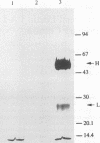
Abstract
Pseudomonas aeruginosa is the most important bacterial pathogen associated with chronic airway infection, especially in cystic fibrosis. We addressed the question of whether the galactophilic internal lectin of P. aeruginosa (PA-I) could represent a virulence factor for the respiratory epithelium. PA-I lectin was localized in all the bacteria of P. aeruginosa ATCC 33347 as determined by immunofluorescence staining. We investigated the dose-dependent effect of P. aeruginosa PA-I lectin on the growth, the ciliary beating frequency, and the morphology of human respiratory cells in primary culture of nasal polyps collected from non-cystic fibrosis patients. PA-I lectin significantly (P < 0.01) inhibited the growth of respiratory cells at a concentration of > or = 10 micrograms/ml. The percentage of active ciliated cell surface of the cultures decreased significantly (P < 0.05) at a PA-I lectin concentration of 50 micrograms/ml. Exposed to a low concentration of PA-I lectin (10 micrograms/ml), respiratory epithelial cells showed intracytoplasmic vacuoles when examined by light and transmission electron microscopy. At a higher concentration of PA-I lectin (100 micrograms/ml), major cell damage and severe epithelial shedding occurred. These results demonstrate that the P. aeruginosa internal PA-I lectin has a dose-dependent cytotoxic effect on respiratory epithelial cells in vitro. The P. aeruginosa PA-I lectin may represent a virulence factor by contributing to the respiratory epithelial damage during P. aeruginosa respiratory infections.
Full text
PDF
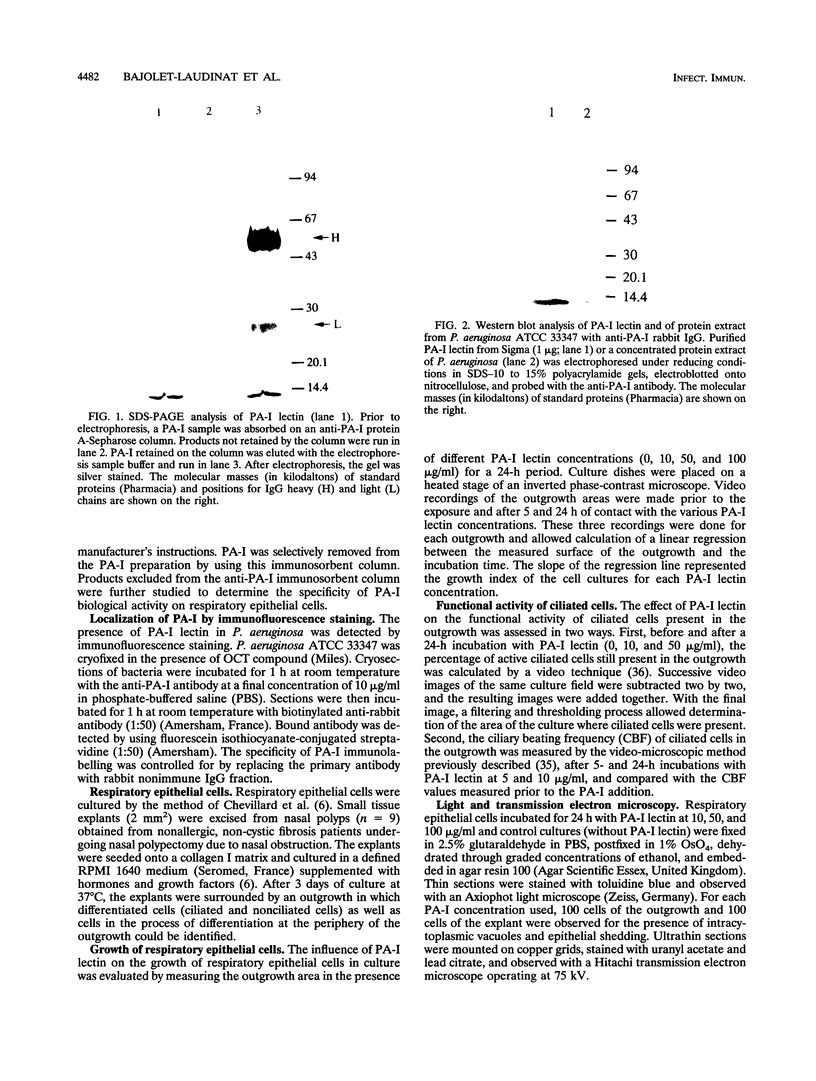
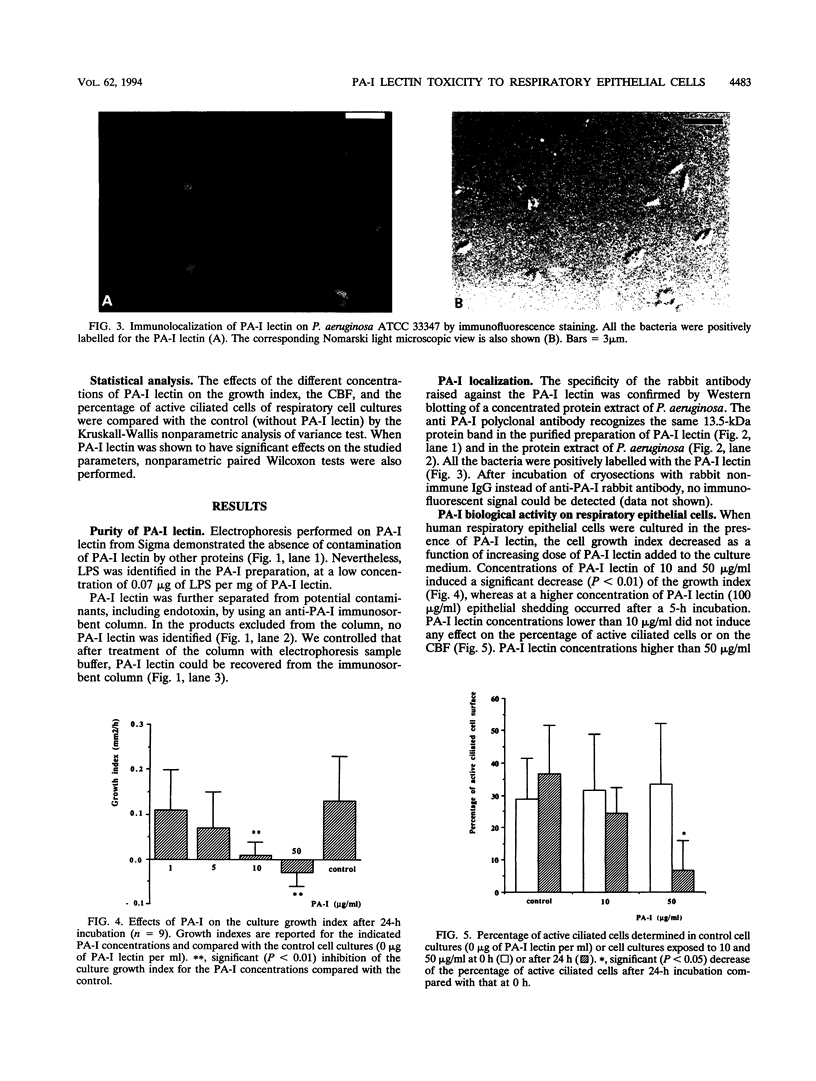




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler K. B., Winn W. C., Jr, Alberghini T. V., Craighead J. E. Stimulatory effect of Pseudomonas aeruginosa on mucin secretion by the respiratory epithelium. JAMA. 1983 Mar 25;249(12):1615–1617. [PubMed] [Google Scholar]
- Avichezer D., Gilboa-Garber N. Antitumoral effects of Pseudomonas aeruginosa lectins on Lewis lung carcinoma cells cultured in vitro without and with murine splenocytes. Toxicon. 1991;29(11):1305–1313. doi: 10.1016/0041-0101(91)90117-a. [DOI] [PubMed] [Google Scholar]
- Avichezer D., Katcoff D. J., Garber N. C., Gilboa-Garber N. Analysis of the amino acid sequence of the Pseudomonas aeruginosa galactophilic PA-I lectin. J Biol Chem. 1992 Nov 15;267(32):23023–23027. [PubMed] [Google Scholar]
