Abstract
Alveolar epithelium is composed of alveolar epithelial cells of type I (AEC I) and type II (AEC II). AEC II secrete lung surfactant by means of exocytosis. P2X7 receptor (P2X7R), a P2 purinergic receptor, has been implicated in the regulation of synaptic transmission and inflammation. Here, we report that P2X7R, which is expressed in AEC I but not AEC II, is a novel mediator for the paracrine regulation of surfactant secretion in AEC II. In primary co-cultures of AEC I and AEC II benzoyl ATP (BzATP; an agonist of P2X7R) increased surfactant secretion, which was blocked by the P2X7R antagonist Brilliant Blue G. This effect was observed in AEC II co-cultured with human embryonic kidney HEK-293 cells stably expressing rat P2X7R, but not when co-cultured with AEC I in which P2X7R was knocked down or in co-cultures of AEC I and AEC II isolated from P2X7R−/− mice. BzATP-mediated secretion involved P2Y2 receptor signaling because it was reduced by the addition of the ATP scavengers apyrase and adenosine deaminase and the P2Y2 receptor antagonist suramin. However, the stimulation with BzATP might also release other substances that potentially increase surfactant secretion as a greater stimulation of secretion was observed in AEC II incubated with BzATP when co-cultured with E10 or HEK-293-P2X7R cells than with ATP alone. P2X7R−/− mice failed to increase surfactant secretion in response to hyperventilation, pointing to the physiological relevance of P2X7R in maintaining surfactant homeostasis in the lung. These results suggest that the activation of P2X7R increases surfactant secretion by releasing ATP from AEC I and subsequently stimulating P2Y2 receptors in AEC II.
Keywords: P2X7 receptor, Exocytosis, Cell–cell communication, Alveolar epithelial cells
Introduction
Pulmonary surfactant, consisting of lipids and surfactant proteins, is synthesized and secreted by alveolar epithelial type II cells (AEC II). Surfactant secretion occurs through the fusion of the limiting membrane of lamellar bodies with the apical plasma membrane, followed by the extrusion of the lamellar body contents into the alveolar space. Numerous surfactant secretagogues such as ATP, terbutaline, phorbol esters, 5′-N-ethylcarboxamidoadenosine (NECA), vasopressin and the Ca2+ ionophore calcimycin (A23187) augment surfactant secretion in cultured AEC II (Andreeva et al., 2007). These secretagogues initiate various signal transduction events. For example, the stimulation of β2-adrenoceptors by terbutaline generates cAMP and activates protein kinase A (PKA) (Dobbs and Mason, 1979), whereas the activation of purinergic P2Y2 receptors (P2Y2Rs) by ATP produces inositol trisphosphate and diacylglycerol and activates protein kinase C (PKC) (Chander et al., 1995; Gobran and Rooney, 1999; Linke et al., 1997).
Pulmonary alveoli are lined by thin flat squamous alveolar type I cells (AEC I) interspersed in the alveolar epithelium. These cells form a nexus with cuboidal AEC II. AEC I have traditionally been thought to play a role in effecting gaseous exchange. However, several new functions of AEC I have been previously uncovered, including their role in protecting the lung from injury (Chen et al., 2006) and the regulation of alveolar fluid homeostasis (Johnson et al., 2006).
Surfactant secretion alters with spatiotemporal Ca2+ changes and signaling (Haller et al., 2001). Intracellular Ca2+ perturbation in AEC I and AEC II allows the cells to communicate bipartitely with each other through Ca2+ wave propagation, by gap junctional intercellular communication or paracrine ATP release (Isakson et al., 2003; Patel et al., 2005). In situ fluorescence imaging has shown that interalveolar Ca2+ conductance initiates in AEC I and stimulates extrusion of lamellar bodies from AEC II (Ichimura et al., 2006). Moreover, Ca2+ oscillations from AEC I communicate with AEC II during lung inflation, suggesting a role for AEC I in surfactant secretion (Ashino et al., 2000). However, the mediators for AEC I and AEC II communication are unclear.
P2X7 receptor (P2X7R) belongs to the P2XR family. P2X7R has a significantly longer intracellular C-terminus where the last 177 amino acids are crucial for the formation of the non-selective pore (North, 2002). Unlike other P2XRs, P2X7R only forms homomultimers, is more potently inhibited by extracellular Ca2+ and/or Mg2+ (Baraldi et al., 2004) and has a very low sensitivity to ATP (Young et al., 2007). The activation of P2X7R leads to the opening of a cationic channel with significant permeability to Ca2+ and intracellular depolarization (Rassendren et al., 1997; Virginio et al., 1997). The cation channel can convert into a non-selective pore, which is permeable to small molecules and ions in the continued presence of ATP and low levels of bivalent cations (North, 2002). The activation of P2X7R also results in the release of various cytokines and signaling molecules. These include ATP released from astrocytes upon the propagation of Ca2+ transmission (Suadicani et al., 2006), interleukin (IL)-1β and matrix metalloproteinase-9 from immune cells as a pro-inflammatory response (Ferrari et al., 2006; Gu and Wiley, 2006), prostaglandin from bone cells during osteogenesis (Li et al., 2005) and the neurotransmitters γ-aminobutyric acid (GABA) and glutamate from the hippocampus (Sperlagh et al., 2002).
P2X7R is predominantly localized in immune cells, glial cells, hematopoietic cells and in various mammalian epithelial cells (Collo et al., 1997). P2X7R has been implicated in inflammation (Hughes et al., 2007), periosteal and cancellous bone formation (Ke et al., 2003), apoptosis (Placido et al., 2006), Alzheimer's disease (Parvathenani et al., 2003) and autoimmune encephalomyelitis (Chen and Brosnan, 2006). We have previously identified P2X7R as a specific marker of AEC I in the lung (Chen, Z. et al., 2004). However, the functional role of P2X7R in the lung remains unexplored. In the present study, using in vitro cell culture and in vivo P2X7R knockout (P2X7R−/−) mice, we demonstrate that, upon the activation of P2X7R, ATP is released from AEC I and modulates surfactant secretion in a paracrine manner through the P2Y2R and PKC-mediated pathway. Our findings reveal a novel function of P2X7R in alveolar epithelial cells and add a new layer to the complex regulation of lung surfactant secretion.
Results
Characterization of a heterocellular culture of AEC I and AEC II
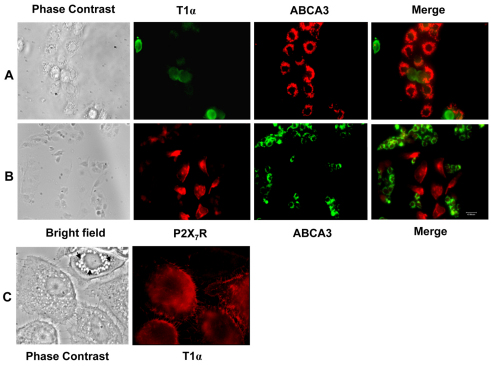
To study the communication of AEC I and AEC II, we first developed a new heterocellular culture of AEC I and AEC II. A mixed preparation of AEC I and AEC II was isolated from rat lungs on the basis of the method modified from the AEC I isolation (Chen, J. et al., 2004). The cell viability was ~97% and the cell yield was ~54×106 cells per rat. Immunophenotyping using specific cell markers revealed that the freshly isolated cells comprised ~31% AEC I and ~64% AEC II (Table 1). This ratio is similar to the AEC I and AEC II numbers in vivo. FACS analysis confirmed that 34% of AEC I existed in the mixed cell preparation. Macrophage contamination was ~3%. Overnight-cultured cells also showed a positive staining of T1α (podoplanin; an AEC I marker) and ATP-binding cassette sub-family A member 3 (ABCA3; an AEC II marker) (Fig. 1A). AEC I displayed the characteristic squamous morphology with the retracted accordion-like appearance of long cytoplasmic extensions (Fig. 1C). The ratio of AEC I and AEC II was essentially the same as that of the freshly isolated cells (Table 1). These results suggest that AEC I and AEC II maintained their phenotypes after the overnight culture. Double-labeling demonstrated that P2X7R was only present in AEC I, but not in AEC II (Fig. 1B), confirming the specific localization of P2X7R in AEC I of the lung (Chen, Z. et al., 2004).
Table 1.
Characterization of AEC I and AEC II preparations
Fig. 1.
Identification of AEC I and AEC II in heterocellular culture. (A) Overnight-cultured cells were double-labeled with polyclonal rabbit anti-T1α (AEC I marker, green) and monoclonal mouse anti-ABCA3 antibodies (AEC II marker, red). (B) Overnight-cultured cells were double-labeled with polyclonal rabbit anti-P2X7R (red) and monoclonal mouse anti-ABCA3 (green) antibodies. (C) Enlarged images showing the AEC I characteristics labeled with AEC-I-specific monoclonal mouse anti-T1α. Arrows indicate characteristic lamellar bodies of AEC II. Scale bars: 40 μm.
Activation of P2X7R in AEC I
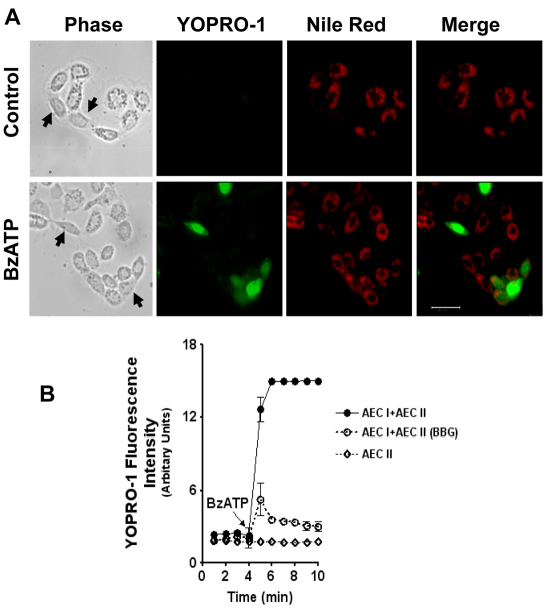
We next determined whether P2X7R could be activated in AEC I of the heterocellular culture of AEC I and AEC II. The activation of P2X7R leads to the opening of non-selective pores that are permeable to small molecules (<900 Da), including the dye YO-PRO-1; the accumulation of the monomeric cyanine nucleic acid chelating dye YO-PRO-1 has been used previously as an indicator of the P2X7R activation (Stokes et al., 2006). The overnight-cultured cells were incubated with YO-PRO-1 in the presence of 2′,3′-O-(4-benzoyl-benzoyl) ATP (BzATP), a specific agonist of P2X7Rs. Nile Red was used to identify AEC II (Chen, J. et al., 2004). As shown in Fig. 2A, the cells that did not have the Nile Red staining took up the dye, indicating the activation of P2X7Rs in AEC I. To quantify the dye uptake, the cells were incubated with YO-PRO-1 for 5 minutes, and YO-PRO-1 fluorescence was measured using a spectrofluorimeter. BzATP caused a rapid increase of the intensity in the heterocellular AEC I and AEC II preparation but had no effects on AEC II alone (Fig. 2B). Brilliant Blue G (BBG) inhibits rat P2X7R at 100 nM and does not affect other P2X receptors even at >10 μM (Jiang et al., 2000). The BzATP-mediated increase of the dye uptake was blocked by 100 nM BBG.
