Abstract
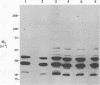
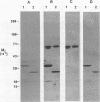
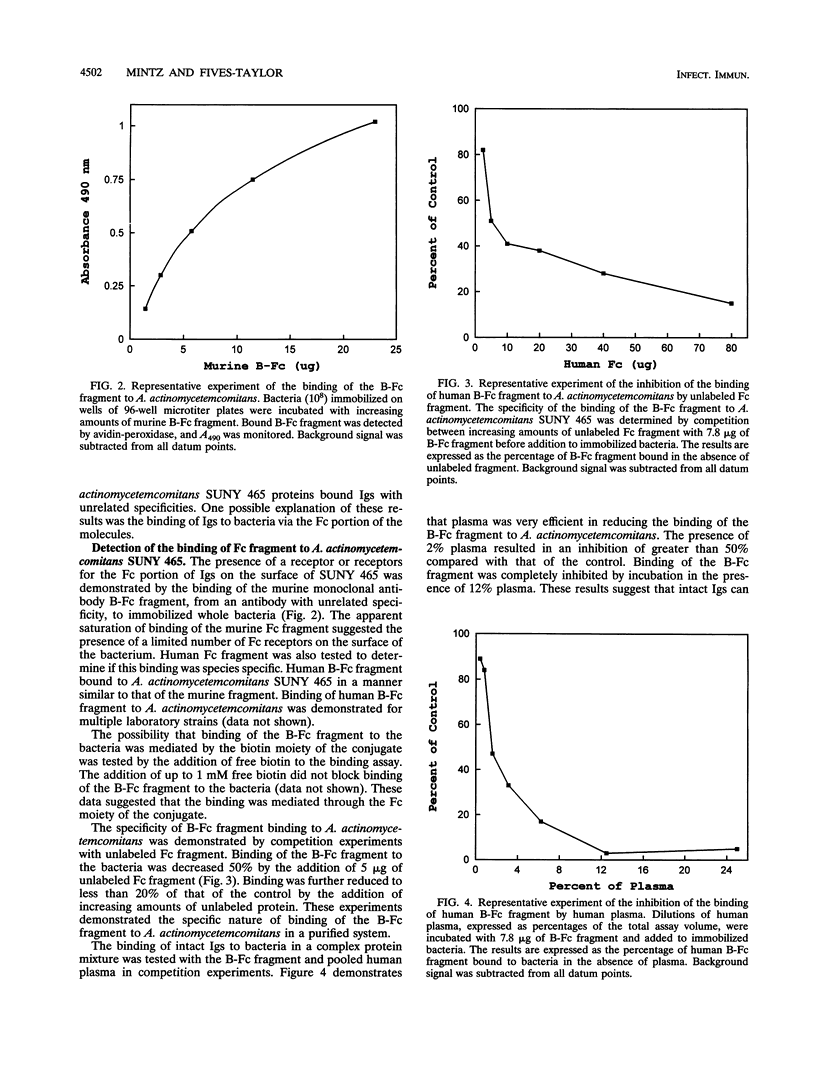
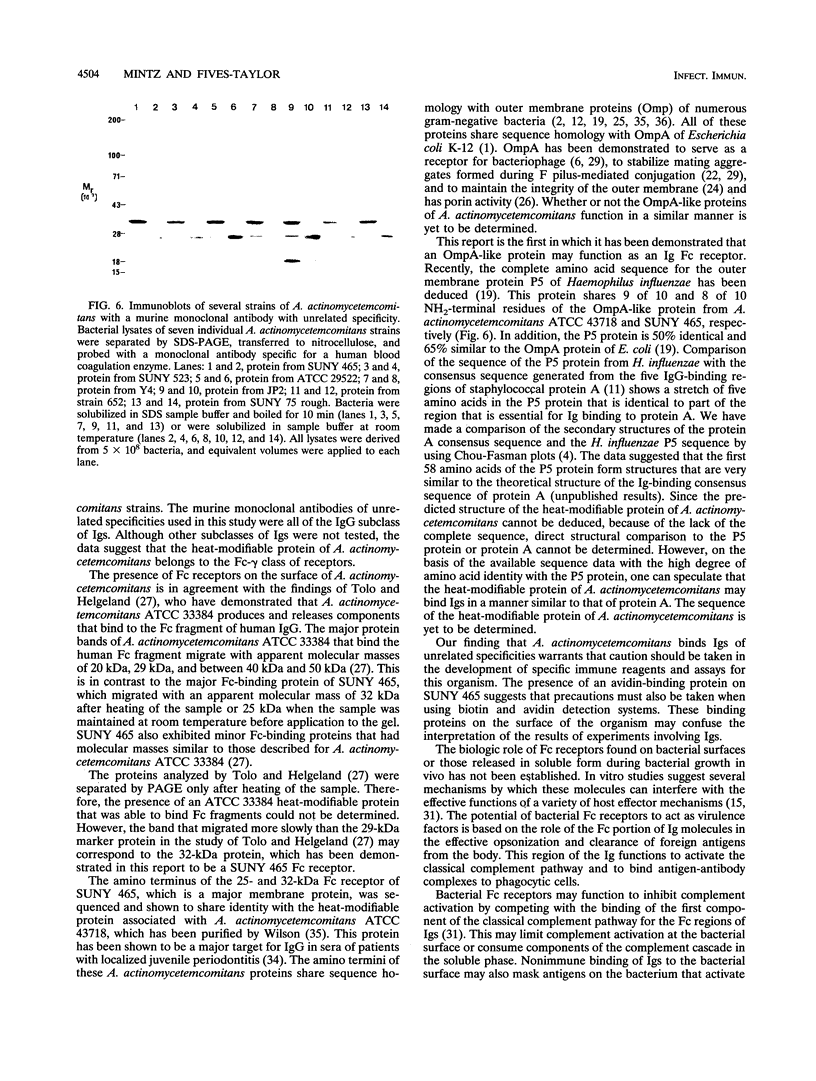
Actinobacillus actinomycetemcomitans expresses proteins that bind to the Fc portion of immunoglobulins. The immunoglobulin Fc receptors on the surface of A. actinomycetemcomitans were detected by the binding of biotinylated human or murine Fc molecules to strain SUNY 465 adsorbed to the bottom of microtiter wells. Biotinylated Fc binding was inhibited by unlabeled Fc molecules and human plasma. Fc receptors were identified by the binding of biotinylated Fc molecules to bacterial membrane proteins separated by polyacrylamide gel electrophoresis and transferred to nitrocellulose. Multiple bands were identified, and the major Fc-binding protein was determined to be a heat-modifiable protein. This protein migrated with approximate molecular weights of 25,000 and 32,000 (unheated and heated, respectively). Amino-terminal sequence analysis of this protein revealed a sequence identical to the heat-modifiable protein described for A. actinomycetemcomitans ATCC 43718. This protein sequence exhibits significant homology with the N termini of outer membrane protein A (OmpA) of Escherichia coli and related OmpA-like proteins from other gram-negative bacteria.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck E., Bremer E. Nucleotide sequence of the gene ompA coding the outer membrane protein II of Escherichia coli K-12. Nucleic Acids Res. 1980 Jul 11;8(13):3011–3027. doi: 10.1093/nar/8.13.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. S., Wetzler L. M., Gotschlich E. C., Rice P. A. Protein III: structure, function, and genetics. Clin Microbiol Rev. 1989 Apr;2 (Suppl):S60–S63. doi: 10.1128/cmr.2.suppl.s60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolstad A. I., Kristoffersen T., Olsen I., Preus H. R., Jesen H. B., Vasstrand E. N., Bakken V. Outer membrane proteins of Actinobacillus actinomycetemcomitans and Haemophilus aphrophilus studied by SDS-PAGE and immunoblotting. Oral Microbiol Immunol. 1990 Jun;5(3):155–161. doi: 10.1111/j.1399-302x.1990.tb00414.x. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Datta D. B., Arden B., Henning U. Major proteins of the Escherichia coli outer cell envelope membrane as bacteriophage receptors. J Bacteriol. 1977 Sep;131(3):821–829. doi: 10.1128/jb.131.3.821-829.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder B. L., Fives-Taylor P. Characterization of monoclonal antibodies specific for adhesion: isolation of an adhesin of Streptococcus sanguis FW213. Infect Immun. 1986 Nov;54(2):421–427. doi: 10.1128/iai.54.2.421-427.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren A., Grubb A. O. Many bacterial species bind human IgD. J Immunol. 1979 Apr;122(4):1468–1472. [PubMed] [Google Scholar]
