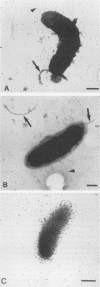
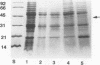
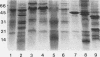
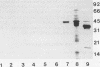
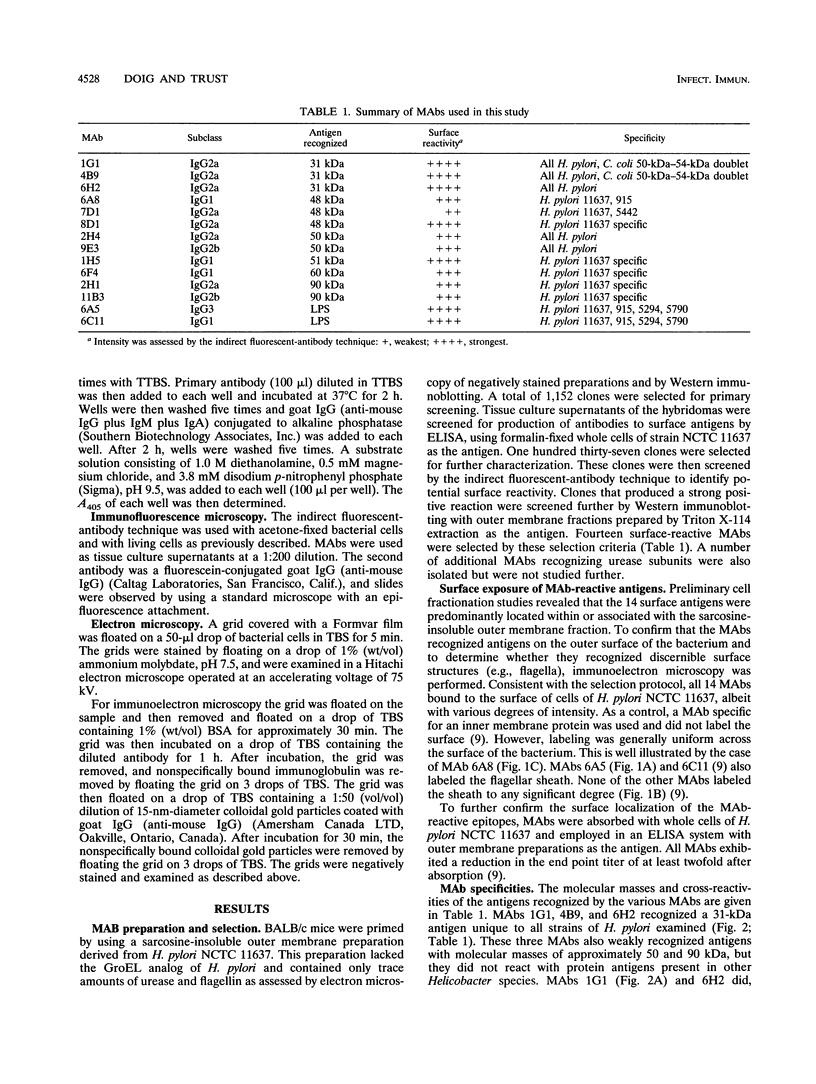
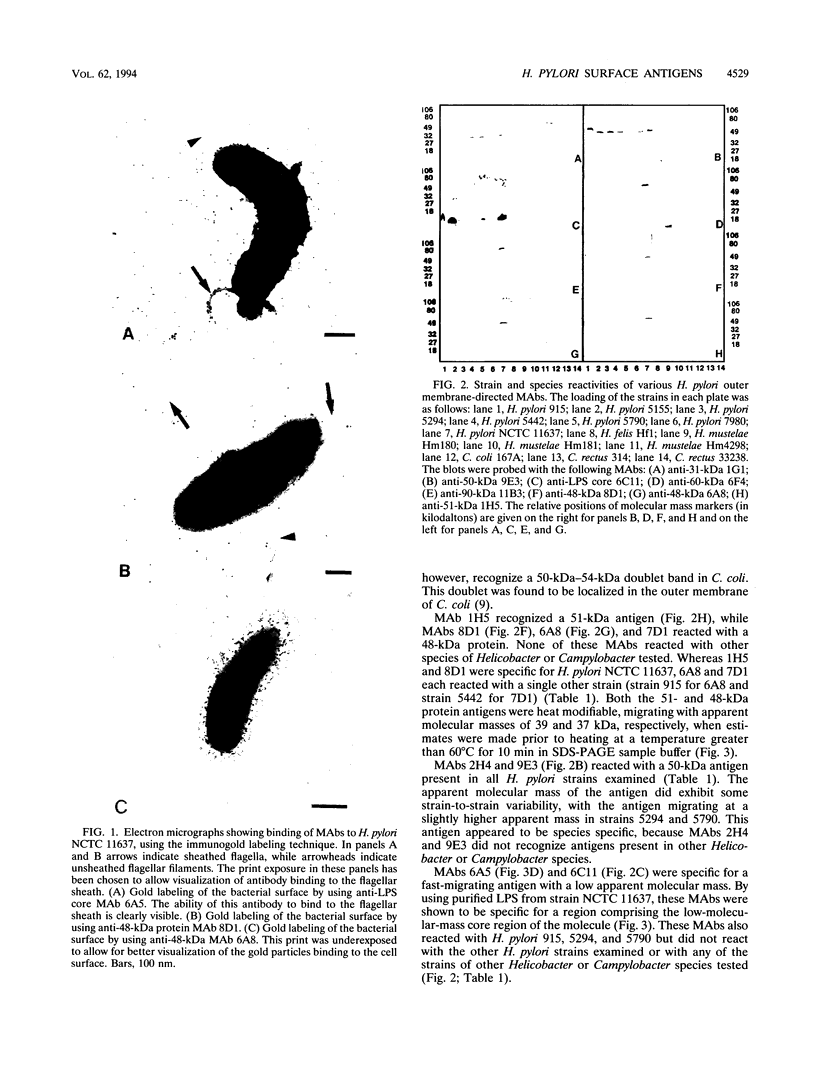
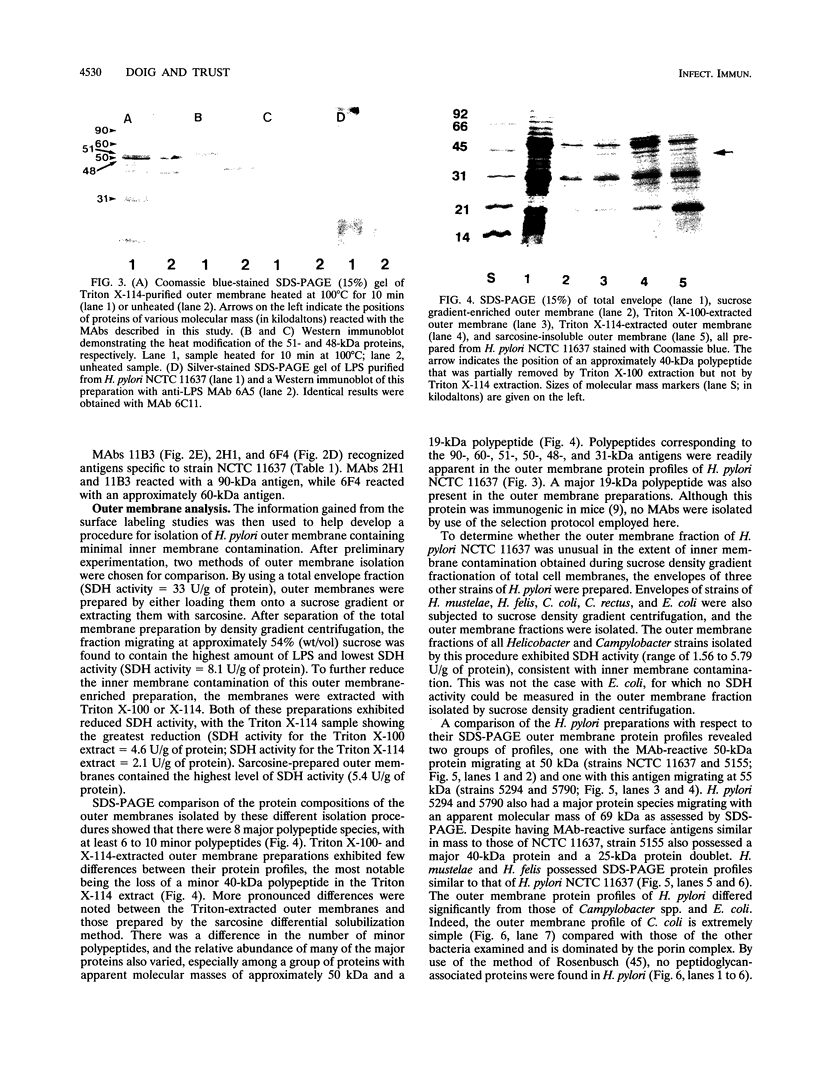
Abstract
Despite the potential significance of surface-localized antigens in the colonization by and disease processes of Helicobacter pylori, few such components have been unequivocally identified and/or characterized. To further investigate the surface of this bacterium, monoclonal antibodies (MAbs) to a sarcosine-insoluble outer membrane fraction prepared from H. pylori NCTC 11637 were raised. MAbs were selected on the basis of their surface reactivity to whole cells by enzyme-linked immunosorbent assay, immunofluorescence, and immunoelectron microscopy. By use of this selection protocol, 14 surface-reactive MAbs were chosen. These MAbs were used to identify six protein antigens (molecular masses, 80, 60, 51, 50, 48, and 31 kDa), all of which were localized within or associated with the outer membrane. Two of the MAbs recognized the core region of lipopolysaccharide (LPS). Only these two anti-LPS MAbs also recognized the flagellar sheath, indicating a structural difference between the sheath and outer membrane. Three of the protein antigens (80, 60, and 