Fig. 2.
BzATP specifically increased YO-PRO-1 uptake in AEC I. (A) Overnight-cultured AEC I and AEC II were incubated with 5 μM YO-PRO-1 in the absence (control) or the presence of 200 μM BzATP for 20 minutes, followed by incubation with 10 μM Nile Red. The cells were fixed and examined with a fluorescence microscope. Arrows point to AEC I. Scale bar, 40 μm. (B) AEC I and AEC II mixture or AEC II alone were incubated with 5 μM YO-PRO-1 for 5 minutes in the absence or presence of 100 nM BBG. The basal line was recorded with an excitation wavelength of 491 nm and an emission wavelength of 509 nm, and 100 μM BzATP was added at 4 minutes. The fluorescence intensities were corrected to the level of cells without the dye. Data are means±s.e.m. for three independent cell preparations.
BzATP stimulates surfactant secretion in a heterocellular culture of AEC I and AEC II
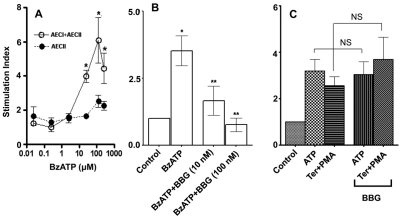
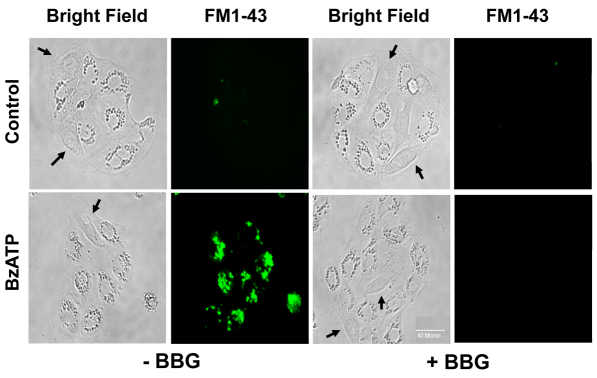
To determine the functional role of P2X7R in surfactant secretion, we stimulated the heterocellular culture of AEC I and AEC II with BzATP and assessed the changes in surfactant secretion. BzATP increased surfactant secretion in a dose-dependent manner, with an EC50 of 4.5±0.3 μM. However, BzATP had little effect when AEC II were seeded alone (Fig. 3A). BBG abolished the BzATP-mediated surfactant secretion (Fig. 3B). BBG was specific to P2X7R because BBG did not affect the surfactant secretion induced by other lung surfactant secretagogues, including ATP, terbutaline and phorbol 12-myristate 13-acetate (PMA) (Fig. 3C). The cell viability was unchanged by BzATP or BBG, as seen in a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay (data not shown). BzATP also increased the fusion pore formation, as determined by FM1-43 staining (Chintagari et al., 2006), which was blocked by BBG (Fig. 4). These results suggest that the activation of P2X7R in AEC I stimulates AEC II to release lung surfactant.
Fig. 3.
Effect of BzATP on surfactant secretion in a heterocellular culture. Freshly isolated AEC I and AEC II or AEC II alone were incubated with [3H]choline overnight. (A) The cells were stimulated with various concentrations of BzATP for 2 hours. (B) AEC I and AEC II were pretreated for 0.5 hour with 10 nM or 100 nM BBG and then incubated with 25 μM BzATP for 2 hours. (C) AEC I and AEC II were pretreated with 100 nM BBG for 30 minutes and then stimulated with 1 mM ATP or 0.1 μM terbutaline plus 1 μM PMA (Ter+PMA) for 2 hours. Surfactant secretion was measured by monitoring the release of [3H]-labeled phosphatidylcholine. The results are expressed as a stimulation index (a ratio of surfactant secretion in stimulated cells over those in unstimulated cells). Data shown are means+s.e.m. In A *P<0.01 compared with unstimulated cells as determined using one-way ANOVA and Tukey's multiple comparison (n=3–7). In B, *P<0.0001 compared with control; **P<0.0001 compared with BzATP stimulation as determined using a Student's t-test (n=5). In C, differences are not significant (NS), P>0.05 (n=3–5).
Fig. 4.
BzATP increases fusion pore formation. AEC I and AEC II were pretreated with 100 nM BBG for 30 minutes and then stimulated with 25 μM BzATP in the presence of 4 μM FM1-43 dye for 1 hour. Cells were washed twice with PBS and fixed for fluorescence microscopy. Arrows point to AEC I. Scale bar: 40 μm.
Co-culture of an AEC-I-like cell line containing P2X7R with AEC II increases surfactant secretion
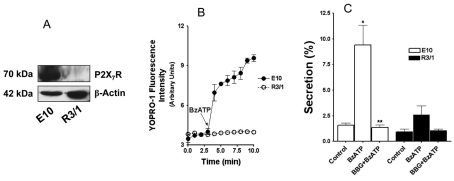
P2X7R is highly expressed in the lung epithelial cell line E10 but not in the R3/1 cell line (Barth et al., 2007). Both cell lines have properties similar to those of AEC I (Kathuria et al., 2007; Koslowski et al., 2004). We confirmed that E10 cells had a very high expression of P2X7R and that R3/1 cells expressed little P2X7R (Fig. 5A). BzATP increased YO-PRO-1 dye uptake in E10 cells but not in R3/1 cells, indicating that P2X7R in E10 cells is functional (Fig. 5B). We then co-cultured isolated AEC II with E10 or R3/1 cells in a ratio of 2:1 and examined the effects of the activation of P2X7R on surfactant secretion in this co-culture system. Upon BzATP stimulation, a significant increase in surfactant secretion from AEC II co-cultured with E10 cells was observed (Fig. 5C). This increase in secretion was blocked when the cells were pretreated with BBG. There was only a slight increase of surfactant secretion in the BzATP-stimulated AEC II and R3/1 co-culture.
Fig. 5.
E10 cells expressing endogenous P2X7R increase surfactant secretion from AEC II upon BzATP stimulation. (A) Expression of endogenous P2X7R in E10 and R3/1 cells. Total cell lysates (30 μg) were probed with anti-P2X7R and anti-β-actin antibodies. (B) Accumulation of YO-PRO-1 dye. E10 and R3/1 cells were incubated with YO-PRO-1 for 5 minutes and fluorescence was monitored. BzATP (200 μM) was added to start the reaction. Data are means±s.e.m. (n=2). (C) AEC II were co-cultured with E10 or R3/1 cells overnight. The cells were then pretreated with 100 nM BBG for 30 minutes and stimulated with 25 μM BzATP for 2 hours and phosphatidylcholine secretion was measured. The results were corrected by subtracting the secretion from E10 or R3/1 cells alone. Data are means+s.e.m. for three independent cell preparations. *P<0.001 compared with control; **P<0.01 compared with BzATP stimulation.
Co-culture of HEK-293 cells stably expressing P2X7R with AEC II increases surfactant secretion
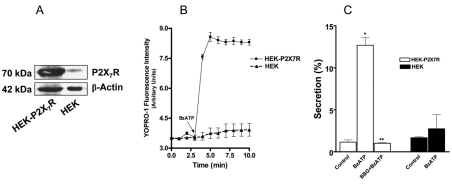
If P2X7R is the mediator for the communication between AEC I and AEC II, any cells that express a functional P2X7R should be able to replace AEC I. We thus utilized human embryonic kidney HEK-293 cells stably expressing rat P2X7R (HEK-P2X7R) (Jiang et al., 2000) for the co-culture experiment. HEK-293 cells were used as a control. Western blot analysis showed that HEK-P2X7R expressed a much higher level of P2X7R than did HEK control cells (Fig. 6A). The ectopically expressed P2X7R was functional given that BzATP markedly increased YO-PRO-1 uptake in HEK-P2X7R in comparison with HEK cells (Fig. 6B). In the co-culture of HEK-P2X7R and AEC II, BzATP dramatically increased surfactant secretion (Fig. 6C). This effect was abolished by BBG. No significant stimulation of surfactant secretion was observed in the co-culture of HEK-293 cells and AEC II.
Fig. 6.
HEK-293 cells stably expressing rat P2X7R increase surfactant secretion from AEC II upon BzATP stimulation. (A) Expression of recombinant rat P2X7R in HEK-P2X7R and HEK cells. Cell lysates (5 μg) were probed with anti-P2X7R and anti-β-actin antibodies. (B) YO-PRO-1 accumulation. HEK-P2X7R and HEK-293 (HEK) cells were incubated with YO-PRO-1 for 5 minutes and the YO-PRO-1 fluorescence was monitored. BzATP (200 μM) was added after 200 seconds. Data are means±s.e.m. (n=2). (C) Surfactant secretion. AEC II were co-cultured with HEK-P2X7R or HEK-293 cells (at a 2:1 ratio) overnight. The cells were pretreated with 100 nM BBG for 30 minutes and stimulated with 25 μM BzATP for 2 hours. Phosphatidylcholine secretion was measured and corrected by subtracting the secretion from HEK-P2X7R or HEK-293 cells alone. Data are means+s.e.m. for three independent cell preparations. *P<0.001 compared with control; **P<0.01 compared with BzATP stimulation.
P2X7R-evoked AEC communication is a paracrine phenomenon
To determine whether the P2X7R-mediated increase in surfactant secretion is mediated through a paracrine stimulation or direct cell–cell contact, we examined the effects of conditioned medium from the BzATP-stimulated E10 or HEK-P2X7R cells on surfactant secretion in AEC II. E10 or HEK-P2X7R cells were incubated with BzATP and the ecto-ATPase inhibitor ARL-67156 for 2 hours. The conditioned medium was then transferred to the recipient AEC II. The conditioned medium from both E10 and HEK-P2X7R dramatically increased surfactant secretion by 9.5- and 7.2-fold, respectively (Fig. 7A). Under the same conditions, AEC-II- or HEK-293-conditioned medium showed no significant increases in surfactant secretion from AEC II.