- Forsgren A., Sjöquist J. "Protein A" from S. aureus. I. Pseudo-immune reaction with human gamma-globulin. J Immunol. 1966 Dec;97(6):822–827. [PubMed] [Google Scholar]
- Foster W. B., Tucker M. M., Katzmann J. A., Miller R. S., Nesheim M. E., Mann K. G. Monoclonal antibodies to human coagulation factor V and factor Va. Blood. 1983 Jun;61(6):1060–1067. [PubMed] [Google Scholar]
- Klugman K. P., Gotschlich E. C., Blake M. S. Sequence of the structural gene (rmpM) for the class 4 outer membrane protein of Neisseria meningitidis, homology of the protein to gonococcal protein III and Escherichia coli OmpA, and construction of meningococcal strains that lack class 4 protein. Infect Immun. 1989 Jul;57(7):2066–2071. doi: 10.1128/iai.57.7.2066-2071.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronvall G. A surface component in group A, C, and G streptococci with non-immune reactivity for immunoglobulin G. J Immunol. 1973 Nov;111(5):1401–1406. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langone J. J. Protein A of Staphylococcus aureus and related immunoglobulin receptors produced by streptococci and pneumonococci. Adv Immunol. 1982;32:157–252. [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Mintz K. P., Mann K. G. Detection of procollagen biosynthesis using peptide-specific antibodies. Matrix. 1990 Jul;10(3):186–199. doi: 10.1016/s0934-8832(11)80168-x. [DOI] [PubMed] [Google Scholar]
- Munson R. S., Jr, Grass S., West R. Molecular cloning and sequence of the gene for outer membrane protein P5 of Haemophilus influenzae. Infect Immun. 1993 Sep;61(9):4017–4020. doi: 10.1128/iai.61.9.4017-4020.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen K., Duncan J. R. Demonstration that nonspecific bovine Brucella abortus agglutinin is EDTA-labile and not calcium-dependent. J Immunol. 1982 Jul;129(1):366–369. [PubMed] [Google Scholar]
- Ouellette L. A., Messier T. L., Church W. R. Neutralization of factor X activity by factor X-specific monoclonal antibodies. Blood Coagul Fibrinolysis. 1992 Oct;3(5):563–574. doi: 10.1097/00001721-199210000-00007. [DOI] [PubMed] [Google Scholar]
- Schweizer M., Henning U. Action of a major outer cell envelope membrane protein in conjugation of Escherichia coli K-12. J Bacteriol. 1977 Mar;129(3):1651–1652. doi: 10.1128/jb.129.3.1651-1652.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenker B. J., McArthur W. P., Tsai C. C. Immune suppression induced by Actinobacillus actinomycetemcomitans. I. Effects on human peripheral blood lymphocyte responses to mitogens and antigens. J Immunol. 1982 Jan;128(1):148–154. [PubMed] [Google Scholar]
