 3 times the risk of defective book reading and lack of learning capabilities in SAC. Taking in account that anemia is most prevalent in developing countries including Serbia, this finding can be explained by the fact that children living in low income families rarely practice healthy lifestyle activities because of their poor household environment and their high prevalence anemia rates.
In Cconclusion
The present study suggests that anemia SAC in Serbia could be reduced by: improvement of SES, introducing diagnostic, monitoring and therapeutic procedures and health education in schools and society. The findings of our study could support health authorities' implementation of national science-based programs for nutritional, educational preventive and control activities for anemia in SAC. However, further investigations are also required to elucidate the causes of anemia in the population group of SAC.
In conclusion, anemia remains an important public health problem in SAC in Serbia. The results of the current cross-sectional survey indicated that age of 12-14 yrs, male gender, refugee campuses residence, lunch skipping, defective fish consumption, irregular poultry intake, disagreement that sport contributes protecting health, absence of learning and defective book reading as lifestyle activities are significant predictors of anemia that need to be considered in intervention programmes aimed to reduce anemia in SAC.
Acknowledgments
The authors thank to Professor Pekka Puska, from the National Public Health Institute (KTL) Finland for his supervising support in realization of the Survey.
Footnotes
Study support
This study has been supported by grants-in-aids by the World Health Organization (2000); United Nations International Children’s Fund (2000) and European Community Humanitarian aid Office ECHO (2000).
References
- 1.Benoist B, McLean E, Cogswell M, Egli I, Wojdyla D. Worldwide prevalence of anemia 1993–2005. WHO Global Database on Anemia. Geneva: World Health Organization; 2008. [Google Scholar]
- 2.Abidoye RO, Akande PA. Nutritional status of public primary school children: a comparison between an upland and riverine area of Ojo LGA, Lagos State Nigeria. Nutr Health. 2000;14:225–240. doi: 10.1177/026010600001400404. [DOI] [PubMed] [Google Scholar]
- 3.Tatala SR, Kihamia CM, Kyungu LH, Svanberg U. Risk factors for anemia in schoolchildren in Tanga Region, Tanzania. Tanzan J Health Res. 2008;10:189–202. doi: 10.4314/thrb.v10i4.45074. [DOI] [PubMed] [Google Scholar]
- 4.Hashizume M, Shimoda T, Sasaki S, Kunii O, Caypil W, Dauletbaev D, et al. Anemia in relation to low bioavailability of dietary iron among school-aged children in the Aral Sea region, Kazakhstan. Int J Food Sci Nutr. 2004;55:37–43. doi: 10.1080/09637480310001642466. [DOI] [PubMed] [Google Scholar]
- 5.National Research Council . Recommended dietary allowances. 10th ed. Washington: National Academy Press; 1989. [Google Scholar]
- 6.Yip R, Dallman PR. Iron. In: Ziegler EE, Filer LJ Jr, editors. Present knowledge in nutrition. 7th ed. Washington: International Life Sciences Institute Press; 1996. [Google Scholar]
- 7.Hallberg L. Iron requirements: comments on methods and some crucial concepts in iron nutrition. Biol Trace Elem Res. 1992;35:25–45. doi: 10.1007/BF02786235. United Nations Children's Fund/United Nations University/World Health Organization. Iron deficiency anaemia. Assessment, prevention and control. A guide for programme managers (WHO/NHD/01.3). Geneva: World Health Organization; 2001. [DOI] [PubMed] [Google Scholar]
- 8.Food and Agriculture Organization/World Health Organization . Food and Agriculture Organization/ World Health Organization. 1992g. Nutrition and development: a global assessment. Rome: International Conference on Nutrition; 1992. [Google Scholar]
- 9.United Nations Children's Fund/ United Nations University/World Health Organization/Micronutrient Initiative . Preventing Iron Deficiency in Women and Children: Background and Consensus on Key Technical Issues and Resource for Advocacy, Planning and Implementing National Programmes. Boston: International Nutrition Foundation; 1999. [Google Scholar]