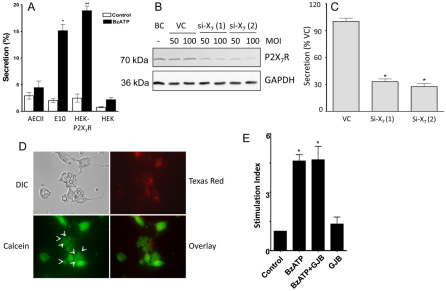
Fig. 7.
Effects of conditioned media from P2X7R-containing cells on surfactant secretion from AEC II. (A) AEC II, E10 cells, HEK-P2X7R or HEK-293 (HEK) cells were incubated in the presence or absence of 25 μM BzATP for 2 hours. The conditioned media were collected and transferred to the recipient AEC II and incubated for an additional 2.5 hours and phosphatidylcholine secretion was measured. Data are means+s.e.m. for three independent experiments. *P<0.001 compared with unstimulated E10 control cells, **P<0.01 compared with HEK-P2X7R unstimulated control cells. (B) E10 cells were transduced with an adenovirus carrying irrelevant shRNA (VC) or shRNA targeting 574–592 [si-X7 (1)] or 669–680 [si-X7 (2)] of mouse P2X7R at an MOI of 50 or 100. Untreated cells were used as a blank control (BC). After 4 days, the cells were collected for western blot analysis. (C) VC, si-X7 (1)- or si-X7 (2)-treated E10 cells (4 days, MOI of 100) were incubated with 25 μM BzATP for 2 hours. The conditioned medium was added to AEC II cells and after 2.5 hours surfactant secretion was measured. *P<0.001 compared with VC (n=3). (D) Preloading assay for dye transfer. E10 cells were preloaded with Texas Red Dextran and calcein-AM and then co-cultured with freshly isolated AEC II for 5 hours. The donor E10 cells showed fluorescence for both Texas Red and calcein. The arrows indicate the acceptor AEC II, which only show calcein fluorescence, indicating the formation of functional gap junctions between E10 cells and AEC II. (E) AEC II and E10 cells were co-cultured (at a 2:1 ratio) for 16 hours and incubated with the gap junction blocker (GJB), 18-α-glycerrhitinic acid (40 μM) for 30 minutes. Cells were further stimulated with 25 μM BzATP for an additional 2 hours. Surfactant secretion was measured by monitoring the release of [3H]-labeled phosphatidylcholine. The results are expressed as a stimulation index (a ratio of surfactant secretion in stimulated cells over those in unstimulated cells). *P<0.001 compared with control (n=3).
To demonstrate further that the observed effects are due to P2X7Rs, we determined the effects of the conditioned medium from the P2X7R-knocked-down E10 cells on surfactant secretion from AEC II. E10 cells were transduced with an adenovirus carrying short hairpin RNA (shRNA) targeting the mouse P2X7R gene, 574–592 [si-X7 (1)] and 669–689 [si-X7 (2)] (nucleotides relative to the transcription start site). Both shRNAs resulted in reduction of P2X7R protein after a 4-day transduction (Fig. 7B). The stimulatory effects on surfactant secretion by the conditioned medium from the P2X7R-knocked-down E10 cells decreased significantly in comparison with that from control virus-treated cells (Fig. 7C).
To address further the possible gap junction contribution to P2X7R-mediated surfactant secretion, we co-cultured freshly isolated AEC II cells and E10 cells in a ratio of 2:1. Using a preloading assay (Abraham et al., 2001), we observed a gap junction formation between E10 cells and type II cells (Fig. 7D). BzATP stimulated surfactant secretion in this co-culture system. However, the gap junction blocker β-glycyrrhetinic acid had no effect on the BzATP-stimulated secretion (Fig. 7E).
ATP is responsible for the P2X7R-mediated surfactant secretion
The activation of P2X7R results in ATP release from rat astrocytes (Suadicani et al., 2006). We therefore examined whether ATP is released from AEC I upon P2X7R activation of AEC I and AEC II cultures. BzATP was able to evoke a robust induction of ATP release in the heterocellular culture of AEC I and AEC II (Fig. 8A). This release was blocked by preincubating the cells with BBG. BzATP did not cause any changes in ATP levels in bulk medium of the AEC II monoculture. The bulk concentration of ATP in medium was measured from 1×106 cells cultured overnight in a 1 ml total volume of medium. After normalization to total cell proteins, we obtained 89 and 197 nM ATP per mg of protein for control and BzATP-stimulated cells, respectively. The average total protein recovered was about 166 μg. Thus, ATP concentrations were in the range of 15 to 33 nM in the medium of each well. These numbers are comparable with those published in a previous report, in which ATP release from stretched type-I-like cells, transdifferentiated from type II cells, was measured (Patel et al., 2005). It should be noted that ATP concentrations on the cell surface are orders of magnitude higher than that in the bulk medium (Beigi et al., 1999; Pellegatti et al., 2005). Furthermore, ATP release from cells is restricted to point-source burst (Arcuino et al., 2002). With consideration to the direct contact of type I and type II cells, ATP might be released at the regions of type I cells that are close to type II cells. In this way, the ATP degradation and the travel distance to type II cells could be reduced. All of the above favor a higher concentration of ATP on the cell surface of the alveolar epithelium and better communication between type I and type II cells through ATP.
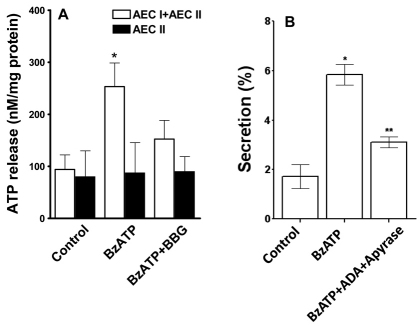
Fig. 8.
P2X7R evokes surfactant secretion through ATP release. (A) Effect of P2X7R modulation on ATP release. AEC I and AEC II (1×106) in 1 ml of medium were pre-incubated with 100 nM BBG for 30 minutes and stimulated with 25 μM BzATP for 5 minutes. The BzATP was removed and 1 ml of fresh medium was added and the cells were incubated for an additional 10 minutes. At the end of the incubation, the medium was collected and ATP was measured. The ATP concentrations ranged from 15 to 33 nM in each well. The results were normalized to the total protein in each well. Data are means+s.e.m. for three independent cell preparations. *P<0.05 compared with control. (B) Effect of the removal of ATP on surfactant secretion. AEC I and AEC II were incubated with adenosine deaminase (ADA, 5 units/ml) and apyrase (10 units/ml) for 30 minutes, followed by stimulation with 25 μM ATP for 2 hours. Surfactant secretion was measured by monitoring the release of [3H]-labeled phosphatidylcholine. Data are means±s.e.m. (n=3–4). *P<0.001 compared with control; **P<0.01 compared with BzATP stimulation (as determined using Student's t-tests).
To determine whether ATP released from AEC I is responsible for the BzATP-mediated surfactant secretion, we used the nucleotide scavengers apyrase and adenosine deaminase to remove ATP, and measured surfactant secretion in the AEC I and AEC II heterocellular culture. Surfactant secretion caused by BzATP was reduced by apyrase and adenosine deaminase treatment (Fig. 8B). These results collectively indicate that P2X7R activation releases ATP from AEC I, which in turn acts on AEC II to stimulate surfactant secretion.
P2X7R-mediated surfactant secretion is via the P2Y2R signaling
Given that the activation of P2X7R releases ATP, we determined whether BzATP-mediated surfactant secretion is due to the activation of the P2Y2R signaling. We measured surfactant secretion after blocking the P2Y2R signaling in the AEC I and AEC II heterocellular culture with suramin (a P2Y2R antagonist), BAPTA-AM [1,2-bis-(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetra(acetoxymethyl); an intracellular Ca2+ chelator] and staurosporine (a PKC inhibitor). As shown in Fig. 9, the BzATP-evoked surfactant secretion was significantly inhibited by all of the agents. However, no significant decrease in secretion was observed in the presence of the PKA inhibitor H89. Taken together, these observations suggest that ATP released from AEC I acts through P2Y2R and a PKC-dependent pathway to promote surfactant secretion in AEC II.
Fig. 9.
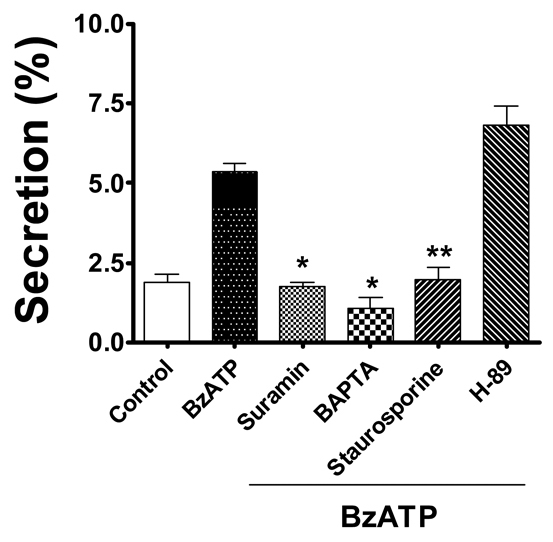
Paracrine control of surfactant secretion is mediated through the P2Y2R and PKC signaling pathway. Overnight-cultured AEC I and AEC II were pretreated with suramin (100 μM), BAPTA-AM (50 μM), staurosporine (100 nM) or H-89 (50 nM) for 30 minutes, followed by stimulation with BzATP (25 μM). Phosphatidylcholine secretion was measured. Data are means+s.e.m. for three or four independent experiments. **P<0.001 compared with control; *P<0.001 compared with BzATP.
BzATP was not able to stimulate surfactant secretion in a heterocellular culture of AEC I and AEC II from P2X7R−/− mice
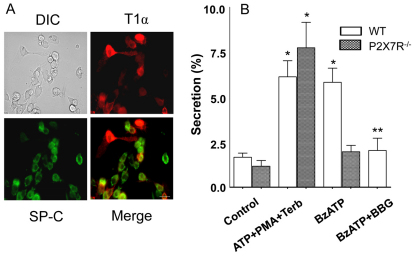
We isolated mouse AEC I and AEC II from C57BL/6 mice. The yield was 8.2±1.3×106 cells per mouse. Double labeling with antibodies against surfactant protein C (SP-C) and T1α (Fig. 10A) revealed 57±1% AEC II and 29±1% AEC I (n=5). Yield, purity and the AEC I:AEC II ratio were not significantly different between wild-type and P2X7R−/− mice. The known lung surfactant secretagogues (ATP, PMA and terbutaline) increased surfactant secretion by approximately threefold in the AEC I and AEC II from wild-type mice (Fig. 10B), which was higher than in the isolated mouse AEC II alone (Gobran and Rooney, 2004; Guttentag et al., 2005; Rice et al., 2002). BzATP also stimulated surfactant secretion, and BBG blocked the BzATP effects, in the wild-type mouse cells. Lung surfactant secretagogues markedly increased surfactant secretion from the P2X7R−/− AEC I and AEC II heteroculture, indicating that AEC II responds to lung surfactant secretagogues normally. However, the AEC I and AEC II heteroculture from P2X7R−/− mice failed to respond to BzATP in surfactant secretion. These results further support our hypothesis that P2X7R is a mediator for AEC I and AEC II communication in the regulation of surfactant secretion.