- Beuth J., Ko H. L., Roszkowski W., Roszkowski K., Ohshima Y. Lectins: mediators of adhesion for bacteria in infectious diseases and for tumor cells in metastasis. Zentralbl Bakteriol. 1990 Dec;274(3):350–358. doi: 10.1016/s0934-8840(11)80692-4. [DOI] [PubMed] [Google Scholar]
- Cash H. A., Straus D. C., Bass J. A. Pseudomonas aeruginosa exoproducts as pulmonary virulence factors. Can J Microbiol. 1983 Apr;29(4):448–456. doi: 10.1139/m83-072. [DOI] [PubMed] [Google Scholar]
- Chevillard M., Hinnrasky J., Zahm J. M., Plotkowski M. C., Puchelle E. Proliferation, differentiation and ciliary beating of human respiratory ciliated cells in primary culture. Cell Tissue Res. 1991 Apr;264(1):49–55. doi: 10.1007/BF00305721. [DOI] [PubMed] [Google Scholar]
- Fick R. B., Jr Pathogenesis of the pseudomonas lung lesion in cystic fibrosis. Chest. 1989 Jul;96(1):158–164. doi: 10.1378/chest.96.1.158. [DOI] [PubMed] [Google Scholar]
- Garber N., Guempel U., Belz A., Gilboa-Garber N., Doyle R. J. On the specificity of the D-galactose-binding lectin (PA-I) of Pseudomonas aeruginosa and its strong binding to hydrophobic derivatives of D-galactose and thiogalactose. Biochim Biophys Acta. 1992 Jun 12;1116(3):331–333. doi: 10.1016/0304-4165(92)90048-y. [DOI] [PubMed] [Google Scholar]
- Gilboa-Garber N., Blonder E. Augmented osmotic hemolysis of human erythrocytes exposed to the galactosephilic lectin of Pseudomonas aeruginosa. Isr J Med Sci. 1979 Jun;15(6):537–539. [PubMed] [Google Scholar]
- Gilboa-Garber N. Pseudomonas aeruginosa lectins as a model for lectin production, properties, applications and functions. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Nov;270(1-2):3–15. doi: 10.1016/s0176-6724(88)80135-4. [DOI] [PubMed] [Google Scholar]
- Gilboa-Garber N. Pseudomonas aeruginosa lectins. Methods Enzymol. 1982;83:378–385. doi: 10.1016/0076-6879(82)83034-6. [DOI] [PubMed] [Google Scholar]
- Gilligan P. H. Microbiology of airway disease in patients with cystic fibrosis. Clin Microbiol Rev. 1991 Jan;4(1):35–51. doi: 10.1128/cmr.4.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girod de Bentzmann S., Bajolet-Laudinat O., Dupuit F., Pierrot D., Fuchey C., Plotkowski M. C., Puchelle E. Protection of human respiratory epithelium from Pseudomonas aeruginosa adherence by phosphatidylglycerol liposomes. Infect Immun. 1994 Feb;62(2):704–708. doi: 10.1128/iai.62.2.704-708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick J., Garber N. The intracellular localization of Pseudomonas aeruginosa lectins. J Gen Microbiol. 1983 Oct;129(10):3085–3090. doi: 10.1099/00221287-129-10-3085. [DOI] [PubMed] [Google Scholar]
