Abstract
Background In utero exposure to tobacco smoking has been suggested to cause persistent alterations in cognitive functioning. We examined if mothers’ smoking during pregnancy (SDP) is associated with long-term impairment in offspring stress coping and the causal mechanism behind a possible link.
Methods We used a large cohort (n = 187 106) of young males in Sweden (mean age = 18.2 years), who underwent a semi-structured psychological assessment in 1997–2006, including an evaluation of stress coping ability, as part of the compulsory military conscript examination. We compared differentially exposed siblings within nuclear families and cousins in extended families and used multilevel structural equation models to disentangle genetic from environmental contributions to the association between SDP and stress coping.
Results SDP and offspring stress coping was moderately strongly associated when comparing unrelated individuals [regression coefficient (b) = −0.38 on a nine-point scale; 95% confidence interval (CI) −0.40 to −0.36, P < 0.0001]. In contrast, it disappeared when siblings were compared (b = 0.11; 95% CI −0.01 to 0.23, P = 0.071). This familial confounding was entirely due to genetic influences.
Conclusions SDP is an established risk factor for pregnancy- and birth-related complications. However, we found no long-term effect of SDP on offspring stress coping. Rather, the observed association was due to familial confounding of genetic origin; women prone to SDP also transmit genes to their children that are associated with poorer coping with stress.
Keywords: Smoking during pregnancy, adolescent stress coping, children-of-sibling model, intergenerational association
Introduction
Maternal smoking during pregnancy (SDP) has been linked to several negative perinatal outcomes, such as low offspring birth weight,1–5 preterm birth,1,4–6 spontaneous abortion4,5,7 and sudden infant death syndrome.4,5,7,8 In addition, long-lasting behaviour problems have been suggested; for example, compared with unrelated controls, offspring who experienced SDP have increased risk of externalizing problems9 including attention-deficit/hyperactivity disorder,7,9 aggression,10 criminality,7,11 poorer general cognitive functioning12 and poorer academic and intellectual performance.13–15
Birth-related SDP outcomes (e.g. lower birth weight, preterm birth) appear causal,5 whereas emerging evidence suggests that the link to most studied long-term behaviours is confounded by other risks or unmeasured familial effects, such as shared environmental or genetic risks.9,11–16
One aspect of individual development that recently received substantial interest is the ability to cope with stress. For instance, increased stress vulnerability has been observed as a consequence of prenatal nicotine exposure in rats.17 Animal studies also suggest that prenatal nicotine exposure increases locomotor activity and causes learning and memory problems.7 Specifically, the fetal programming hypothesis18,19 includes suggestions that the major regulatory systems involved in stress responses, the autonomic nervous system and the hypothalamic–pituitary–adrenal axis, could be permanently altered early in life; both pre- and post-natally.18,19 These alterations could, for example, be caused by restricted access to food or specific nutrients, maternal adversity or exposure to synthetic glucocorticoids (e.g. cortisol).19 Birth weight has often been used as a proxy for measuring adverse fetal environment,18,19 and prior Swedish studies suggested that offspring size at birth is related to the measure of stress susceptibility used in the current study.20–22
Other possible teratogenic SDP-related mechanisms include disturbed placenta function and impaired transport of nutrients and oxygen to the fetus,7 and nicotine-caused intrauterine hypoxia or birth asphyxia7 leading to fetal death or long-term neurological deficits, or cellular alterations to the central and peripheral nervous systems.7,23 The decrease in ‘fetal breathing’ (fetal thoracic movements), reported to occur after nicotine exposure, causes lung maturation to slow down and reduces the blood flow to the fetus.7 Additionally, activation of neurotransmitter receptors in the fetal brain could lead to epigenetic alterations involving permanent change in cell functioning that might not be detected until much later in the more developed, adolescent, brain.7,24
However, another important mechanism through which SDP could effect offspring behaviour is the passing of genetic vulnerability from parent to offspring; a passive gene–environment correlation.25 In effect, repeated results from studies of behavioural problems (e.g. externalizing behaviour,9 criminality11 and poor academic achievement14,15) in offspring exposed to SDP were later found to be entirely confounded by familial risks. That is, a selective mechanism for SDP exposure exists so that mothers who smoke during pregnancy share also other risk factors with their children; hence, these other risk factors cause the observed adverse outcomes rather than SDP per se. Variables such as maternal age, education or socio-economic status might be the source of this selection,7 but also unmeasured familial environment similarity and genetic risks.
We aimed to investigate whether the association between intrauterine exposure to SDP and stress coping in Swedish late adolescent men persisted after controlling for measured and unmeasured confounding caused by intrafamilial similarity.
Methods
Study population
We linked several nationwide longitudinal registries, maintained by government agencies in Sweden, using the unique personal identification number given to all Swedish citizens. We used data from the Multi-Generation26 and Education Registers,27 the 1990 Swedish Census,28 and the Conscript,29 Medical Birth,30,31 Total Population and National Crime Registers.32 Eligible for the study were all male youth in Sweden who underwent an evaluation regarding suitability for duty by a clinical psychologist at compulsory conscription for military services during 1997–2006 and born 1982–88 (SDP registration at antenatal care started in 1982). For this study, we used data collected regarding stress coping (n = 187 106). Military conscription was mandatory for Swedish men until 2008 and enforced by law. The majority of conscripts were 18 years old [79.3%; mean age = 18.2 years, standard deviation (SD) = 0.4, median = 18.2, range 17.1–24.3 years]. Individuals were linked to their siblings and cousins via parents and grandparents using the Multi-Generation Register, thus identifying extended and nuclear families. This register links all children born 1932 or later in Sweden to both their parents. Nuclear families were indexed by the mothers (164 563 mothers with at least one child), whereas extended families were indexed by the maternal or paternal grandmother. There were 150 268 extended families with at least one individual in the offspring generation. Offspring with both maternal and paternal cousins could be included in two extended families.