Fig. 10.
Effect of BzATP on surfactant secretion in AEC I and AEC II heteroculture from wild-type and P2X7R−/− mice. (A) Wild-type mouse AEC I and AEC II cells were double-labeled with rabbit anti-SP-C (type II cell, green) and hamster anti-T1α (type I cells, red) antibodies. Scale bar: 40 μm. (B) AEC I and AEC II from wild-type (WT) or P2X7R−/− mice were cultured overnight and stimulated for 2 hours with lung surfactant secretagogues (100 μM ATP, 0.1 μM PMA and 10 μM terbutaline; ATP+PMA+Terb) or 25 μM BzATP with or without 100 nM BBG. Phosphatidylcholine secretion was measured. Data shown are means+s.e.m. for three independent cell preparations. *P<0.001 compared with control; **P<0.001 compared with BzATP stimulation.
Hyperventilation increases surfactant secretion in wild-type mice but not in P2X7R−/− mice
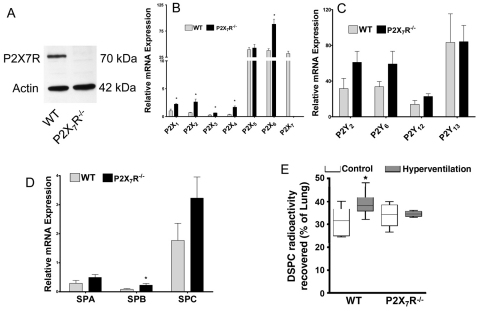
Hyperventilation has been shown to increase surfactant secretion (Hildebran et al., 1981; Nicholas and Barr, 1983; Oyarzun and Clements, 1978), possibly through mechanical stretch (Wirtz and Dobbs, 1990). Given that P2X7R interacts with the proteins that are associated with mechanotransduction (Kim et al., 2001; Pavalko et al., 1998; von Wichert et al., 2003), we investigated potential defects in the response of P2X7R−/− mice to hyperventilation. Western blot analysis confirmed the absence of P2X7R in the lungs of P2X7R−/− mice (Fig. 11A). Real-time PCR analysis revealed that the relative mRNA expression of the P2X receptors P2X2 and P2X4 was increased severalfold in P2X7R−/− mice in comparison with that of wild-type mice (Fig. 11B), whereas that for the P2X1, P2X3 and P2X6 receptors was only modestly increased. No changes were observed for the P2X5 receptors. P2Y receptors were not significantly affected by P2X7R knockout (Fig. 11C). Although surfactant protein B (SP-B) mRNA expression increased about twofold in the P2X7R−/− mice, SP-A and SP-C were not changed (Fig. 11D). T1α, a type I cell marker showed no changes between wild-type and P2X7R−/− mice (data not shown).
Fig. 11.
Comparison of surfactant secretion in P2X7R−/− and wild-type mice. (A) Western blot of lung tissues from wild-type (WT) and P2X7R−/− mice. (B–D) Real-time PCR analysis of P2X receptors (B), P2Y receptors (C) and surfactant proteins (D). Data were normalized to 18S rRNA. *P<0.05 (n=3 animals, assayed in duplicate). (E) Surfactant secretion. Mice were intraperitoneally injected with 0.5 μCi/g [3H]choline and hyperventilated for 30 minutes. Lipids in lavage and lung tissue were extracted and saturated phosphatidylcholine isolated. Surfactant secretion was calculated as a percentage (d.p.m. in lavage over d.p.m. in lavage and lung tissue). *P<0.05. Animal number: wild-type, 10; wild-type with hyperventilation, 8; P2X7R−/−, 5; and P2X7R−/− with hyperventilation, 4.
We also compared surfactant lipid secretion in the whole animal between wild-type and P2X7R−/− mice according to the method of Ikegami and colleagues (Ikegami et al., 2000). There was no significant difference in basal surfactant secretion between wild-type and P2X7R−/− mice. However, hyperventilation increased surfactant secretion in wild-type mice but not in P2X7R−/− mice (Fig. 11E). No significant changes were observed in total cell counts or total proteins in lavage under all of the conditions (data not shown), indicating that no lung injury had occurred. Note that the secretions shown in Fig. 11E are not net secretions, but the percentages of saturated phosphatidylcholine in lavage over the total saturated phosphatidylcholine in lavage plus lung tissue (after 16 hours of [3H]choline labeling and following a 30-minute hyperventilation if indicated). The percentages of secretion in wild-type mice without and with hyperventilation were 31% and 38%, respectively. This is comparable with a previous report (Ikegami et al., 2000). Thus, net secretion should be less than 7% even under a 30-minute hyperventilation condition.
Hyperventilation causes mechanical stretch and alkalosis. To determine whether there is a difference in alkalosis between wild-type and P2X7R−/− mice, we performed blood gas analysis. As shown in Table 2, hyperventilation did not cause significant changes in arterial blood oxygen (PaO2) levels in wild-type and P2X7R−/− mice. However, hyperventilation increased arterial blood pH and decreased arterial blood CO2 (PaCO2) in both wild-type and P2X7R−/− mice. These results indicate that there were no significant differences in hyperventilation-induced alkalosis between wild-type and P2X7R−/− mice.
Table 2.
Arterial blood gas analysis
Discussion
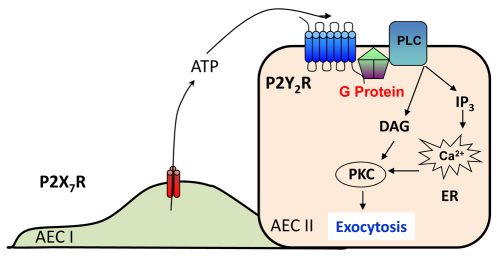
It has been suggested that AEC I contribute to the regulation of surfactant secretion in AEC II (Ashino et al., 2000; Isakson et al., 2003; Patel et al., 2005). However, the factors in AEC I that mediate AEC I and AEC II communication are unknown. Here, we report that P2X7R in AEC I is responsible for the communication between AEC I and AEC II during the regulation of surfactant secretion. The results from the present study show that, upon P2X7R activation, AEC I releases ATP, which acts on AEC II in a paracrine manner to trigger surfactant release though the P2Y2R and PKC pathway (Fig. 12).
Fig. 12.
A proposed model for P2X7R-mediated surfactant secretion. DAG, diaclyglycerol; ER, endoplasmic reticulum; IP3, inositol trisphosphate; PLC, phospholipase C.
We depend upon cell culture models to analyze the functions of AEC I. Owing to the difficulty in isolating AEC I in high purity and yield, AEC-I-like cells transdifferentiated from freshly isolated AEC II have been used previously to study the communication between AEC I and AEC II (Isakson et al., 2003; Patel et al., 2005). These AEC-I-like cells might not represent the in vivo AEC I phenotype as DNA microarray analysis reveals different sets of gene expression between isolated AEC I and the AEC-I-like cells (Gonzalez et al., 2005). In the present study, we developed a heterocellular culture of AEC I and AEC II directly isolated from rat and mouse lungs. This system has a 1:2 ratio of AEC I and AEC II numbers that is similar to that of lungs in vivo. Furthermore, the model maintains AEC I and AEC II phenotypes, including cell marker expression, functional receptors (P2X7R and P2Y2R) and lung surfactant secretion.
Several lines of evidence support the model that the activation of P2X7R couples AEC I and AEC II during the regulation of surfactant secretion. First, BzATP, an agonist of P2X7R, increased surfactant secretion and formation of the fusion pore in a heterocellular culture of AEC I and AEC II. The increase in secretion is due to the specific activation of P2X7R in AEC I because: (1) P2X7R is specifically expressed in AEC I but not in AEC II (Chen, Z. et al., 2004); (2) BzATP specifically activated P2X7R in AEC I, as determined by the YO-PRO-1 dye uptake; (3) BzATP did not affect surfactant secretion in AEC II alone; (4) BBG, an antagonist of P2X7R, blocked the BzATP-induced surfactant secretion (the specificity of BBG was demonstrated by its failure to inhibit surfactant secretion stimulated by other lung surfactant secretagogues in the heterocellular culture of AEC I and AEC II); and (5) BzATP had no effects on surfactant secretion from AEC I and AEC II isolated from P2X7 R−/− mice.
Another line of evidence favoring P2X7R as a mediator for the increase in surfactant secretion comes from co-culture of P2X7R-expressing cells with AEC II. Two lung cell lines, E10 and R3/1, have AEC-I-like properties (Kathuria et al., 2007; Koslowski et al., 2004). However, E10 cells express a high level of P2X7R, whereas R3/1 cells have little P2X7R (Barth et al., 2007). Co-culture of E10 cells with AEC II results in a robust increase in surfactant secretion in response to BzATP stimulation. This increase in secretion was not observed when R3/1 cells were co-cultured with AEC II. E10 and R3/1 cells were established from a clonal outgrowth of adult mouse lung tissue and an explant-replica of the E20 fetal lungs of Han–Wistar rats, respectively. It is possible that the observed differences between E10 and R3/1 cells are due to other properties of the cell lines rather than P2X7R. To eliminate this possibility, rat P2X7R was overexpressed in HEK-293 cells, which do not express endogenous P2X7R and are not related to AEC I. The HEK-293 cells stably expressing P2X7R, but not the control HEK-293 cells, when co-cultured with AEC II, elicited a marked increase in surfactant secretion upon stimulation with BzATP. Furthermore, knockdown of P2X7R in E10 cells abolished its stimulatory effects on surfactant secretion. These results suggest that P2X7R is one of the key components in AEC I that regulates surfactant secretion.
