Abstract
Renal cell carcinoma comprises 80%–85% of kidney malignancies. For early presentations, nephrectomy provides a high cure rate, but patients usually present at advanced stages, leading to poor outcomes. Even for patients without metastatic spread who undergo nephrectomy, metastatic recurrence is frequent. We report the case of a patient who underwent nephrectomy for stage iii renal cell carcinoma and who presented 20 months later with respiratory symptoms consistent with pneumonia, influenza, or (less likely) congestive heart failure or a cardiac event. Persistent right pleural effusion on serial chest radiographs despite treatment prompted computed tomography evaluation, which revealed lymphangitic carcinomatosis, a very rare form of renal cell carcinoma metastasis to the lung. This preliminary finding was confirmed by right middle lobe tissue biopsy through bronchoscopy and cytopathology examination.
Keywords: Renal cell carcinoma, recurrence, lung metastasis, lymphangitic carcinomatosis
1. CASE DESCRIPTION
A 68-year-old obese non-smoking white man, with a past medical history of coronary article disease, diabetes mellitus, hypertension, and T3bN0M0 stage iii left renal cell carcinoma (rcc) treated 20 months earlier with left radical nephrectomy, presented with 3 days of worsening cough, dyspnea, and pleuritic chest pain. The rcc had been grade 3/4 clear cell adenocarcinoma, 10 cm in the greatest dimension, with spread to the left renal vein. The patient’s relevant preoperative laboratory values were blood urea nitrogen (bun) 23 mg/dL, serum creatinine (scr) 1.1 mg/dL, serum Ca (sCa) 9.7 mg/dL, white blood cells (wbcs) 8400/μL, hemoglobin 11.2 g/dL, and platelets 621000/μL. Liver function tests (lfts) were within normal limits except for serum albumin 3.8 g/dL. Erythrocyte sedimentation rate (esr) and C-reactive protein (crp) were never measured.
On current presentation, the patient also complained of fatigue and decreased appetite, and denied fevers, chills, nausea, vomiting, diarrhea, and constipation. Respiratory exam revealed decreased right lower lobe breath sounds and moderate crackles in the right middle lobe. Laboratory studies were consistent with his postoperative baselines: bun 25 mg/dL, scr 1.4 mg/dL, sCa 8.8 mg/dL, wbcs 7300/μL, hemoglobin 9.5 g/dL, platelets 431000/μL, and lfts within normal limits except for albumin 2.8 g/dL, with esr and crp again not measured. Troponin and creatine kinase–mb were negative for 3 consecutive drawings (ruling out a cardiac cause). Chest radiography demonstrated a right-sided pleural effusion, and an electrocardiogram revealed normal sinus rhythm at 58 bpm.
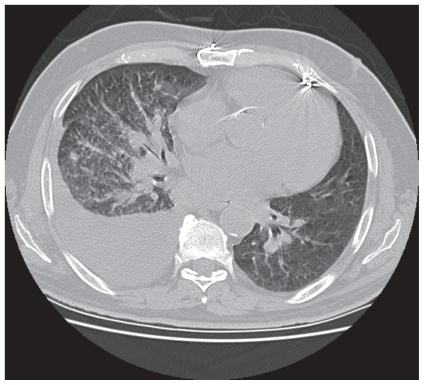
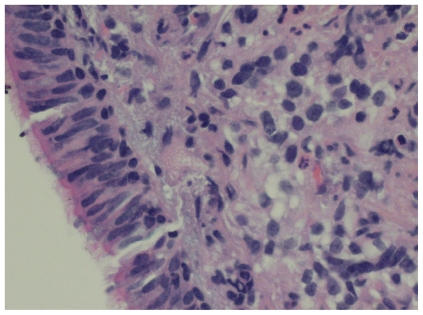
Because the patient had presented to a New York City hospital in July 2009 (during an H1N1 swine flu epidemic), he was initially diagnosed with pneumonia (with H1N1 influenza also a consideration), and he was treated with cefepime, vancomycin, and oseltamivir. Although he improved symptomatically, serial chest radiography demonstrated a persistent right pleural effusion. Intravenous (IV)–contrast computed tomography (ct) imaging of the chest revealed diffuse metastatic tissue in the right lung consistent with pulmonary lymphangitic carcinomatosis (Figure 1). The patient subsequently underwent right middle lobe tissue biopsy through bronchoscopy with endobronchial ultrasonography. Cytopathology examination confirmed rcc grade 4/4 metastases with lymphangitic spread (Figure 2).