- Gray L., Kreger A. Microscopic characterization of rabbit lung damage produced by Pseudomonas aeruginosa proteases. Infect Immun. 1979 Jan;23(1):150–159. doi: 10.1128/iai.23.1.150-159.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingley S. T., Hastie A. T., Kueppers F., Higgins M. L. Disruption of respiratory cilia by proteases including those of Pseudomonas aeruginosa. Infect Immun. 1986 Nov;54(2):379–385. doi: 10.1128/iai.54.2.379-385.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingley S. T., Hastie A. T., Kueppers F., Higgins M. L., Weinbaum G., Shryock T. Effect of ciliostatic factors from Pseudomonas aeruginosa on rabbit respiratory cilia. Infect Immun. 1986 Jan;51(1):254–262. doi: 10.1128/iai.51.1.254-262.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro N. C., Barker A., Rutman A., Taylor G., Watson D., McDonald-Gibson W. J., Towart R., Taylor W. A., Wilson R., Cole P. J. Effect of pyocyanin and 1-hydroxyphenazine on in vivo tracheal mucus velocity. J Appl Physiol (1985) 1989 Jul;67(1):316–323. doi: 10.1152/jappl.1989.67.1.316. [DOI] [PubMed] [Google Scholar]
- Pier G. B. Pulmonary disease associated with Pseudomonas aeruginosa in cystic fibrosis: current status of the host-bacterium interaction. J Infect Dis. 1985 Apr;151(4):575–580. doi: 10.1093/infdis/151.4.575. [DOI] [PubMed] [Google Scholar]
- Plotkowski M. C., Beck G., Tournier J. M., Bernardo-Filho M., Marques E. A., Puchelle E. Adherence of Pseudomonas aeruginosa to respiratory epithelium and the effect of leucocyte elastase. J Med Microbiol. 1989 Dec;30(4):285–293. doi: 10.1099/00222615-30-4-285. [DOI] [PubMed] [Google Scholar]
- Ramphal R., Vishwanath S. Why is Pseudomonas the colonizer and why does it persist? Infection. 1987 Jul-Aug;15(4):281–287. doi: 10.1007/BF01644139. [DOI] [PubMed] [Google Scholar]
- Read R. C., Roberts P., Munro N., Rutman A., Hastie A., Shryock T., Hall R., McDonald-Gibson W., Lund V., Taylor G. Effect of Pseudomonas aeruginosa rhamnolipids on mucociliary transport and ciliary beating. J Appl Physiol (1985) 1992 Jun;72(6):2271–2277. doi: 10.1152/jappl.1992.72.6.2271. [DOI] [PubMed] [Google Scholar]
- Sharon N. Bacterial lectins, cell-cell recognition and infectious disease. FEBS Lett. 1987 Jun 15;217(2):145–157. doi: 10.1016/0014-5793(87)80654-3. [DOI] [PubMed] [Google Scholar]