Exposure
Starting in 1982, all pregnant women in contact with public tax-funded antenatal care in Sweden are asked by their personal midwife about SDP; this information is included in the Medical Birth Register. The coverage is excellent; >98% of all births are recorded in the register.30 SDP data were available for 162 371 of the 187 106 (86.8%) pregnancies in the study. Of these, 44 550 women reported SDP (27.4%), which is comparable with earlier findings.5,9,11,12 The validity of self-reported SDP is high in general33 and previous studies suggest good validity also in the current sample.2,34
Outcome
A clinical psychologist rated individual psychological functioning (PF) at conscription, purportedly reflecting stress coping during wartime,20,35 based on a standardized, 20–25-min semi-structured interview. PF was rated 1–9 on a nine-point Likert-type scale; higher values indicate better coping. The distribution was stipulated to be normal with mean = 5 and SD = 2 (χ2 goodness-of-fit test with nine categories; P = 0.23, indicating no reason to reject the normality assumption). The individual PF score was used as a proxy for general ability to cope with stress.
Covariates
We adjusted our analyses using offspring, nuclear family and extended family confounders and mediators.
Offspring confounders
Maternal age was divided into five categories: <20, 20–24, 25–29, 30–34 and >34 years. Birth year was used alone and together with Conscript Register data to stratify age at conscription into categories; ≤17.50, 17.51–18.50 and >18.50 years. A birth order variable for male nuclear family offspring was also constructed.
Offspring mediators
We considered two mediators, both obtained from the Medical Birth Register: gestational time divided into categories: <32, 32–36, 37–41 and >41 weeks; and birth weight.
Parental confounders
Parental occupation, divided into seven categories,36 income and cohabitation status were all based on the 1990 Census. The Register of Education for 2004 provided highest parental educational level, classified into seven categories.27 Parental criminal convictions for 1973–2004 were collected from the National Crime Register. The Total Population Register supplied mother’s country of birth divided into 12 categories according to geographic and demographic similarities. Finally, we included a variable indicating if a half-sibship existed for offspring within the nuclear family.
Extended family confounders
We also included a variable on whether an individual had maternal or paternal half-cousins.
Statistical methods
To analyse the effect of SDP on PF, we used linear regression treating PF as a normally distributed variable. Results are presented both crude (unadjusted) and adjusted for possible confounders. To handle possible period effects, we adjusted the crude unrelated and cousin models for birth year and the crude sibling model for birth order.
Since birth weight and gestational age could be mediators of the association between SDP and PF, additional analyses were run to investigated whether these covariates mediated the association between SDP and PF as proposed by the fetal programming hypothesis.
Since we aimed at isolating a possible direct effect of SDP on PF by eliminating possible familial confounding, we ran additional models to test if familial effects distorted the association. With these, we compared PF in siblings and cousins differentially exposed to SDP to explore if the association remained when looking at within-family effects (i.e. if differentially exposed siblings/cousins also differed in PF). Hence, we used the extended family and nuclear family as clusters and sub-clusters to capture similarities within families. These analyses were performed with hierarchical linear models (HLM)37,38 using SAS Proc Mixed.39 Thus, unmeasured variables common to individuals in the nuclear or extended family (i.e. shared genes and environments) were accounted for.9 We call this approach the Children-of-Siblings model since it is similar to the statistical methods used when examining variables for children of twins in conjunction with variables for their parents, or the Children-of-Twins model.9,37,40,41 SDP and continuous covariates were centred around the cluster means (for both nuclear and extended family) which yielded covariates equivalent to fixed effects.15 Furthermore, this procedure reduced possible bias due to correlation between covariates and residual errors.42 We utilized an informed backwards elimination process when deciding which covariates to use; thus, these may differ across models.
We performed sibling–sibling and cousin–cousin comparisons on two different data subsets. Siblings were compared using a subset consisting of two siblings within a nuclear family. Nuclear families were solely indexed by mothers, since 91% of children in Sweden stays with their mother when parents divorce or separate,43 and our explicit aim was to capture possible familial effects. There were 26 118 individual siblings within 13 059 nuclear families. Cousin comparisons were made on a subset consisting of two cousins within an extended family. There were 52 888 individuals from 47 684 nuclear families within 26 421 extended families included in the subset (individuals eligible for comparisons within two extended families might be included twice in the analysis). To examine the difference between how SDP influence PF in full- and half-sibling/cousins, we conducted the analyses separately. The concordance of SDP in sibling and cousin pairs are presented in Table 1.
Table 1.