FIGURE 1.
Axial computed tomography image of the mid-thorax, demonstrating a large right pleural effusion and lymphangitic thickening consistent with right-sided pulmonary lymphangitic carcinomatosis.
FIGURE 2.
Biopsy confirming metastatic renal cell carcinoma.
The patient next presented to an outside hospital’s cancer center for further evaluation and treatment, where he was started on palliative sunitinib without surgery or radiotherapy. Over the next 4 months, serial chest and abdominal ct imaging revealed growing metastases in both lungs and the right kidney, and a mass in the left posterolateral pelvis. Head ct imaging demonstrated a single brain metastasis, which was treated with stereotactic radiosurgery. In January 2010, the patient was intubated for respiratory compromise and soon died.
2. DISCUSSION AND CONCLUSIONS
Renal cell carcinoma (also called renal adenocarcinoma) originates within the renal cortex and represents 80%–85% of adult kidney malignancies 1. In 2009, approximately 62,400 people in Canada and the United States were diagnosed with rcc, and about 14,600 people died from the malignancy 2,3. For unknown reasons, rcc incidence rates have been steadily increasing, particularly among African-Americans 4.
This malignancy remains clinically silent for most of its natural history, and patients therefore often present with advanced disease, with approximately 30% of patients presenting with metastases 5. However, surgical resection achieves high cure rates for localized disease. Table I demonstrates the prognoses from several studies based on rcc stage.
TABLE I.
Five-year survival in renal cell carcinoma by TNM stage
Renal cell carcinoma metastasizes most frequently to the lungs (50%–60% of patients with metastases) and also commonly to the bones, liver, renal fossae, brain, and, by direct extension, beyond Gerota’s fascia 9. Metastasis usually occurs by hematogenous spread to the parenchyma of other organs. For patients with lung metastases, hilar or mediastinal lymph node involvement (or both) occurs in 22%–30% of cases and is associated with worse outcomes 10,11. Pulmonary lymphangitic carcinomatosis (diffuse spread of the tumour to the pulmonary lymphatic system) is exceedingly rare.
Our patient’s T3bN0M0 rcc diagnosis was a locoregionally- advanced stage iii malignancy 12. Approximately 33%–42% of patients who undergo nephrectomy for nonmetastatic T3 tumours have metastases by 5 years after the surgery 13,14. For all patients with recurrent rcc, lung metastases occur in 29%–54% of cases 13,15,16.
With such a serious risk of recurrence, suggested follow-up guidelines derived from retrospective studies for T3b rcc after nephrectomy include chest radiographs and routine laboratory studies every 6 months for 5 years, plus abdominal ct imaging (preferably with IV contrast, if renal function permits) at year 2 and year 4 or 5 13,16,17. One treatment group recommended increasing the frequency of ct imaging to 6, 12, 24, and 36 months postoperatively 14. With surveillance imaging, more than 90% of lung metastases are discovered while the patient is still asymptomatic, thereby improving outcomes 13,16,17. Based on a retrospective study, resection of all known pulmonary metastases without evidence of other metastatic spread appears to confer a survival advantage, with 33% survival at 5 years 18.
During our patient’s postoperative surveillance period, he presented every 3–4 months for laboratory studies, but was mostly noncompliant with the imaging studies. His laboratory values were generally within normal limits, aside from a postoperative wbc elevation to 17400/μL and a 1-year postoperative acute scr rise to 2.5 mg/dL, secondary to furosemide overdiuresis. Immediately after the nephrectomy, ct imaging of chest and abdomen with IV contrast did not reveal any residual tumour or metastases. Magnetic resonance imaging of the abdomen 4 months later was also negative, as was chest radiography the following month. He underwent no additional imaging until he presented with respiratory symptoms.