Communication between AEC I and AEC II can be accomplished by means of two major routes: gap junction and paracrine communication (Koval, 2002). What are the mechanisms for P2X7R-mediated AEC I and AEC II communication? Cell-to-cell contact and/or gap junction communication as a major mechanism is unlikely given that the gap junction blocker β-glycyrrhetinic acid did not block BzATP-stimulated surfactant secretion. Furthermore, the conditioned medium from BzATP-stimulated E10 or HEK-P2X7R cells showed that there was a stimulation of surfactant secretion comparable with that seen during the co-culture of these cells with AEC II. Thus, the release of soluble factors from AEC I upon the activation of P2X7R is probably responsible for AEC I and AEC II communication. One such factor is ATP. Indeed, P2X7R activation in the heterocellular culture of AEC I and AEC II caused release of ATP into the bulk milieu, which was blocked by BBG. This effect was completely absent in the AEC II monoculture. The removal of ATP from the medium significantly inhibited the BzATP-stimulated surfactant secretion, further supporting the hypothesis that ATP released from AEC I, upon the activation of P2X7R, functions as a soluble mediator to enhance surfactant secretion. There is evidence showing that P2X7R mediates ATP release in other cell systems (Arcuino et al., 2002; Pellegatti et al., 2005; Suadicani et al., 2006). However, given BzATP-stimulated surfactant secretion was higher than ATP-stimulated secretion in AEC I and AEC II co-culture, it is possible that, in addition to ATP, other soluble factors such as ATP metabolites (ADP, AMP or adenosine) might contribute to P2X7R-mediated secretion. It is also possible that the stimulation of P2X7R with BzATP might release other substances that potentially increase surfactant secretion.
Hypotonic swelling and the activation of protease-activated receptor 3 (PAR-3, also known as F2RL2) by thrombin is also known to result in the release of ATP from the lung epithelial type II cell line A549 in a Ca2+-dependent manner (Tatur et al., 2007; Seminario-Vidal et al., 2009). Therefore, it is possible that, under certain conditions, AEC II might release ATP and stimulate surfactant secretion in an autocrine manner. However, the release of ATP from AEC II is not due to P2X7R, as these cells do not contain P2X7R. The relative importance of ATP release from AEC I compared with other lung cells in vivo remains to be determined.
Regulated surfactant secretion in AEC II responds to extracellular ATP stimulation through P2Y2R (Linke et al., 1997). The activation of P2Y2R in AEC II stimulates G-protein-coupled phospholipase C, resulting in the generation of diacylglycerol and Ins(1,4,5)P3, and the activation of PKC, in a Ca2+-dependent manner (Chander et al., 1995). AEC II express several PKC isoforms (α, β1, β2, η, δ, μ and ξ) that potentiate protein phosphorylation and final fusion events (Gobran and Rooney, 1999). The blocking of P2Y2R by suramin, chelation of intracellular Ca2+ by BAPTA and inhibition of PKC by staurosporine all abolished the BzATP-evoked increase in surfactant secretion in the heterocellular culture of AEC I and AEC II. ATP can be degraded into adenosine (Fields and Burnstock, 2006), which can stimulate adenosine A2 receptors, activate PKA and increase cAMP levels in the cells (Griese et al., 1991). However, the increase in surfactant secretion caused by BzATP was unaffected by the PKA inhibitor H89, indicating that the PKA pathway is not involved in the P2X7R-mediated surfactant secretion. One of the possibilities is that ATP release from cells is restricted to a point-source burst (Arcuino et al., 2002), which prevents the production of its metabolites, including adenosine, that act on other receptors.
The P2X7R−/− mice are viable and fertile (Solle et al., 2001). However, some defects have been observed previously. For example, although peritoneal macrophages from P2X7R−/− and wild-type mice produce similar amounts of pro-IL-1β after lipopolysaccharide (LPS) challenge, the macrophages from P2X7R−/− mice do not generate mature IL-1β in response to ATP (Solle et al., 2001). These mice also have a decreased periosteal bone formation and an increased resorption in trabecular bone tissue (Ke et al., 2003). The lack of P2X7R results in a reduction of mechanical loading-stimulated bone growth (Li et al., 2005). However, no information on the lung phenotype is available in these mice. In the present study, we have shown that BzATP did not stimulate surfactant secretion in AEC I and AEC II isolated from P2X7R−/− mice. Furthermore, when they were challenged with hyperventilation, wild-type mice showed an enhanced surfactant secretion but P2X7R−/− mice did not. Hyperventilation is known to increase surfactant secretion (Hildebran et al., 1981; Nicholas and Barr, 1983; Oyarzun and Clements, 1978), probably through mechanical stretch (Wirtz and Dobbs, 1990), and P2X7R is known to be involved in mechanotransduction (Kim et al., 2001; Pavalko et al., 1998; von Wichert et al., 2003). Thus, the lack of response to hyperventilation in P2X7R−/− mice might be due to a defect in the sensing of mechanical stretch in AEC I via P2X7R. We attempted to measure ATP concentrations in the broncho alveolar lavage fluid (BALF) samples from mice. Unfortunately, the variability among animals, even in control mice, prevents us from drawing any conclusions. The technical difficulty is probably due to degradation of ATP, even though we included ARL67156 (an ecto-ATPase inhibitor) in the lavage buffer. Another possible reason is that lavage itself might induce ATP release.
In addition to P2X7R, expression of P2X4R has been reported in AEC I and also in bronchial epithelial cells (Leipziger, 2003; Qiao et al., 2003). P2X4R has been proposed to be involved in Cl− secretion in human airway epithelium (Leipziger, 2003); however, its functions in the lung are largely unknown. P2X4R and P2X7R form a complex with caveolin-1 in a type I cell line (Weinhold et al., 2010). There is also evidence of P2X4/7 heteromers in other systems (Casas-Pruneda et al., 2009). Interestingly, we found an increased expression of P2X4R in P2X7R−/− mouse lungs compared with that in wild-type controls. Further studies are warranted to address whether P2X4R modulates P2X7R functions in AEC I.
In conclusion, our results demonstrate that the activation of P2X7R, possibly by mechanical stress, releases ATP from AEC I and stimulates surfactant secretion, in a paracrine manner, from AEC II through the P2Y2R and PKC-mediated signaling pathway (Fig. 12). This finding reveals a novel function of P2X7R in the lung by which AEC I communicates with AEC II via P2X7R, contributing to the fine-tuning of surfactant balance. The present study also reinforces the idea that AEC I are not a simple barrier for gas exchange, but are functional cells in the lung.
Materials and Methods
Reagents
2′,3′-O-(4-benzoyl-benzoyl) ATP (BzATP), Brilliant Blue G (BBG), adenosine deaminase (ADA), apyrase, suramin, staurosporine, ARL67156, the ATP bioluminescent assay kit (FLAA), polyclonal rabbit anti-P2X7R, anti-P2X7R (extracellular)–FITC and anti-podoplanin (anti-T1α) antibodies were obtained from Sigma. Elastase was from Worthington Biochemical (Lakewood, NJ). Mouse anti-LB-180 (ABCA3) antibody was from Covance (Berkeley, CA). Alexa-Fluor-546 and -488-conjugated anti-rabbit and anti-mouse secondary antibodies were from Molecular Probes (Eugene, OR). Cy3-conjugated Affini-pure goat anti-(mouse IgG) and anti-(hamster IgG) were from Jackson ImmunoResearch Laboratories (West Grove, PA). The Dc protein assay kit was from Bio-Rad (Hercules, CA). The enhanced chemiluminescence detection system was from Amersham Biosciences (Piscataway, NJ). Anti-(rat IgG)- and anti-(mouse IgG)-conjugated magnetic beads were from Dynal Biotech (Lake Success, NY). Mouse anti-T1α antibody was a generous gift from Mary Williams (Boston University, Boston, MA). Mouse anti-CD45 (anti-leukocyte common antigen or anti-LC) antibody was from BD Biosciences (Franklin Lakes, NJ). YO-PRO-1 dye was from Invitrogen (Carlsbad, CA). Human embryonic kidney HEK-293 cells stably expressing rat P2X7R (HEK-P2X7R), E10 cells and R3/1 cells were kindly provided by Annmarie Surprenant (University of Manchester, Manchester, UK), Mary Williams and Roland Koslowski (Dresden University of Technology, Dresden, Germany).
Isolation of rat AEC I and AEC II
Rat AEC I and AEC II were isolated from the lungs of pathogen-free male Sprague–Dawley rats (150–200 g) by modifying our previous method for isolating AEC I (Chen, J. et al., 2004), in which the elastase concentration was increased from 4.5 units/ml to 8 units/ml. The Oklahoma State University Animal Care and Use Committee approved all the animal procedures used in the present study. Rats were anesthetized with an intraperitoneal injection of ketamine (40 mg/kg of body weight) and xylazine (8 mg/kg of body weight). A tracheotomy was performed and rats were ventilated with a rodent ventilator throughout the process of perfusion. The rats were exsanguinated by abdominal aorta and heart transection. A catheter was placed in the pulmonary artery and the lungs were perfused with solution II (10 mM HEPES, pH 7.4, 0.9% NaCl, 0.1% glucose, 5 mM KCl, 1.3 mM MgSO4, 1.7 mM CaCl2, 0.1 mg/ml streptomycin sulfate, 0.06 mg/ml penicillin G, 3 mM Na2HPO4 and 3 mM NaH2PO4), followed by instilling solution I (solution II plus 0.06 mg/ml EGTA) through the trachea. Lungs were removed and lavaged with solution I. The lungs were then digested by instilling warm elastase three times (8 units/ml final concentration in 7 ml of solution II) for 20 min each at 37°C to release AEC I and AEC II. The lung tissue was chopped and the cell suspension was mixed with 100 μg/ml DNase I and incubated for 5 minutes at 37°C with gentle rotation. The cell suspension was then filtered through 160- and 37-μm gauge nylon meshes once, followed by a 15-μm gauge nylon mesh twice. The cells were incubated in rat IgG-coated 100-mm-diameter polystyrene bacteriological Petri dishes (3 mg of IgG per dish for each rat) at 37°C for 45 minutes. The unattached cells were centrifuged at 250 g for 10 minutes and resuspended with 1 ml of solution III [RPMI 1640 medium containing 25 mM HEPES and 1% fetal bovine serum (FBS)].
To remove further the remaining leukocytes, the cells were resuspended in 0.5 ml of solution III and incubated with rat IgG (1 mg/ml) and anti-LC (16 μg/ml) at 4°C for 20 minutes with gentle rotation. After being washed with solution III twice, the cells were incubated with sheep anti-(rat IgG) (150 μl per rat) and goat anti-(mouse IgG) (150 μl per rat) Dynabeads at 4°C for 15 minutes. A magnetic field was applied to remove the cells attached to the magnetic beads. Finally, the cells were harvested and resuspended in Dulbecco's modified Eagle's medium (DMEM) containing 10% FBS, 0.1 mM nonessential amino acids, 1000 units/ml penicillin G and 100 μg/ml streptomycin, and cultured overnight for further experiments. The viabilities of the cell preparations were determined by the Trypan Blue dye exclusion method.