Concordance regarding exposure to maternal smoking during pregnancy in sibling and cousin pairs among all male children born 1982–88 in Sweden and assessed for PF at age 18 years as part of mandatory military conscript evaluation
| Relation | SDP = 0 | SDP = 1 | Total |
|---|---|---|---|
| Full siblings | 25 452 | ||
| Concordant | 18 702 | 4450 | 23 152 |
| Discordant | 1150 | 1150 | 2300 |
| Half siblings | 666 | ||
| Concordant | 256 | 292 | 548 |
| Discordant | 59 | 59 | 118 |
| Full cousins | 50 038 | ||
| Concordant | 27 480 | 5106 | 32 586 |
| Discordant | 8726 | 8726 | 17 452 |
| Half cousins | 2850 | ||
| Concordant | 978 | 492 | 1470 |
| Discordant | 690 | 690 | 1380 |
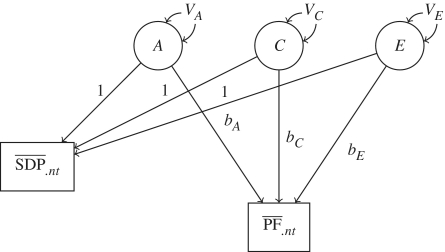
Finally, we aimed to disentangle the source of the familial confounding of the association between maternal SDP and her son’s PF by partitioning the variance of the intergenerational association in a two-level hierarchical structural equation model (SEM) using the statistical software program Mplus.44,45 Analyses were first performed with PF, and then on the residuals of PF retrieved from a linear regression model using the covariates. Because the results of these two analyses were very similar, we present only the analyses of the former. Results from the latter analyses are presented in the Supplementary Figure S1 (Supplementary data are available at IJE online). Wherever possible, we picked one pair of sisters and their children for each extended family (24 468 children from 11 485 sister pairs). The two SEM levels refer to within-mothers, comparing the association between mothers’ SDP and offspring’s PF within each of the nuclear families, and between-mothers, comparing the average SDP association with the average SDP between nuclear families. The variance was partitioned into three parts A, C and E, corresponding to genetics, shared environment (makes siblings similar) and non-shared environment (makes siblings different)9 (Figure 1). The partitioning of the variance comes from taking into account the genetic relatedness between the mothers; full siblings share 50% of their co-segregating genes, while half-siblings share 25%. This notion was incorporated in the SEM as constraints on the genetic variance parameter modelled (VA in Figure 1). Another constraint was that the modelled shared environment variance parameter VC was equal within full sibling pairs and maternal half-siblings, whereas this parameter was set to 0 for paternal half-siblings (again, because 91% of the children remain with their mother when parents separate).43 This way of modelling allows us to draw conclusions about which of the intergenerational paths (bA, bC and bE) that explain the association between SDP and PF. The method, as applied in Children-of-Twins model, has been described elsewhere.9,41
Figure 1.
Variance partitioning for A, genetics; C, the shared environment and E, non-shared environment. The graph represents the model for one of two sibling mothers. SDP: mean smoking during pregnancy exposure; PF: mean psychological functioning capacity (stress coping); A: latent variable representing the genes; C: latent variable representing the shared environment; E: latent variable representing the non-shared environment; VA: variance of latent variable A; VC: variance of latent variable C; VE: variance of latent variable E; bA: regression coefficient for PF regressed on A; bC: regression coefficient for PF regressed on C; bE: regression coefficient for PF regressed on E
Results
Mean PF scores are presented in Table 2 for SDP and offspring covariates and in Table 3 for parental covariates.
Table 2.
Offspring characteristics for all 187 106 male children born 1982–88 in Sweden and assessed for PF at age 18 years as part of mandatory military conscript evaluation
| Characteristic | n (%) | PF, mean (SD) |
|---|---|---|
| Smoking during pregnancy | ||
| Yes | 44 550 (23.8) | 4.6 (1.8) |
| No | 117 822 (63.0) | 5.0 (1.8) |
| Missing | 24 734 (13.2) | 4.9 (1.8) |
| Gestational time, weeks | ||
| 28–31 | 654 (0.3) | 4.5 (1.7) |
| 32–36 | 8966 (4.8) | 4.8 (1.8) |
| 37–41 | 162 873 (87.4) | 4.9 (1.8) |
| >41 | 13 812 (7.4) | 4.9 (1.8) |
| Missing | 801 (0.4) | 4.7 (1.7) |
| Age at conscription, years | ||
| ≤17.50 | 2245 (1.2) | 5.0 (1.7) |
| 17.51–18.50 | 158 625 (84.8) | 4.9 (1.8) |
| >18.50 | 26 236 (14.0) | 4.6 (1.9) |
| Year of birth | ||
| 1982 | 29 971 (16.0) | 4.8 (1.8) |
| 1983 | 28 231 (15.1) | 4.9 (1.8) |
| 1984 | 25 915 (13.9) | 5.1 (1.7) |
| 1985 | 30 243 (16.2) | 4.8 (1.9) |
| 1986 | 27 017 (14.4) | 4.8 (1.8) |
| 1987 | 25 801 (13.8) | 4.7 (1.8) |
| 1988 | 19 928 (10.7) | 4.9 (1.7) |
| Birth order | ||
| 1 | 169702 (90.7) | 4.9 (1.8) |
| 2 | 16 812 (9.0) | 4.9 (1.8) |
| 3 | 578 (0.3) | 4.8 (1.8) |
| 4 | 10 (0.0) | 4.5 (1.5) |
| 5 | 1 (0.0) | 7 (−) |
| Missing | 3 (0.0) | 4.3 (2.1) |
| Birth weight, kg | ||
| <1.50 | 1641 (0.9) | 4.7 (1.7) |
| 1.50–1.99 | 1006 (0.5) | 4.6 (1.8) |
| 2.00–2.49 | 3503 (1.9) | 4.6 (1.8) |
| 2.50–2.99 | 16 233 (8.7) | 4.7 (1.8) |
| 3.00–3.49 | 55 210 (29.5) | 4.8 (1.8) |
| 3.50–3.99 | 68 784 (36.8) | 4.9 (1.8) |
| ≥4 | 40 729 (21.8) | 4.9 (1.8) |
| Mother’s age at delivery, years | ||
| <20 | 5491 (2.9) | 4.3 (1.8) |
| 20–24 | 43 338 (23.2) | 4.7 (1.8) |
| 25–29 | 69 977 (37.4) | 4.9 (1.8) |
| 30–34 | 47 551 (25.4) | 5.0 (1.8) |
| >34 | 20 749 (11.1) | 4.9 (1.8) |
| Total | 187 106 | 4.9 (1.8) |
Table 3.