As mentioned earlier, rcc lung metastasis usually occurs by hematogenous spread to the parenchyma and less frequently involves the lymphogenous route. Pulmonary lymphangitic carcinomatosis occurs infrequently with breast, lung, stomach, thyroid, pancreatic, laryngeal, colonic, prostatic, and cervical malignancies, but it is extremely rare with renal tumours 19. We identified 7 other cases of pulmonary lymphangitic carcinomatosis from a renal malignancy in the literature, with most patients also presenting with rapidly progressive dyspnea 20–23. Our case appears to demonstrate the first presentation of unilateral pulmonary lymphangitic carcinomatosis secondary to a renal malignancy.
Because the lung is a major site of metastasis for many malignancies, pulmonary metastases should be considered for patients with respiratory symptoms who have a history of cancer. However, in rare instances, an unusual converse situation may arise: a patient who presents with respiratory symptoms may have an occult malignancy that has already metastasized to the lungs. Clinicians who diagnose parenchymal, lymphogenous, or lymphangitic pulmonary metastasis should consider spread from rcc and other malignancies that frequently present at late stages or that have high rates of metastatic recurrence. Additionally, it is critically important that patients be compliant with their surveillance imaging after nephrectomy for rcc.
Footnotes
3. CONFLICT OF INTEREST DISCLOSURES
The authors of this manuscript affirm that they do not have relationships with pharmaceutical companies or employment contracts, consultancy, advisory boards, speaker bureaus, membership of Board of Directors, or stock ownership that could represent a financial conflict of interest.
4. REFERENCES
- 1.Kosary CL, McLaughlin JK. Kidney and renal pelvis. In: Miller BA, Ries LAG, Hankey BF, et al., editors. SEER Cancer Statistics Review, 1973–1990 nih publication no. 93–2789. Bethesda, MD: National Cancer Institute; 1993. pp. XI.1–XI.22. [Google Scholar]
- 2.Canadian Cancer Society and the National Cancer Institute of Canada. Canadian Cancer Statistics 2008. Toronto: Canadian Cancer Society; 2008. p. 12. [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics 2009. CA Cancer J Clin. 2009;9:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 4.Chow WH, Devesa SS, Warren JL, Fraumeni JF., Jr Rising incidence of renal cell cancer in the United States. JAMA. 1999;281:1628–31. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 5.van der Poel HG, Roukema JA, Horenblas S, van Geel AN, Debruyne FM. Metastasectomy in renal cell carcinoma: a multicenter retrospective analysis. Eur Urol. 1999;35:197–203. doi: 10.1159/000019849. [DOI] [PubMed] [Google Scholar]
- 6.Tsui KH, Shvarts O, Smith RB, Figlin RA, deKernion JB, Belldegrun A. Prognostic indicators for renal cell carcinoma: a multivariate analysis of 643 patients using the revised 1997 TNM staging criteria. J Urol. 2000;163:1090–5. doi: 10.1016/S0022-5347(05)67699-9. [DOI] [PubMed] [Google Scholar]
- 7.Javidan J, Stricker HJ, Tamboli P, et al. Prognostic significance of the 1997 TNM classification of renal cell carcinoma. J Urol. 1999;162:1277–81. doi: 10.1016/S0022-5347(05)68264-X. [DOI] [PubMed] [Google Scholar]