- Sonntag I., Schwarz H., Hirota Y., Henning U. Cell envelope and shape of Escherichia coli: multiple mutants missing the outer membrane lipoprotein and other major outer membrane proteins. J Bacteriol. 1978 Oct;136(1):280–285. doi: 10.1128/jb.136.1.280-285.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinola S. M., Griffiths G. E., Shanks K. L., Blake M. S. The major outer membrane protein of Haemophilus ducreyi is a member of the OmpA family of proteins. Infect Immun. 1993 Apr;61(4):1346–1351. doi: 10.1128/iai.61.4.1346-1351.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara E., Nikaido H. Pore-forming activity of OmpA protein of Escherichia coli. J Biol Chem. 1992 Feb 5;267(4):2507–2511. [PubMed] [Google Scholar]
- Tolo K., Helgeland K. Fc-binding components: a virulence factor in Actinobacillus actinomycetemcomitans? Oral Microbiol Immunol. 1991 Dec;6(6):373–377. doi: 10.1111/j.1399-302x.1991.tb00509.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Alphen L., Havekes L., Lugtenberg B. Major outer membrane protein d of Escherichia coli K12. Purification and in vitro activity of bacteriophages k3 and f-pilus mediated conjugation. FEBS Lett. 1977 Mar 15;75(1):285–290. doi: 10.1016/0014-5793(77)80104-x. [DOI] [PubMed] [Google Scholar]
- Van Dyke T. E., Bartholomew E., Genco R. J., Slots J., Levine M. J. Inhibition of neutrophil chemotaxis by soluble bacterial products. J Periodontol. 1982 Aug;53(8):502–508. doi: 10.1902/jop.1982.53.8.502. [DOI] [PubMed] [Google Scholar]
- Widders P. R., Dorrance L. A., Yarnall M., Corbeil L. B. Immunoglobulin-binding activity among pathogenic and carrier isolates of Haemophilus somnus. Infect Immun. 1989 Feb;57(2):639–642. doi: 10.1128/iai.57.2.639-642.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widders P. R., Stokes C. R., Newby T. J., Bourne F. J. Nonimmune binding of equine immunoglobulin by the causative organism of contagious equine metritis, Taylorella equigenitalis. Infect Immun. 1985 May;48(2):417–421. doi: 10.1128/iai.48.2.417-421.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. E. IgG antibody response of localized juvenile periodontitis patients to the 29 kilodalton outer membrane protein of Actinobacillus actinomycetemcomitans. J Periodontol. 1991 Mar;62(3):211–218. doi: 10.1902/jop.1991.62.3.211. [DOI] [PubMed] [Google Scholar]
- Wilson M. E. The heat-modifiable outer membrane protein of Actinobacillus actinomycetemcomitans: relationship to OmpA proteins. Infect Immun. 1991 Jul;59(7):2505–2507. doi: 10.1128/iai.59.7.2505-2507.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff W. A., Hancock R. E. Pseudomonas aeruginosa outer membrane protein F: structural role and relationship to the Escherichia coli OmpA protein. J Bacteriol. 1989 Jun;171(6):3304–3309. doi: 10.1128/jb.171.6.3304-3309.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnall M., Widders P. R., Corbeil L. B. Isolation and characterization of Fc receptors from Haemophilus somnus. Scand J Immunol. 1988 Aug;28(2):129–137. doi: 10.1111/j.1365-3083.1988.tb02424.x. [DOI] [PubMed] [Google Scholar]