- 10.Looker AC, Dallman PR, Carroll MD, Gunter EW, Johnson CL. Prevalence of iron deficiency in the United States. JAMA. 1997;277:973–976. doi: 10.1001/jama.1997.03540360041028. [DOI] [PubMed] [Google Scholar]
- 11.Boutry M, Needlman R. Use of diet history in the screening of iron deficiency. Pediatrics. 1996;98:1138–1142. [PubMed] [Google Scholar]
- 12.Oken E, Rifas-Shiman SL, Kleinman KP, Scanlon KS, Rich-Edwards JW, Gillman MW. Trends in childhood anemia in a Massachusetts health maintenance organization, 1987-2001. MedGenMed. 2006;8:58. [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed F, Khan MR, Islam M, Kabir I, Fuchs GJ. Anemia and iron deficiency among adolescent schoolgirls in peri-urban Bangladesh. Eur J Clin Nutr. 2000;54:678–683. doi: 10.1038/sj.ejcn.1601073. [DOI] [PubMed] [Google Scholar]
- 14.Alaofè H, Zee J, Dossa R, O'Brien HT. Education and improved iron intakes for treatment of mild iron-deficiency anemia in adolescent girls in southern Benin. Food Nutr Bull. 2009;30:24–36. doi: 10.1177/156482650903000103. [DOI] [PubMed] [Google Scholar]
- 15.Perry GS, Byers T, Yip R, Margen S. Iron nutrition does not account for the hemoglobin differences between blacks and whites. J Nutr. 1992;122:1417–1424. doi: 10.1093/jn/122.7.1417. [DOI] [PubMed] [Google Scholar]
- 16.Kikafunda JK, Lukwago FB, Turyashemererwa F. Anemia and associated factors among under-fives and their mothers in Bushenyi district, western Uganda. Public Health Nutr. 2009;12:2302–2308. doi: 10.1017/S1368980009005333. [DOI] [PubMed] [Google Scholar]
- 17.Kent S, Dunn D. Anemia and the transition of nomadic huntergatherers to a sedentary life-style: follow-up study of a Kalahari community. Am J Phys Anthropol. 1996;99:455–472. doi: 10.1002/(SICI)1096-8644(199603)99:3<455::AID-AJPA7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 18.Shell-Duncan B, McDade T. Cultural and environmental barriers to adequate iron intake among northern Kenyan schoolchildren. Food Nutr Bull. 2005;26:39–48. doi: 10.1177/156482650502600105. [DOI] [PubMed] [Google Scholar]
- 19.Creed Kanashiro HM, Uribe TG, Bartolini RM, Fukumoto MN, Lopez TT, Zavaleta NM, et al. Improving dietary intake to prevent anemia in adolescent girls through community kitchens in a periurban population of Lima, Peru. J Nutr. 2000;130:459S–461S. doi: 10.1093/jn/130.2.459S. [DOI] [PubMed] [Google Scholar]
- 20.Bhargava A, Bouis HE, Scrimshaw NS. Dietary intakes and socioeconomic factors are associated with the hemoglobin concentration of bangladeshi women. J Nutr. 2001;131:758–764. doi: 10.1093/jn/131.3.758. [DOI] [PubMed] [Google Scholar]
- 21.Khatib IM, Elmadf I. Poor nutritional health of Bedouin preschool children in Jordan: the irony of urbanization. Ann Nutr Metab. 2009;54:301–309. doi: 10.1159/000239847. [DOI] [PubMed] [Google Scholar]
- 22.Seal AJ, Creeke PI, Mirghani Z, Abdalla F, McBurney RP, Pratt LS, et al. Iron and vitamin A deficiency in long-term African refugees. J Nutr. 2005;135:808–813. doi: 10.1093/jn/135.4.808. [DOI] [PubMed] [Google Scholar]
- 23.Kohli-Kumar M. Screening for anemia in children: AAP recommendations: a critique. Pediatrics. 2001;108:1–2. doi: 10.1542/peds.108.3.e56. [DOI] [PubMed] [Google Scholar]
- 24.Summary of Policy Recommendations for Clinical Preventive Services [homepage on the Internet] Leawood: American Academy of Family Physicians; 2010. [updated 2010 January; cited 2010 Jun 1]. Available from: http://www.aafp.org/online/en/home/clinical/exam.html. [Google Scholar]
- 25.Center for Disease Control and Prevention Reports Recommendations to prevent and control iron deficiency in the United States. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47:1–29. [PubMed] [Google Scholar]
- 26.American Academy of Pediatrics . Iron deficiency. In: Kleinman R, editor. Pediatric Nutrition Handbook. 4th ed. Elk Grove Village: American Academy of Pediatrics; 1998. [Google Scholar]
- 27.United States Preventive Services Task Force . Guide to clinical preventive services. 2d ed. Baltimore: Williams and Wilkins; 1996. [Google Scholar]
- 28.Dallman PR, Rudolph AM, editors. Pediatrics. 16th ed. New York: Appelton Century Crofts; 1977. [Google Scholar]