- 8.Kinouchi T, Saiki S, Meguro N, et al. Impact of tumor size on the clinical outcomes of patients with Robson state I renal cell carcinoma. Cancer. 1999;85:689–95. doi: 10.1002/(SICI)1097-0142(19990201)85:3<689::AID-CNCR19>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996;335:865–75. doi: 10.1056/NEJM199609193351207. [DOI] [PubMed] [Google Scholar]
- 10.Fourquier P, Regnard JF, Rea S, Levi JF, Levasseur P. Lung metastases of renal cell carcinoma: results of surgical resection. Eur J Cardiothorac Surg. 1997;11:17–21. doi: 10.1016/S1010-7940(96)01013-5. [DOI] [PubMed] [Google Scholar]
- 11.Pfannschmidt J, Hoffmann H, Muley T, Krysa S, Trainer C, Dienemann H. Prognostic factors for survival after pulmonary resection of metastatic renal cell carcinoma. Ann Thorac Surg. 2002;74:1653–7. doi: 10.1016/S0003-4975(02)03803-1. [DOI] [PubMed] [Google Scholar]
- 12.Greene FL, Page DL, Fleming ID, et al., editors. AJCC Cancer Staging Manual. 6th ed. New York: Springer–Verlag; 2002. [Google Scholar]
- 13.Levy DA, Slaton JW, Swanson DA, Dinney CP. Stage specific guidelines for surveillance after radical nephrectomy for local renal cell carcinoma. J Urol. 1998;159:1163–7. doi: 10.1016/S0022-5347(01)63541-9. [DOI] [PubMed] [Google Scholar]
- 14.Stephenson AJ, Chetner MP, Rourke K, et al. Guidelines for the surveillance of localized renal cell carcinoma based on the patterns of relapse after nephrectomy. J Urol. 2004;172:58–62. doi: 10.1097/01.ju.0000132126.85812.7d. [DOI] [PubMed] [Google Scholar]
- 15.Hafez KS, Novick AC, Campbell SC. Patterns of tumor recurrence and guidelines for followup after nephron sparing surgery for sporadic renal cell carcinoma. J Urol. 1997;157:2067–70. doi: 10.1016/S0022-5347(01)64675-5. [DOI] [PubMed] [Google Scholar]
- 16.Ljungberg B, Alamdari FI, Rasmuson T, Roos G. Follow-up guidelines for nonmetastatic renal cell carcinoma based on the occurrence of metastases after radical nephrectomy. BJU Int. 1999;84:405–11. doi: 10.1046/j.1464-410x.1999.00202.x. [DOI] [PubMed] [Google Scholar]
- 17.Sandock DS, Seftel AD, Resnick MI. A new protocol for the follow-up of renal cell carcinoma based on pathological stage. J Urol. 1995;154:28–31. doi: 10.1016/S0022-5347(01)67215-X. [DOI] [PubMed] [Google Scholar]
- 18.Hofmann HS, Neef H, Krohe K, Andreev P, Silber RE. Prognostic factors and survival after pulmonary resection of metastatic renal cell carcinoma. Eur Urol. 2005;48:77–81. doi: 10.1016/j.eururo.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Bruce DM, Heys SD, Eremin O. Lymphangitis carcinomatosa: a literature review. JR Coll Surg Edinb. 1996;41:7–13. [PubMed] [Google Scholar]
- 20.Reinke RT, Higgins CB, Niwayama G, Harris RH, Friedman PJ. Bilateral pulmonary hilar lymphadenopathy. An unusual manifestation of metastatic renal cell carcinoma. Radiology. 1976;121:49–53. doi: 10.1148/121.1.49. [DOI] [PubMed] [Google Scholar]
- 21.Nunez D, Jr, Gonzalez–Serva L, Galloway SJ. Pulmonary lymphangitic carcinomatosis in renal adenocarcinoma. Br J Radiol. 1977;50:142–3. doi: 10.1259/0007-1285-50-590-142. [DOI] [PubMed] [Google Scholar]
- 22.Vanclaire J, Bodart E, Schlesser P, Francotte N, Thiry G, Hainaut H. Pulmonary carcinomatous lymphangitis and renal adenocarcinoma [French] Arch Fr Pediatr. 1990;47:735–6. [PubMed] [Google Scholar]
- 23.Kirk JE, Kumaran M. Lymphangitis carcinomatosa as an unusual presentation of renal cell carcinoma: a case report. J Med Case Reports. 2008;2:19. doi: 10.1186/1752-1947-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]