- Somerville M., Richardson P. S., Rutman A., Wilson R., Cole P. J. Stimulation of secretion into human and feline airways by Pseudomonas aeruginosa proteases. J Appl Physiol (1985) 1991 May;70(5):2259–2267. doi: 10.1152/jappl.1991.70.5.2259. [DOI] [PubMed] [Google Scholar]
- Somerville M., Taylor G. W., Watson D., Rendell N. B., Rutman A., Todd H., Davies J. R., Wilson R., Cole P., Richardson P. S. Release of mucus glycoconjugates by Pseudomonas aeruginosa rhamnolipid into feline trachea in vivo and human bronchus in vitro. Am J Respir Cell Mol Biol. 1992 Jan;6(1):116–122. doi: 10.1165/ajrcmb/6.1.116. [DOI] [PubMed] [Google Scholar]
- Vasil M. L., Graham L. M., Ostroff R. M., Shortridge V. D., Vasil A. I. Phospholipase C: molecular biology and contribution to the pathogenesis of Pseudomonas aeruginosa. Antibiot Chemother (1971) 1991;44:34–47. doi: 10.1159/000420295. [DOI] [PubMed] [Google Scholar]
- Wilson R., Pitt T., Taylor G., Watson D., MacDermot J., Sykes D., Roberts D., Cole P. Pyocyanin and 1-hydroxyphenazine produced by Pseudomonas aeruginosa inhibit the beating of human respiratory cilia in vitro. J Clin Invest. 1987 Jan;79(1):221–229. doi: 10.1172/JCI112787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R., Roberts D., Cole P. Effect of bacterial products on human ciliary function in vitro. Thorax. 1985 Feb;40(2):125–131. doi: 10.1136/thx.40.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R., Sykes D. A., Watson D., Rutman A., Taylor G. W., Cole P. J. Measurement of Pseudomonas aeruginosa phenazine pigments in sputum and assessment of their contribution to sputum sol toxicity for respiratory epithelium. Infect Immun. 1988 Sep;56(9):2515–2517. doi: 10.1128/iai.56.9.2515-2517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Straus D. C., Johanson W. G., Jr, Berry V. K., Bass J. A. Role of pili in adherence of Pseudomonas aeruginosa to mammalian buccal epithelial cells. Infect Immun. 1980 Sep;29(3):1146–1151. doi: 10.1128/iai.29.3.1146-1151.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm J. M., Chevillard M., Puchelle E. Wound repair of human surface respiratory epithelium. Am J Respir Cell Mol Biol. 1991 Sep;5(3):242–248. doi: 10.1165/ajrcmb/5.3.242. [DOI] [PubMed] [Google Scholar]
- Zahm J. M., Lamiot E., Pierrot D., Chevillard M., Hinnrasky J., Puchelle E. Quantitation of in vitro ciliated cell growth through image analysis. In Vitro Cell Dev Biol. 1990 Nov;26(11):1063–1067. doi: 10.1007/BF02624441. [DOI] [PubMed] [Google Scholar]