Isolation of mouse AEC I and AEC II
Mouse AEC I and AEC II were isolated from male C57BL/6 mice (6–8 weeks of age) according to the previously reported procedure, with some modification (Bortnick et al., 2003). Mice were anesthetized with ketamine (80 mg/kg of body weight) and xylazine (10 mg/kg of body weight). The abdominal cavity was opened, exsanguinated and cannulated with a 20-gauge luer stub adapter through the intratracheal route. Lungs were perfused with solution II, followed by instilling 1 ml of the digestion cocktail (dispase, 2500 caseinolytic units/ml, and 4 units/ml elastase in solution II) directly through the trachea. Three lungs were isolated, pooled into a beaker containing ~17 ml of the digestion cocktail and incubated at 37°C for 15 minutes. After incubation, the lungs were chopped small. Lung tissues were further digested with the addition of DNase I (100 μg/ml) for 45 minutes at 37°C, with intermittent shaking. The digested lungs were filtered through 160-, 37- and 15-μm gauge nylon mesh sequentially. The filtrate was centrifuged at 250 g for 10 minutes. The cell pellet was resuspended in DMEM and incubated in a 100-mm-diameter Petri dish coated with mouse IgG (75 μg per dish) for 1 hour. The cells were spun down at 250 g for 10 minutes and resuspended in DMEM containing 10% FBS. The yield was ~8×106 cells per mouse and the cell viability was >95%.
Immunocytochemistry
Freshly isolated AEC I and AEC II were fixed with 4% paraformaldehyde for 30 minutes, followed by cytospining them onto glass slides at 600 g for 10 minutes. For overnight cultured cells, the cells were cultured on coverslips overnight and washed three times with PBS (pH 7.4) before fixation with 4% paraformaldehyde for 30 minutes. Cells were permeablized with 0.1% Triton X-100 for 15 minutes and blocked with 5% FBS for 1 hour. Cells were incubated with mouse monoclonal anti-ABCA3 (1:200), anti-T1α (E11) (1:100) and anti-CD45 (1:100) antibodies, and rabbit polyclonal anti-P2X7R (1:100) antibodies overnight at 4°C. Cells were then washed and incubated with Alexa-Fluor-546-conjugated anti-(rabbit Ig) and Alexa-Fluor-488-conjugated anti-(mouse Ig) secondary antibodies, or Cy3-conjugated Affini-Pure anti-mouse IgG (1:250). DAPI (2.5 mg/ml) staining was used for counting cells. Cells were viewed on a Nikon Eclipse E600 fluorescence microscope or Nikon Eclipse TE 2000 U inverted fluorescence microscope.
Cytometry
Cytometry of isolated AEC I and AEC II was performed by resuspending cells in the staining buffer (Hanks balanced salt solution without Phenol Red plus 1% bovine serum albumin and 0.1% sodium azide) containing 10 μl of P2X7R (ecto)–FITC antibody per 106 cells and incubated for 20 minutes at 4°C with constant rotation. The cells were then washed twice with the staining buffer and gated for P2X7R (ecto)–FITC-positive cells in a single argon laser cytofluorometer (FACSCalibur; BD Biosciences).
FM1-43 staining
Fusion pore formation was monitored by FM1-43 staining as previously described (Chintagari et al., 2006). Overnight cultured AEC I and AEC II were preincubated with 100 nM BBG for 30 minutes and then stimulated for 1 hour with 25 μM BzATP. FM1-43 dye (4 μM final concentration) was added along with BzATP. Cells were washed, fixed with 4% ice-cold paraformaldehyde and examined by fluorescence microscopy.
YO-PRO-1 dye uptake
Accumulation of the monomeric cyanine nucleic acid chelating dye YO-PRO-1 (molecular mass of 375 Da) was used as an indicator of P2X7R activation (Stokes et al., 2006). Freshly isolated AEC I and AEC II or cell lines (E10, R3/1 and HEK-P2X7R; 1×106 cells per ml) were incubated with the assay buffer (15 mM HEPES, pH 7.4, 135 mM NaCl, 5 mM KCl, 1.8 mM CaCl2 and 0.8 mM MgCl2) in the presence or absence of 100 nM BBG for 20 minutes. YO-PRO-1 at a 5 μM final concentration was added and incubated for an additional 5 minutes. The cells were transferred into a cuvette under continuous stirring, and YO-PRO-1 fluorescence was measured by a FluoroMax-3 spectrofluorimeter (HORIBA; Jobin Yvon, Edison, NJ) using an excitation and emission wavelength of 491 nm and 509 nm, respectively. BzATP (200 μM) was added after recording the baseline for 3–4 minutes. Fluorescence intensity was corrected using the cells without dye. In some cases, baselines were adjusted for comparisons between the different cells.
Dye uptake was also monitored directly by fluorescence microscopy in order to assess P2X7R activation in a specific type of cell in the AEC I and AEC II heterocellular culture. Overnight-cultured cells were washed twice and incubated with the assay buffer for 15 minutes. BzATP (200 μM) was added and incubated with YO-PRO-1 (5 μM) for 20 minutes. Cells were then incubated with Nile Red (10 μM) for an additional 2 minutes, washed twice with PBS, fixed with 4% ice-cold paraformaldehyde and examined by fluorescence microscopy.
Surfactant secretion assay
Surfactant secretion was performed as previously described (Chintagari et al., 2006). The freshly isolated AEC I and AEC II (1×106 cells in a 35-cm-diameter dish for rat and 0.8×106 cells in each well of a 12-well plate for mouse) were cultured overnight in the presence of [3H]choline [0.6 μCi per 106 cells (2.2×104 Bq)]. The heterocellular culture was incubated with or without various inhibitors or antagonists for 30 minutes. One set of the dishes was removed at this timepoint for analyzing the value at time zero. The cells were then incubated with BzATP or other secretagogues for 2 hours. At the end of incubation, lipids in the medium and cells were extracted using a one-step phosphotidylcholine extraction method (Vassar et al., 2007), and the radioactivities were counted. Surfactant secretion was expressed as a percentage [d.p.m. in medium/d.p.m. in medium and cells) ×100]. All of the secretion data were corrected by subtracting the value at time zero. A stimulation index was defined as a ratio of stimulated secretion to basal secretion.
MTT assay
Cell viability was assessed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) dye conversion or Trypan Blue exclusion assays, as described previously (Chintagari et al., 2006).
Western blot
Cells were lysed in lysis buffer (10 mM Tris-HCl, pH 7.5, 1% Triton X-100, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotonin and 10 μg/ml leupeptin). The protein concentration was determined using the DC protein assay kit. Proteins were separated by SDS-PAGE (10% gels) and transferred onto a nitrocellulose membrane. The membrane was stained with Ponceau S to ensure proper transfer and blocked overnight with 5% dried skimmed milk powder in 100 mM Tris-buffered saline plus 0.1% Tween 20 (TBST). The membranes were incubated with anti-P2X7R antibodies at a 1:1000 dilution or anti-β actin antibodies at a 1:2000 dilution overnight at 4°C. After being washed with TBST three times, the membranes were incubated with horseradish-peroxidase-conjugated anti-(rabbit IgG) (1:2000) for 1 hour. The blots were washed again and individual target proteins were visualized using the enhanced chemiluminescence detection system.
Co-culture
HEK-293 cells stably expressing rat P2X7R (HEK-P2X7R) and HEK-293 cells were cultured in DMEM and F12 medium (1:1) containing 10% FBS, 2 mM glutamine, 1000 units/ml penicillin G and 100 μg/ml streptomycin. E10 and R3/1 cells were cultured in CMRL and Ham's F12 medium containing 10% FBS, 2 mM glutamine, 1000 units/ml penicillin G and 100 μg/ml streptomycin, respectively. All the cells were grown to confluence, released and co-cultured with freshly isolated AEC II in a 1:2 ratio in DMEM for 16–18 hours. Cells were labeled and assayed for surfactant secretion as described above. The radioactivity counts in E10, R3/1, HEK-P2X7R and HEK-293 cells were less than 10% of those in AEC II.
To determine the effects of conditioned media on surfactant secretion, E10 cells, HEK-P2X7R and HEK 293 cells were grown to confluence in the respective culture medium, as described above. Freshly isolated AEC II was cultured for 16–18 hours in complete DMEM. These cells were washed three times with DMEM and incubated with 100 μM ARL67156 and 25 μM BzATP for 2 hours. The conditioned medium was removed and centrifuged at 250 g for 5 minutes. The supernatant, without dialysis or concentration, was transferred to recipient [3H]choline-labeled AEC II. Cells were further incubated for 2 hours and surfactant secretion was assayed.
Measurement of ATP concentration
The cells were washed three times with 1 ml of DMEM and preincubated with or without 100 nM BBG for 15 minutes. BzATP (25 μM) was then added for 10 minutes to stimulate the cells. The medium was then removed to avoid the interference of BzATP in the ATP assay. Fresh medium containing 100 μM ARL67156 was added and was incubated with the cells for 20 minutes. The medium was then centrifuged at 600 g for 10 minutes, and the supernatant was collected and frozen in aliquots at −20°C for subsequent ATP assay.
ATP concentration was measured using the chemiluminescent luciferin–luciferase assay kit. Media (25 μl) were placed in a white opaque 96-well plate. An equal volume of luciferin–luciferase assay solution was added to each well. Luminescence was recorded using a FLUOSTAR microplate reader (BMG, Labtech, Germany). Luminescence measurements were taken for 5 minutes with an integration time of 60 ms per well. A standard curve was generated for each experiment with ATP standards up to 1 μM. The results were expressed as nM ATP in bulk medium per mg of total cellular protein.
Knockdown of P2X7R
Adenoviral shRNA vectors were constructed as previously described (Gou et al., 2007). Two shRNA sequences targeting 574–592 and 669–689 of mouse P2X7R were chosen (Jun et al., 2007; Lu et al., 2007) and were named as si-X7 (1) and si-X7 (2). E10 cells were transduced with virus control, si-X7 (1) and si-X7 (2) for 4 days at a multiplicity of infection (MOI) of 0–100.
Preloading assay for dye transfer
This was performed according to the methodology previously described (Abraham et al., 2001). E10 cells cultured in 35-mm-diameter tissue culture dishes were incubated with DMEM containing 1 mg/ml of Texas-Red–dextran (molecular mass of 10,000 kDa) and 10 μM calcein-AM for 30 minutes at 37°C. Freshly isolated unlabeled acceptor AEC II were co-cultured with the double-labeled E10 cells for 5 hours and fluorescence images were taken.