Characteristics for parents of all 187 106 male children born 1982–88 in Sweden and assessed for PF at age 18 years as part of mandatory military conscript evaluation
| Characteristic | Maternal characteristics |
Paternal characteristics |
||
|---|---|---|---|---|
| n (%) | PF, mean (SD) | n (%) | PF, mean (SD) | |
| Parent's occupation | ||||
| Unskilled blue-collar worker | 48 941 (26.2) | 4.6 (1.7) | 34 349 (18.4) | 4.6 (1.8) |
| Skilled blue collar | 20 845 (11.1) | 4.8 (1.7) | 37 737 (20.2) | 4.7 (1.7) |
| Low-level white collar | 28 390 (15.2) | 5.0 (1.7) | 16 086 (8.6) | 5.0 (1.8) |
| Intermediate-level white collar | 36 426 (19.5) | 5.2 (1.8) | 32 521 (17.4) | 5.1 (1.8) |
| High-level white collar | 13 395 (7.2) | 5.3 (1.8) | 28 976 (15.5) | 5.2 (1.8) |
| Self employed | 5615 (3.0) | 5.0 (1.8) | 14 889 (8.0) | 4.9 (1.8) |
| No information/uncategorized | 10 576 (5.7) | 4.7 (1.8) | 9692 (5.2) | 4.7 (1.8) |
| Missinga | 22 918 (12.2) | 4.6 (1.8) | 12 856 (6.9) | 4.5 (1.8) |
| Parent’s income, Swedish kronor | ||||
| <100 000 | 91 964 (49.2) | 4.7 (1.8) | 25 538 (13.6) | 4.5 (1.8) |
| 100 000–199 900 | 89 125 (47.6) | 4.9 (1.8) | 96 755 (51.7) | 4.7 (1.8) |
| 200 000–299 900 | 5194 (2.8) | 5.4 (1.8) | 50 536 (27.0) | 5.1 (1.8) |
| 300 000–399 900 | 686 (0.37) | 5.4 (1.8) | 10 059 (5.4) | 5.4 (1.7) |
| ≥400 000 | 137 (0.07) | 5.6 (2.0) | 4218 (2.3) | 5.6 (1.8) |
| Parent’s highest education at childbirth | ||||
| <9 years | 4179 (2.2) | 4.3 (1.8) | 10 649 (5.7) | 4.5 (1.7) |
| 9 years | 17 955 (9.6) | 4.4 (1.8) | 26 099 (13.9) | 4.6 (1.7) |
| 1–2 years upper secondary education | 67 436 (36.0) | 4.7 (1.7) | 61 131 (32.7) | 4.7 (1.8) |
| 3 years upper secondary education | 23 762 (12.7) | 4.9 (1.8) | 23 859 (12.8) | 5.0 (1.8) |
| <3 years post-secondary education | 33 622 (18.0) | 5.1 (1.8) | 25 175 (13.5) | 5.2 (1.8) |
| >3 years post-secondary education | 36 056 (19.3) | 5.2 (1.8) | 29 013 (15.5) | 5.2 (1.8) |
| Postgraduate education | 992 (0.5) | 5.3 (1.9) | 2862 (1.5) | 5.2 (1.8) |
| Missing | 3104 (1.7) | 4.5 (1.9) | 8318 (4.4) | 4.5 (1.9) |
| Parent convicted of a criminal offenceb | ||||
| No | 167 303 (89.4) | 4.9 (1.8) | 117 597 (62.9) | 5.0 (1.8) |
| Yes | 19 803 (10.6) | 4.5 (1.8) | 69 509 (37.1) | 4.6 (1.8) |
| Mother’s country of birth | ||||
| Sweden | 170 017 (90.9) | 4.9 (1.8) | n/a | n/a |
| Scandinavia except Sweden | 7466 (4.0) | 4.6 (1.8) | n/a | n/a |
| 25 European Union member states except Scandinavia and former Eastern Europe | 1367 (0.7) | 4.7 (1.8) | n/a | n/a |
| Former Eastern Europe | 1745 (0.9) | 4.7 (1.9) | n/a | n/a |
| Europe except Scandinavia, 25 European Union member states and former Eastern Europe | 2439 (1.3) | 4.4 (1.7) | n/a | n/a |
| Former USSR | 113 (0.1) | 4.6 (1.8) | n/a | n/a |
| Africa | 507 (0.3) | 4.4 (1.6) | n/a | n/a |
| Canada or USA | 261 (0.1) | 4.7 (1.9) | n/a | n/a |
| Rest of North America | 64 (0.0) | 4.7 (1.8) | n/a | n/a |
| South America | 814 (0.4) | 4.4 (1.7) | n/a | n/a |
| Asia | 2165 (1.2) | 4.3 (1.7) | n/a | n/a |
| Oceania | 35 (0.0) | 4.5 (1.4) | n/a | n/a |
| Missing | 113 (0.1) | 4.7 (1.7) | n/a | n/a |
| Half-sibshipc | n/a | n/a | ||
| No | 186 157 (99.5) | 4.9 (1.8) | n/a | n/a |
| Yes | 949 (0.5) | 4.5 (1.8) | n/a | n/a |
| Cohabitation status | n/a | n/a | ||
| Parents cohabiting | 159 102 (85.0) | 4.9 (1.8) | n/a | n/a |
| Parents not cohabiting | 7998 (4.3) | 4.5 (1.8) | n/a | n/a |
| Missing | 20 006 (10.7) | 4.9 (1.8) | n/a | n/a |
aMissing values from the 1990 Census was due to the reason that some Swedes failed to respond as required by law.
bCriminal convictions were obtained from the national crime registry; hence, there were no missing data.
cThe low prevalence of half-siblings was due to the short time interval (7 years, 1982–88), during which both the index children and the half-siblings had to be born, and that only male children with a score for PF at conscription were included.
n/a: not applicable; either only maternal values are present or there is only one value per nuclear family.