- 29.Mills A, Meadows N. Screening for anemia: evaluation of a haemoglobinometer. Arch Dis Child. 1989;64:1468–1471. doi: 10.1136/adc.64.10.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen PP, Short TG, Leung DH, Oh TE. A clinical evaluation of the Hemocue haemoglobinometer using capillary, venous and arterial samples. Anaesth Intensive Care. 1992;20:497–503. doi: 10.1177/0310057X9202000419. [DOI] [PubMed] [Google Scholar]
- 31.Ferreira MU, da Silva-Nunes M, Bertolino CN, Malafronte RS, Muniz PT, Cardoso MA. Anemia and iron deficiency in school children, adolescents, and adults: a community-based study in rural Amazonia. Am J Public Health. 2007;97:237–239. doi: 10.2105/AJPH.2005.078121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aranceta Bartrina J, Pιrez Rodrigo C, Marzana Sanz I, Egileor Gurtubai I, Gondra Rezola J, Gonzαlez de Galdeano L, et al. Prevalence of iron-deficiency anemia in the Basque Country. Aten Primaria. 1998;22:353–361. [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention Nutritional assessment of adolescent refugees--Nepal, 1999. MMWR Morb Mortal Wkly Rep. 2000;49:864–867. [PubMed] [Google Scholar]
- 34.Anonymous The health and nutritional status of schoolchildren in Africa: evidence from school-based health programmes in Ghana and Tanzania. The partnership for child development. Trans R Soc Trop Med Hyg. 1998;92:254–261. doi: 10.1016/s0035-9203(98)90999-3. [DOI] [PubMed] [Google Scholar]
- 35.Lozoff B, Jimenez E, Smith JB. Double burden of iron deficiency in infancy and low socioeconomic status: a longitudinal analysis of cognitive test scores to age 19 years. Arch Pediatr Adolesc Med. 2006;160:108–113. doi: 10.1001/archpedi.160.11.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin JD, Lin PY, Lin LP, Hsu SW, Loh CH, Yen CF, et al. Prevalence and associated risk factors of anemia in children and adolescents with intellectual disabilities. Res Dev Disabil. 2010;31:25–32. doi: 10.1016/j.ridd.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 37.Stoltzfus RJ, Chwaya HM, Tielsch JM, Schulze KJ, Albonico M, Savioli L. Epidemiology of iron deficiency anemia in Zanzibari schoolchildren: the importance of hookworms. Am J Clin Nutr. 1997;65:153–159. doi: 10.1093/ajcn/65.1.153. [DOI] [PubMed] [Google Scholar]
- 38.Brabin L, Brabin BJ. The cost of successful adolescent growth and development in girls in relation to iron and vitamin A status. Am J Clin Nutr. 1992;55:955–958. doi: 10.1093/ajcn/55.5.955. [DOI] [PubMed] [Google Scholar]
- 39.Yip R. Iron deficiency: contemporary scientific issues and international programmatic approaches. J Nutr. 1994;124:1479S–1490S. doi: 10.1093/jn/124.suppl_8.1479S. [DOI] [PubMed] [Google Scholar]
- 40.Woodruff BA, Blanck HM, Slutsker L, Cookson ST, Larson MK, Duffield A, et al. Anemia, iron status and vitamin A deficiency among adolescent refugees in Kenya and Nepal. Public Health Nutr. 2006;9:26–34. doi: 10.1079/phn2005825. [DOI] [PubMed] [Google Scholar]
- 41.Alaofe H, Zee J, Dossa R, Turqeon O'Brien H. Iron status of adolescent girls from two boarding schools in southern Benin. Public Health Nutr. 2008;11:737–746. doi: 10.1017/S1368980008001833. [DOI] [PubMed] [Google Scholar]
- 42.Choudhary A, Moses PD, Mony P, Mathai M. Prevalence of anemia among adolescent girls in the urban slums of Vellore, South India. Trop Doct. 2006;36:167–169. doi: 10.1258/004947506777978253. [DOI] [PubMed] [Google Scholar]
- 43.Kurniawan YA, Muslimatun S, Achadi EL, Sastroamidjojo S. Anemia and iron deficiency anemia among young adolescent girls from the peri urban coastal area of Indonesia. Asia Pac J Clin Nutr. 2006;15:350–356. [PubMed] [Google Scholar]
- 44.Soekarjo DD, de Pee S, Bloem MW, Tjiong R, Yip R, Schreurs WH, et al. Socio-economic status and puberty are the main factors determining anemia in adolescent girls and boys in East Java, Indonesia. Eur J Clin Nutr. 2001;55:932–939. doi: 10.1038/sj.ejcn.1601247. [DOI] [PubMed] [Google Scholar]
- 45.Alderman H, Linnemayr S. Anemia in low-income countries is unlikely to be addressed by economic development without additional programs. Food Nutr Bull. 2009;30:265–269. doi: 10.1177/156482650903000308. [DOI] [PubMed] [Google Scholar]