Real-time PCR
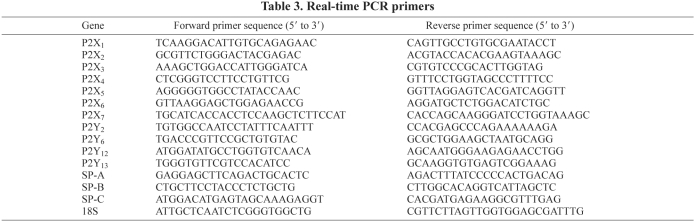
Real-time PCR analysis was performed as previously described (Jin et al., 2006). The primers used are listed in Table 3. Data were normalized to the levels of 18S rRNA.
Table 3.
Real-time PCR primers
Surfactant secretion in mice
Surfactant secretion in wild-type and P2X7R−/− mice was measured according to the method of Ikegmai and colleagues (Ikegami et al., 2000). P2X7R−/− mice on a C57BL/6 background were purchased from the Jackson Laboratory (strain: B6.129P2-P2rx7tm1Gab/J) and bred in the Laboratory Animal Resources Unit at Oklahoma State University. The wild-type (C57BL/6) and P2X7R−/− mice were given 0.5 μCi [3H]choline per gram of body weight intraperitoneally and housed for 16 hours. The mice were hyperventilated for 30 minutes with a tidal volume (Vt) of 30 ml/kg of body weight and a positive end-expiratory pressure (PEEP) of 5 cm of H2O, as previously described (Wyszogrodski et al., 1975). Under these conditions, surfactant secretion was increased and the inactivation of surfactant did not occur because of the use of PEEP (Wyszogrodski et al., 1975). The mice were then killed, and the lavage and lung tissue were collected. Lipids were extracted and saturated phosphatidylcholine isolated according to the method of Mason and colleagues (Mason et al., 1976). Secretion was expressed as [(d.p.m. in lavage)/(d.p.m. in lavage and lung tissue)]×100%.
Statistical analysis
Data were expressed as means±s.e.m. Statistical analysis was performed by one-way analysis of variance, followed by Tukey's analysis or Student's t-test using Graphpad prism version 4. A P-value of <0.05 was considered significant.
Acknowledgments
We thank Mary Williams (Boston University) for providing E10 cells, with the permission of Al Malkinson (University of Colorado), Annmarie Surprenant (University of Manchester) for HEK-293 cells stably expressing rat P2X7R and Roland Koslowski (Dresden University of Technology) for R3/1 cells. We also thank Mike Davis (Oklahoma State University) for the use of his blood analyzer. This work was supported by the NIH [grant numbers R01 HL-052146, R01 HL-071628 and R01 HL-083188 (to L.L.)]. Deposited in PMC for release after 12 months.
References
- Abraham V., Chou M. L., George P., Pooler P., Zaman A., Savani R. C., Koval M. (2001). Heterocellular gap junctional communication between alveolar epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 280, L1085-L1093 [DOI] [PubMed] [Google Scholar]
- Andreeva A. V., Kutuzov M. A., Voyno-Yasenetskaya T. A. (2007). Regulation of surfactant secretion in alveolar type II cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 293, L259-L271 [DOI] [PubMed] [Google Scholar]
- Arcuino G., Lin J. H., Takano T., Liu C., Jiang L., Gao Q., Kang J., Nedergaard M. (2002). Intercellular calcium signaling mediated by point-source burst release of ATP. Proc. Natl. Acad. Sci. USA 99, 9840-9845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashino Y., Ying X., Dobbs L. G., Bhattacharya J. (2000). [Ca(2+)](i) oscillations regulate type II cell exocytosis in the pulmonary alveolus. Am. J. Physiol. Lung Cell. Mol. Physiol. 279, L5-L13 [DOI] [PubMed] [Google Scholar]
- Baraldi P. G., Di V. F., Romagnoli R. (2004). Agonists and antagonists acting at P2X7 receptor. Curr. Top. Med. Chem. 4, 1707-1717 [DOI] [PubMed] [Google Scholar]
- Barth K., Weinhold K., Guenther A., Young M. T., Schnittler H., Kasper M. (2007). Caveolin-1 influences P2X7 receptor expression and localization in mouse lung alveolar epithelial cells. FEBS J. 274, 3021-3033 [DOI] [PubMed] [Google Scholar]
- Beigi R., Kobatake E., Aizawa M., Dubyak G. R. (1999). Detection of local ATP release from activated platelets using cell surface-attached firefly luciferase. Am. J. Physiol. 276, C267-C278 [DOI] [PubMed] [Google Scholar]
- Bortnick A. E., Favari E., Tao J. Q., Francone O. L., Reilly M., Zhang Y., Rothblat G. H., Bates S. R. (2003). Identification and characterization of rodent ABCA1 in isolated type II pneumocytes. Am. J. Physiol. Lung Cell. Mol. Physiol. 285, L869-L878 [DOI] [PubMed] [Google Scholar]
- Casas-Pruneda G., Reyes J. P., Perez-Flores G., Perez-Cornejo P., Arreola J. (2009). Functional interactions between P2X4 and P2X7 receptors from mouse salivary epithelia. J. Physiol. 587, 2887-2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chander A., Sen N., Wu A. M., Spitzer A. R. (1995). Protein kinase C in ATP regulation of lung surfactant secretion in type II cells. Am. J. Physiol. 268, L108-L116 [DOI] [PubMed] [Google Scholar]
- Chen J., Chen Z., Narasaraju T., Jin N., Liu L. (2004). Isolation of highly pure alveolar epithelial type I and type II cells from rat lungs. Lab. Invest. 84, 727-735 [DOI] [PubMed] [Google Scholar]
- Chen J., Chen Z., Chintagari N. R., Bhaskaran M., Jin N., Narasaraju T., Liu L. (2006). Alveolar type I cells protect rat lung epithelium from oxidative injury. J. Physiol. 572, 625-638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Brosnan C. F. (2006). Exacerbation of experimental autoimmune encephalomyelitis in P2X7R−/− mice: evidence for loss of apoptotic activity in lymphocytes. J. Immunol. 176, 3115-3126 [DOI] [PubMed] [Google Scholar]
- Chen Z., Jin N., Narasaraju T., Chen J., McFarland L. R., Scott M., Liu L. (2004). Identification of two novel markers for alveolar epithelial type I and II cells. Biochem. Biophys. Res. Commun. 319, 774-780 [DOI] [PubMed] [Google Scholar]
- Chintagari N. R., Jin N., Wang P., Narasaraju T. A., Chen J., Liu L. (2006). Effect of cholesterol depletion on exocytosis of alveolar type II cells. Am. J. Respir. Cell Mol. Biol. 34, 677-687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collo G., Neidhart S., Kawashima E., Kosco-Vilbois M., North R. A., Buell G. (1997). Tissue distribution of the P2X7 receptor. Neuropharmacology. 36, 1277-1283 [DOI] [PubMed] [Google Scholar]
- Dobbs L. G., Mason R. J. (1979). Pulmonary alveolar type II cells isolated from rats. Release of phosphatidylcholine in response to beta-adrenergic stimulation. J. Clin. Invest. 63, 378-387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari D., Pizzirani C., Adinolfi E., Lemoli R. M., Curti A., Idzko M., Panther E., Di Virgilio F. (2006). The P2X7 receptor: a key player in IL-1 processing and release. J. Immunol. 176, 3877-3883 [DOI] [PubMed] [Google Scholar]
- Fields R. D., Burnstock G. (2006). Purinergic signalling in neuron-glia interactions. Nat. Rev. Neurosci. 7, 423-436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobran L. I., Rooney S. A. (1999). Surfactant secretagogue activation of protein kinase C isoforms in cultured rat type II cells. Am J. Physiol. 277, L251-L256 [DOI] [PubMed] [Google Scholar]
- Gobran L. I., Rooney S. A. (2004). Pulmonary surfactant secretion in briefly cultured mouse type II cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 286, L331-L336 [DOI] [PubMed] [Google Scholar]
- Gonzalez R., Yang Y. H., Griffin C., Allen L., Tigue Z., Dobbs L. (2005). Freshly-isolated rat alveolar type I cells, type II cells, and cultured type II cells have distinct molecular phenotypes. Am. J. Physiol. Lung Cell. Mol. Physiol. 288, L179-L189 [DOI] [PubMed] [Google Scholar]
- Gou D., Weng T., Wang Y., Wang Z., Zhang H., Gao L., Chen Z., Wang P., Liu L. (2007). A novel approach for the construction of multiple shRNA expression vectors. J. Gene Med. 9, 751-763 [DOI] [PubMed] [Google Scholar]
- Griese M., Gobran L. I., Rooney S. A. (1991). A2 and P2 purine receptor interactions and surfactant secretion in primary cultures of type II cells. Am. J. Physiol. 261, L140-L147 [DOI] [PubMed] [Google Scholar]
- Gu B. J., Wiley J. S. (2006). Rapid ATP-induced release of matrix metalloproteinase 9 is mediated by the P2X7 receptor. Blood 107, 4946-4953 [DOI] [PubMed] [Google Scholar]
- Guttentag S. H., Akhtar A., Tao J. Q., Atochina E., Rusiniak M. E., Swank R. T., Bates S. R. (2005). Defective surfactant secretion in a mouse model of Hermansky-Pudlak syndrome. Am. J. Respir. Cell Mol. Biol. 33, 14-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller T., Dietl P., Pfaller K., Frick M., Mair N., Paulmichl M., Hess M. W., Furst J., Maly K. (2001). Fusion pore expansion is a slow, discontinuous, and Ca2+-dependent process regulating secretion from alveolar type II cells. J. Cell Biol. 155, 279-289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebran J. N., Goerke J., Clements J. A. (1981). Surfactant release in excised rat lung is stimulated by air inflation. J. Appl. Physiol. 51, 905-910 [DOI] [PubMed] [Google Scholar]
- Hughes J. P., Hatcher J. P., Chessell I. P. (2007). The role of P2X(7) in pain and inflammation. Purinergic Signal. 3, 163-169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura H., Parthasarathi K., Lindert J., Bhattacharya J. (2006). Lung surfactant secretion by interalveolar Ca2+ signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 291, L596-L601 [DOI] [PubMed] [Google Scholar]
- Ikegami M., Whitsett J. A., Jobe A., Ross G., Fisher J., Korfhagen T. (2000). Surfactant metabolism in SP-D gene-targeted mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 279, L468-L476 [DOI] [PubMed] [Google Scholar]