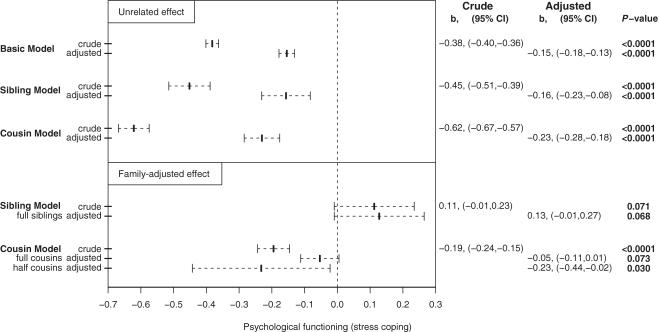
The crude estimate of the association of SDP with PF was −0.38 [95% confidence interval (CI) −0.40 to −0.36, P < 0.0001; Figure 2, for results from the regression analysis: Supplementary Table S1 (Supplementary data are available at IJE online)]. This means that a child exposed to SDP had, on average, a PF or stress coping score 0.38 points lower on the nine-point scale than an unrelated child unexposed to SDP. The following analyses explored the mechanisms behind this negative association.
Figure 2.
Regression coefficient estimates and 95% CIs for PF as a function of maternal smoking during pregnancy among male offspring born 1982–88 in Sweden and assessed for PF at age 18 years as part of mandatory military conscript evaluation
Each of the included covariates individually predicted PF (analyses not shown). When adjusting for these, the association was attenuated [b = −0.15; 95% CI −0.18 to −0.13, P < 0.0001; Figure 2 and Supplementary Table S2 (Supplementary data are available at IJE online)].
Having adjusted for known confounders, we investigated if the association was due to unmeasured familial confounding by performing sibling and cousin analyses with HLM. The crude and adjusted between extended family estimates (‘unrelated’ estimates) remained approximately similar (Figure 2). Thus, offspring in extended families where SDP had occurred also had lower mean PF scores, regardless of if a mother smoked during his own or somebody else’s pregnancy (sibling’s or a cousin’s). As seen in Figure 2, family-adjusted effects (from the sibling and cousin models) differed from ‘unrelated’ effects. When accounting for covariates for SDP-discordant siblings, the within-regression effect of SDP on PF completely disappeared (b = 0.13; 95% CI −0.01 to 0.27, P = 0.068; Figure 2), providing strong evidence for familial confounding. Another way to address the mechanism behind this would be to study also half-siblings, but because of too few half-siblings discordant for SDP (59 pairs, Table 1) the statistical power was minimal [Supplementary Table S6 (Supplementary data are available at IJE online)]. When full cousins were studied, the adjusted within-regression parameter was close to zero (b = −0.05; 95% CI −0.11 to 0.01, P = 0.073; Figure 2) and reduced compared with the within-half-cousins effect (b = −0.23; 95% CI −0.44 to −0.02, P = 0.030; Figure 2) and to the between extended family parameter, again indicating substantial familial confounding. For full tables of regression coefficients from sibling and cousin analyses, see Supplementary Tables S3–S10 (Supplementary data are available at IJE online).
We found minimal influence of birth weight and gestational age on the association between SDP and PF, suggesting that these covariates did not mediate neither the crude nor the adjusted association that we could verify. This held true for unrelated comparisons as well as sibling and cousin comparisons (analyses not shown).
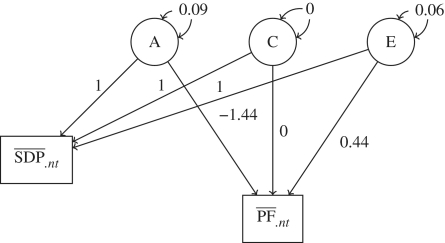
Because the HLM analyses indicated substantial familial confounding, we tried to estimate genetic and environmental effects on the association using SEM. When fitting the ACE model, both the intergenerational paths bC and bE had very large standard errors [bA = −1.48, standard error (SE) = 0.90, P = 0.10; bC = −1.31, SE = 2.65 P = 0.62; bE = 0.18, SE = 0.41, P = 0.66], which indicates that one latent variance parameter in the model is negligible.41 To test whether the ACE model fitted the data better than the AE and/or the CE model, two scaled-difference χ2-tests46 were performed. We found no evidence that the ACE model explained the data better than the AE model [χ2 = 1.2, degrees of freedom (df) = 2, P = 0.56]. In contrast, when comparing the ACE model with the CE model, the result was in favour of the ACE model (χ2 = 11.2, df = 2, P = 0.004). We used the Bayesian Information Criterion (BIC) to determine which of the models ACE and AE that fitted the data best. The AE model outperformed the ACE (BICAE = 126 493, BICACE = 126 511; The Akaike Information Criterion (AIC) yielded a similar result: AICAE = 126 277, AICACE = 126 280). Therefore, in subsequent models the shared environment parameters were set to zero.9,41 Results from the model fitting are presented in Figure 3. The regression coefficient bA expressing the genetic intergenerational transmission on PF was negative (bA = –1.44, SE = 0.13, P < 0.0001) while the non-shared environmental regression coefficient was positive (bE = 0.44, SE = 0.21, P = 0.037). For the full model, see Supplementary Figure S2 (Supplementary data are available at IJE online).
Figure 3.
Variance partitioning for A, genetics; C, the shared environment and E, non-shared environment. The graph represents the fitted model for one of two sibling mothers. SDP: mean smoking during pregnancy exposure; PF: mean psychological functioning capacity (stress coping); A: latent variable representing the genes; C: latent variable representing the shared environment; E: latent variable representing the non-shared environment, variance of latent variable A = 0.09 (P < 0.0001), variance of latent variable E = 0.06 (P < 0.0001), regression coefficient for PF regressed on A = −1.44 (P < 0.0001), regression coefficient for PF regressed on E = 0.44 (P = 0.037)
Discussion
We aimed to investigate the effect of intrauterine exposure to SDP on offspring stress coping in late adolescence. We used nationwide longitudinal registers, to compare not only unrelated individuals differentially exposed to SDP, but also relatives (siblings and cousins) to explore possible familial confounding and estimate the roles of genetic and environmental determinants.
Our main finding was that the observed association between SDP and poorer PF was entirely confounded by familial factors. Since the association could not be entirely explained by selected a priori confounders, we applied models that investigated unmeasured confounding based on similarities within nuclear and extended families. Familial confounding was evident; the association between SDP and poorer offspring stress coping decreased when half-cousin comparisons were used instead of unrelated individuals, and disappeared completely in within full-cousin and full-sibling comparisons. A possible reason for mothers to change smoking habits between pregnancies is if a life-altering event has occurred. However, a Swedish study on whether smoking habit changes after an adverse pregnancy outcome found only modest effects on continued smoking in next pregnancy.47 Thus, this is probably not a major reason for the familial confounding, especially since such effects are even less influential in the comparison between smoking discordant sisters. Additionally, data suggested that genetic effects entirely accounted for this familial confounding. The present results concur with previous studies in humans, suggesting that associations between SDP and cognitive/behavioural outcomes in adolescent offspring are not causal but subject to substantial familial confounding,9,11–15 primarily due to genetic rather than environmental mechanisms. One possible mechanism is that mothers transmit smoking liability to offspring and offspring smoking influenced stress reactivity. We could not test this since no data on smoking were available for the conscripts. Regardless, to be informative, studies of the effect of SDP and other parental risk factors on offspring must take familial confounding into account.
We also tested the fetal programming hypothesis, the study of which often used low birth weight as a proxy for adverse fetal environment.18,19 When comparing siblings or cousins differently exposed to SDP, the inclusion of birth weight as a potential mediator of the link between SDP and offspring PF, the latter remained essentially unchanged. Thus, either the lowering of birth weight due to SDP is not in the same causal pathway as the effect on PF or the effect of low birth weight on PF is also due to familial confounding.
Our study had several strengths, particularly its size and longitudinal total population-based design with high coverage of exposure data and outcome. As supported by Swedish data, we assumed that children are primarily raised by their biological mothers when parents divorce or separate.43 Since PF was assessed by professionals employed by the Swedish armed forces, the rating was classified. Hence, although previous studies used PF as a stress coping measure,20–22,35 we could not explicitly validate it. As indirect support, however, the test has been used for several decades, and it has been validated in that it correlates with military rank at the completion of military service.48 Regarding exposure, we used mothers’ smoking status at prenatal care registration (approximately the first trimester) as SDP measure. Self-reported SDP might be less reliable in later years, since the stigma associated with smoking while pregnant has increased. However, studies support its validity,2,33,34 the period when SDP was measured was quite short (1982–88), and we controlled for period effects when including birth year/birth order as a covariate. We cannot differentiate between prenatal only and prenatal plus postnatal smoking; therefore, the associations examined could be due to postnatal smoking as well as SDP. Another limitation of the study is that analyses were done in men, hence generalization to women cannot be assumed.
Finally, we want to stress that mothers’ SDP is associated with numerous adverse outcomes, especially related to birth and infancy.5,49 However, our results add to the accumulating evidence that SDP have no, or only minor, long-term causal effects on offspring cognitive functioning.9,11–15 Other factors accounts for the association, and in the case of stress coping the confounds seem to be mainly of genetic origin. To conclude, mothers prone to SDP also transmit genes to their children, which cause poorer stress coping in the latter.
Supplementary Data
Supplementary data are available at IJE online.
Funding
Swedish National Prison and Probation R&D; the Swedish Research Council − Medicine; the Indiana University Faculty Research Support Program, NARSAD; the National Institute of Child Health and Human Development (grant number HD056354).
Conflict of interest: None declared.
KEY MESSAGES.
Fetal exposure to tobacco smoking is suggested to cause long-term alterations to regulatory systems involved in stress response.
We found an association between SDP and worse stress coping in male offspring at 18 years of age.
This association is not causal but subject to substantial familial confounding.
The familial confounding seems to be primarily of genetic origin.