- Isakson B. E., Seedorf G. J., Lubman R. L., Evans W. H., Boitano S. (2003). Cell-cell communication in heterocellular cultures of alveolar epithelial cells. Am. J. Respir. Cell Mol. Biol. 29, 552-561 [DOI] [PubMed] [Google Scholar]
- Jiang L. H., Mackenzie A. B., North R. A., Surprenant A. (2000). Brilliant blue G selectively blocks ATP-gated rat P2X(7) receptors. Mol. Pharmacol. 58, 82-88 [PubMed] [Google Scholar]
- Jin N., Kolliputi N., Gou D., Weng T., Liu L. (2006). A novel function of ionotropic gamma -aminobutyric acid receptors involving alveolar fluid homeostasis. J. Biol. Chem. 281, 36012-36020 [DOI] [PubMed] [Google Scholar]
- Johnson M. D., Bao H. F., Helms M. N., Chen X. J., Tigue Z., Jain L., Dobbs L. G., Eaton D. C. (2006). Functional ion channels in pulmonary alveolar type I cells support a role for type I cells in lung ion transport. Proc. Natl. Acad. Sci. USA 103, 4964-4969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun D. J., Kim J., Jung S. Y., Song R., Noh J. H., Park Y. S., Ryu S. H., Kim J. H., Kong Y. Y., Chung J. M., et al. (2007). Extracellular ATP mediates necrotic cell swelling in SN4741 dopaminergic neurons through P2X7 receptors. J. Biol. Chem. 282, 37350-37358 [DOI] [PubMed] [Google Scholar]
- Kathuria H., Cao Y., Hinds A., Ramirez M. I., Williams M. C. (2007). ERM is expressed by alveolar epithelial cells in adult mouse lung and regulates caveolin-1 transcription in mouse lung epithelial cell lines. J. Cell. Biochem. 102, 13-27 [DOI] [PubMed] [Google Scholar]
- Ke H. Z., Qi H., Weidema A. F., Zhang Q., Panupinthu N., Crawford D. T., Grasser W. A., Paralkar V. M., Li M., Audoly L. P., et al. (2003). Deletion of the P2X7 nucleotide receptor reveals its regulatory roles in bone formation and resorption. Mol. Endocrinol. 17, 1356-1367 [DOI] [PubMed] [Google Scholar]
- Kim M., Jiang L. H., Wilson H. L., North R. A., Surprenant A. (2001). Proteomic and functional evidence for a P2X7 receptor signalling complex. EMBO J. 20, 6347-6358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koslowski R., Barth K., Augstein A., Tschernig T., Bargsten G., Aufderheide M., Kasper M. (2004). A new rat type I-like alveolar epithelial cell line R3/1: bleomycin effects on caveolin expression. Histochem. Cell Biol. 121, 509-519 [DOI] [PubMed] [Google Scholar]
- Koval M. (2002). Sharing signals: connecting lung epithelial cells with gap junction channels. Am. J. Physiol. Lung Cell. Mol. Physiol. 283, L875-L893 [DOI] [PubMed] [Google Scholar]
- Leipziger J. (2003). Control of epithelial transport via P2 receptors. Am. J. Physiol. Renal Physiol. 284, F419-F432 [DOI] [PubMed] [Google Scholar]
- Li J., Liu D., Ke H. Z., Duncan R. L., Turner C. H. (2005). The P2X7 nucleotide receptor mediates skeletal mechanotransduction. J. Biol. Chem. 280, 42952-42959 [DOI] [PubMed] [Google Scholar]
- Linke M. J., Burton F. M., Fiedeldey D. T., Rice W. R. (1997). Surfactant phospholipid secretion from rat alveolar type II cells: possible role of PKC isozymes. Am. J. Physiol. 272, L171-L177 [DOI] [PubMed] [Google Scholar]
- Lu H., Burns D., Garnier P., Wei G., Zhu K., Ying W. (2007). P2X7 receptors mediate NADH transport across the plasma membranes of astrocytes. Biochem. Biophys. Res. Commun. 362, 946-950 [DOI] [PubMed] [Google Scholar]
- Mason R. J., Nellenbogen J., Clements J. A. (1976). Isolation of disaturated phosphatidylcholine with osmium tetroxide. J. Lipid Res. 17, 281-284 [PubMed] [Google Scholar]
- Nicholas T. E., Barr H. A. (1983). The release of surfactant in rat lung by brief periods of hyperventilation. Respir. Physiol. 52, 69-83 [DOI] [PubMed] [Google Scholar]
- North R. A. (2002). Molecular physiology of P2X receptors. Physiol. Rev. 82, 1013-1067 [DOI] [PubMed] [Google Scholar]
- Oyarzun M. J., Clements J. A. (1978). Control of lung surfactant by ventilation, adrenergic mediators, and prostaglandins in the rabbit. Am. Rev. Respir. Dis. 117, 879-891 [DOI] [PubMed] [Google Scholar]
- Parvathenani L. K., Tertyshnikova S., Greco C. R., Roberts S. B., Robertson B., Posmantur R. (2003). P2X7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer's disease. J. Biol. Chem. 278, 13309-13317 [DOI] [PubMed] [Google Scholar]
- Patel A. S., Reigada D., Mitchell C. H., Bates S. R., Margulies S. S., Koval M. (2005). Paracrine stimulation of surfactant secretion by extracellular ATP in response to mechanical deformation. Am. J. Physiol. Lung Cell. Mol. Physiol. 289, L489-L496 [DOI] [PubMed] [Google Scholar]
- Pavalko F. M., Chen N. X., Turner C. H., Burr D. B., Atkinson S., Hsieh Y. F., Qiu J., Duncan R. L. (1998). Fluid shear-induced mechanical signaling in MC3T3-E1 osteoblasts requires cytoskeleton-integrin interactions. Am. J. Physiol. 275, C1591-C1601 [PubMed] [Google Scholar]
- Pellegatti P., Falzoni S., Pinton P., Rizzuto R., Di Virgilio F. (2005). A novel recombinant plasma membrane-targeted luciferase reveals a new pathway for ATP secretion. Mol. Biol. Cell 16, 3659-3665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placido R., Auricchio G., Falzoni S., Battistini L., Colizzi V., Brunetti E., Di Virgilio F., Mancino G. (2006). P2X(7) purinergic receptors and extracellular ATP mediate apoptosis of human monocytes/macrophages infected with Mycobacterium tuberculosis reducing the intracellular bacterial viability. Cell. Immunol. 244, 10-18 [DOI] [PubMed] [Google Scholar]
- Qiao R., Zhou B., Liebler J. M., Li X., Crandall E. D., Borok Z. (2003). Identification of three genes of known function expressed by alveolar epithelial cells. Am. J. Respir. Cell Mol. Biol. 29, 95-105 [DOI] [PubMed] [Google Scholar]
- Rassendren F., Buell G. N., Virginio C., Collo G., North R. A., Surprenant A. (1997). The permeabilizing ATP receptor, P2X7. Cloning and expression of a human cDNA. J. Biol. Chem. 272, 5482-5486 [DOI] [PubMed] [Google Scholar]
- Rice W. R., Conkright J. J., Na C. L., Ikegami M., Shannon J. M., Weaver T. E. (2002). Maintenance of the mouse type II cell phenotype in vitro. Am. J. Physiol. Lung Cell. Mol. Physiol. 283, L256-L264 [DOI] [PubMed] [Google Scholar]
- Seminario-Vidal L., Kreda S., Jones L., O'Neal W., Trejo J., Boucher R. C., Lazarowski E. R. (2009) Thrombin promotes release of ATP from lung epithelial cells through coordinated activation of rho- and Ca2+-dependent signaling pathways. J. Biol. Chem. 284, 20638-20648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solle M., Labasi J., Perregaux D. G., Stam E., Petrushova N., Koller B. H., Griffiths R. J., Gabel C. A. (2001). Altered cytokine production in mice lacking P2X(7) receptors. J. Biol. Chem. 276, 125-132 [DOI] [PubMed] [Google Scholar]
- Sperlagh B., Kofalvi A., Deuchars J., Atkinson L., Milligan C. J., Buckley N. J., Vizi E. S. (2002). Involvement of P2X7 receptors in the regulation of neurotransmitter release in the rat hippocampus. J. Neurochem. 81, 1196-1211 [DOI] [PubMed] [Google Scholar]
- Stokes L., Jiang L. H., Alcaraz L., Bent J., Bowers K., Fagura M., Furber M., Mortimore M., Lawson M., Theaker J., et al. (2006). Characterization of a selective and potent antagonist of human P2X(7) receptors, AZ11645373. Br. J. Pharmacol. 149, 880-887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suadicani S. O., Brosnan C. F., Scemes E. (2006). P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J. Neurosci. 26, 1378-1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatur S., Groulx N., Orlov S. N., Grygorczyk R. (2007). Ca2+-dependent ATP release from A549 cells involves synergistic autocrine stimulation by coreleased uridine nucleotides. J. Physiol. 584, 419-435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar V., Hagen C., Ludwig J., Thomas R., Zhou J. (2007). One-step method of phosphatidylcholine extraction and separation. Biotechniques 42, 442, 444 [DOI] [PubMed] [Google Scholar]
- Virginio C., Church D., North R. A., Surprenant A. (1997). Effects of divalent cations, protons and calmidazolium at the rat P2X7 receptor. Neuropharmacology 36, 1285-1294 [DOI] [PubMed] [Google Scholar]
- von Wichert G., Jiang G., Kostic A., De V. K., Sap J., Sheetz M. P. (2003). RPTP-alpha acts as a transducer of mechanical force on alphav/beta3-integrin-cytoskeleton linkages. J. Cell Biol. 161, 143-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhold K., Krause-Buchholz U., Rodel G., Kasper M., Barth K. (2010). Interaction and interrelation of P2X7 and P2X4 receptor complexes in mouse lung epithelial cells. Cell. Mol. Life Sci. 67, 2631-2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz H. R., Dobbs L. G. (1990). Calcium mobilization and exocytosis after one mechanical stretch of lung epithelial cells. Science 250, 1266-1269 [DOI] [PubMed] [Google Scholar]
- Wyszogrodski I., Kyei-Aboagye K., Taeusch H. W., Jr, Avery M. E. (1975). Surfactant inactivation by hyperventilation: conservation by end-expiratory pressure. J. Appl. Physiol. 38, 461-466 [DOI] [PubMed] [Google Scholar]
- Young M. T., Pelegrin P., Surprenant A. (2007). Amino acid residues in the P2X7 receptor that mediate differential sensitivity to ATP and BzATP. Mol. Pharmacol. 71, 92-100 [DOI] [PubMed] [Google Scholar]