References
- 1.Andres RL, Day MC. Perinatal complications associated with maternal tobacco use. Semin Neonatol. 2000;5:231–41. doi: 10.1053/siny.2000.0025. [DOI] [PubMed] [Google Scholar]
- 2.Cnattingius S, Haglund B. Decreasing smoking prevalence during pregnancy in Sweden: the effect on small-for-gestational-age births. Am J Public Health. 1997;87:410–13. doi: 10.2105/ajph.87.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardy JB, Mellits ED. Does maternal smoking during pregnancy have a long-term effect on the child? Lancet. 1972;2:1332–36. doi: 10.1016/s0140-6736(72)92777-8. [DOI] [PubMed] [Google Scholar]
- 4.The 2004 United States Surgeon General's Report. The health consequences of smoking. NSW Public Health Bull. 2004;15:107. [PubMed] [Google Scholar]
- 5.Cnattingius S. The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob Res. 2004;6(Suppl 2):S125–40. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- 6.Kyrklund-Blomberg NB, Cnattingius S. Preterm birth and maternal smoking: risks related to gestational age and onset of delivery. Am J Obstet Gynecol. 1998;179:1051–55. doi: 10.1016/s0002-9378(98)70214-5. [DOI] [PubMed] [Google Scholar]
- 7.Ernst M, Moolchan ET, Robinson ML. Behavioral and neural consequences of prenatal exposure to nicotine. J Am Acad Child Adolesc Psychiatry. 2001;40:630–41. doi: 10.1097/00004583-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Haglund B, Cnattingius S. Cigarette smoking as a risk factor for sudden infant death syndrome: a population-based study. Am J Public Health. 1990;80:29–32. doi: 10.2105/ajph.80.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Onofrio BM, Van Hulle CA, Waldman ID, et al. Smoking during pregnancy and offspring externalizing problems: an exploration of genetic and environmental confounds. Dev Psychopathol. 2008;20:139–64. doi: 10.1017/S0954579408000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huijbregts SC, Seguin JR, Zoccolillo M, Boivin M, Tremblay RE. Associations of maternal prenatal smoking with early childhood physical aggression, hyperactivity-impulsivity, and their co-occurrence. J Abnorm Child Psychol. 2007;35:203–15. doi: 10.1007/s10802-006-9073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Onofrio BM, Singh AL, Iliadou A, et al. Familial confounding of the association between maternal smoking during pregnancy and offspring criminality: a population-based study in Sweden. Arch Gen Psychiatry. 2010;67:529–38. doi: 10.1001/archgenpsychiatry.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kafouri S, Leonard G, Perron M, et al. Maternal cigarette smoking during pregnancy and cognitive performance in adolescence. Int J Epidemiol. 2009;38:158–72. doi: 10.1093/ije/dyn250. [DOI] [PubMed] [Google Scholar]
- 13.Lundberg F, Cnattingius S, D’Onofrio BM, et al. Maternal smoking during pregnancy and intellectual performance in young adult Swedish male offspring. Paediatr Perinatal Epidemiol. 2010;24:79–87. doi: 10.1111/j.1365-3016.2009.01073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lambe M, Hultman C, Torrång A, Maccabe J, Cnattingius S. Maternal smoking during pregnancy and school performance at age 15. Epidemiology. 2006;17:524–30. doi: 10.1097/01.ede.0000231561.49208.be. [DOI] [PubMed] [Google Scholar]
- 15.D'Onofrio BM, Singh AL, Iliadou A, et al. A quasi-experimental approach of maternal smoking during pregnancy and offspring academic achievement. Child Dev. 2010;81:80–100. doi: 10.1111/j.1467-8624.2009.01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knopik VS. Maternal smoking during pregnancy and child outcomes: real or spurious effect? Dev Neuropsychol. 2009;34:1–36. doi: 10.1080/87565640802564366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaglenova J, Birru S, Pandiella NM, Breese CR. An assessment of the long-term developmental and behavioral teratogenicity of prenatal nicotine exposure. Behav Brain Res. 2004;150:159–70. doi: 10.1016/j.bbr.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Phillips DI. Programming of the stress response: a fundamental mechanism underlying the long-term effects of the fetal environment? J Intern Med. 2007;261:453–60. doi: 10.1111/j.1365-2796.2007.01801.x. [DOI] [PubMed] [Google Scholar]
- 19.Phillips DI, Jones A. Fetal programming of autonomic and HPA function: do people who were small babies have enhanced stress responses? J Physiol. 2006;572:45–50. doi: 10.1113/jphysiol.2005.104695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nilsson PM, Nyberg P, Östergren PO. Increased susceptibility to stress at a psychological assessment of stress tolerance is associated with impaired fetal growth. Int J Epidemiol. 2001;30:75–80. doi: 10.1093/ije/30.1.75. [DOI] [PubMed] [Google Scholar]
- 21.Nilsson PM, Nilsson JA, Östergren PO, Rasmussen F. Fetal growth predicts stress susceptibility independent of parental education in 161991 adolescent Swedish male conscripts. J Epidemiol Community Health. 2004;58:571–73. doi: 10.1136/jech.2003.015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lundgren EM, Cnattingius S, Jonsson B, Tuvemo T. Intellectual and psychological performance in males born small for gestational age. Horm Res. 2003;59(Suppl 1):139–41. doi: 10.1159/000067850. [DOI] [PubMed] [Google Scholar]
- 23.Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther. 1998;285:931–45. [PubMed] [Google Scholar]
- 24.Huizink AC, Mulder EJ. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neurosci Biobehav Rev. 2006;30:24–41. doi: 10.1016/j.neubiorev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Rutter M. Proceeding from observed correlation to causal inference. Perspectives Psychol Sci. 2007;2:377–95. doi: 10.1111/j.1745-6916.2007.00050.x. [DOI] [PubMed] [Google Scholar]
- 26.Multi-generation Register 2005 − A Description of Contents and Quality. Örebro: Statistics Sweden; 2006. [(Accessed July 19, 2010)]. http://www.scb.se/statistik/_publikationer/BE9999_2005A01_BR_BE96ST0606.pdf. [Google Scholar]
- 27.Utbildningsregistret. Statistics Sweden. [(Accessed July 19, 2010)]. Available from: http://www.scb.se/statistik/UF/UF0506/_dokument/UF0506_DO_2004.pdf.
- 28.Folk- och bostadsräkningen 1990, FoB 90. Stockholm: Statistics Sweden; 1999. [(Accessed July 19, 2010)]. http://www.scb.se/statistik/BE/BE0205/_dokument/BE0205_DO_1990.pdf. [Google Scholar]
- 29.Magnusson PK, Gunnell D, Tynelius P, Davey Smith G, Rasmussen F. Strong inverse association between height and suicide in a large cohort of Swedish men: evidence of early life origins of suicidal behavior? Am J Psychiatry. 2005;162:1373–75. doi: 10.1176/appi.ajp.162.7.1373. [DOI] [PubMed] [Google Scholar]
- 30.The Swedish Medical Birth Register − A Summary of Content and Quality. Stockholm: Centre for Epidemiology, 2003. http://www.socialstyrelsen.se/publikationer2003/2003-112-3 (Accessed July 19, 2010)
- 31.Cnattingius S, Ericson A, Gunnarskog J, Källén B. A quality study of a medical birth registry. Scand J Soc Med. 1990;18:143–48. doi: 10.1177/140349489001800209. [DOI] [PubMed] [Google Scholar]
- 32.Fazel S, Grann M. The population impact of severe mental illness on violent crime. Am J Psychiatry. 2006;163:1397–403. doi: 10.1176/ajp.2006.163.8.1397. [DOI] [PubMed] [Google Scholar]
- 33.Lindqvist R, Lendahls L, Tollbom Ö, Åberg H, Hakansson A. Smoking during pregnancy: comparison of self-reports and cotinine levels in 496 women. Acta Obstet Gynecol Scand. 2002;81:240–44. doi: 10.1034/j.1600-0412.2002.810309.x. [DOI] [PubMed] [Google Scholar]
- 34.Hatziandreu EJ, Pierce JP, Fiore MC, et al. The reliability of self-reported cigarette consumption in the United States. Am J Public Health. 1989;79:1020–23. doi: 10.2105/ajph.79.8.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leboeuf-Yde C, Larsen K, Ahlstrand I, Volinn E. Coping and back problems: analysis of multiple data sources on an entire cross-sectional cohort of Swedish military recruits. BMC Musculoskelet Disord. 2006;7:39. doi: 10.1186/1471-2474-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Socioekonomisk indelning (SEI) 1982. Stockholm: Statistics Sweden. http://www.scb.se/Grupp/Hitta_statistik/Forsta_Statistik/Klassifikationer/_Dokument/SEI-MIS.pdf (Accessed July 19, 2010)
- 37.Harden KP, Lynch SK, Turkheimer E, et al. A behavior genetic investigation of adolescent motherhood and offspring mental health problems. J Abnorm Psychol. 2007;116:667–83. doi: 10.1037/0021-843X.116.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raudenbush SW, Bryk AS. Hierarchical Linear Models Applications and Data Analysis Methods. 2nd. Thousand Oaks, CA: Sage Publications; 2002. [Google Scholar]
- 39.Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schnabenberger O. SAS for Mixed Models. Cary, NC: SAS Press; 2006. [Google Scholar]
- 40.D'Onofrio BM, Turkheimer E, Emery RE, et al. A genetically informed study of marital instability and its association with offspring psychopathology. J Abnorm Psychol. 2005;114:570–86. doi: 10.1037/0021-843X.114.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D'Onofrio BM, Turkheimer EN, Eaves LJ, et al. The role of the children of twins design in elucidating causal relations between parent characteristics and child outcomes. J Child Psychol Psychiatry. 2003;44:1130–44. doi: 10.1111/1469-7610.00196. [DOI] [PubMed] [Google Scholar]
- 42.Neuhaus JM, McCulloch CE. Separating between- and within-cluster covariate effects by using conditional and partitioning methods. J Roy Stat Soc. 2006;68:859–72. [Google Scholar]
- 43.Fakta om den svenska familjen. Demografiska rapporter (1994:2) Stockholm: Statistics Sweden; 1994. [Google Scholar]
- 44.Muthén BO. Mplus Technical Appendices. Los Angeles, CA: Muthén & Muthén; 1998–2004. [Google Scholar]
- 45.Muthén LK, Muthén BO. Mplus User's Guide. 5. Los Angeles, CA: Muthén & Muthén; 1998–2007. [Google Scholar]
- 46.Satorra A, Bentler PM. A scaled difference chi-square test statistic for moment structure analysis. Psychometrika. 2001;66:507–14. doi: 10.1007/s11336-009-9135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cnattingius S, Akre O, Lambe M, Ockene J, Granath F. Will an adverse pregnancy outcome influence the risk of continued smoking in the next pregnancy? Am J Obstet Gynecol. 2006;195:1680–86. doi: 10.1016/j.ajog.2006.06.071. [DOI] [PubMed] [Google Scholar]
- 48.Carlstedt B. Validering av inskrivningsprövningen mot vitsord från den militära grundutbildningen. Karlstad: Försvarshögskolan, Klara AB Tryckeri i Karlstad; 1999. [Google Scholar]
- 49.Johansson AL, Dickman PW, Kramer MS, Cnattingius S. Maternal smoking and infant mortality: does quitting smoking reduce the risk of infant death? Epidemiology. 2009;20:590–97. doi: 10.1097/EDE.0b013e31819dcc6a. [DOI] [PubMed] [Google Scholar]