51 kDa) were strain specific, while the other three antigens were present in other strains of H. pylori. Both the 51- and 48-kDa antigens were heat modifiable and likely are porins. A conserved 31-kDa protein may represent another species of porin. A method involving sucrose density ultracentrifugation and Triton extraction that allows the preparation of H. pylori outer membranes with minimal inner membrane contamination is described. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis showed that the protein content of the H. pylori outer membrane is similar structurally to those of other species of Helicobacter but markedly different from those of taxonomically related Campylobacter spp. and Escherichia coli. H. pylori also appeared to lack peptidoglycan-associated proteins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin J. W., Doig P., Stewart M., Trust T. J. Structural comparison of urease and a GroEL analog from Helicobacter pylori. J Bacteriol. 1992 Nov;174(22):7470–7473. doi: 10.1128/jb.174.22.7470-7473.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Blaser M. J. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J Infect Dis. 1990 Apr;161(4):626–633. doi: 10.1093/infdis/161.4.626. [DOI] [PubMed] [Google Scholar]
- Blaser M. J. Helicobacter pylori: microbiology of a 'slow' bacterial infection. Trends Microbiol. 1993 Oct;1(7):255–260. doi: 10.1016/0966-842x(93)90047-u. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Doig P., Austin J. W., Kostrzynska M., Trust T. J. Production of a conserved adhesin by the human gastroduodenal pathogen Helicobacter pylori. J Bacteriol. 1992 Apr;174(8):2539–2547. doi: 10.1128/jb.174.8.2539-2547.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doig P., Austin J. W., Trust T. J. The Helicobacter pylori 19.6-kilodalton protein is an iron-containing protein resembling ferritin. J Bacteriol. 1993 Jan;175(2):557–560. doi: 10.1128/jb.175.2.557-560.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouet E. B., Denoyel G. A., Boude M., Wallano E., Andujar M., de Montclos H. P. Characterization of an immunoreactive species-specific 19-kilodalton outer membrane protein from Helicobacter pylori by using a monoclonal antibody. J Clin Microbiol. 1991 Aug;29(8):1620–1624. doi: 10.1128/jcm.29.8.1620-1624.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn B. E., Campbell G. P., Perez-Perez G. I., Blaser M. J. Purification and characterization of urease from Helicobacter pylori. J Biol Chem. 1990 Jun 5;265(16):9464–9469. [PubMed] [Google Scholar]
- Dunn B. E., Roop R. M., 2nd, Sung C. C., Sharma S. A., Perez-Perez G. I., Blaser M. J. Identification and purification of a cpn60 heat shock protein homolog from Helicobacter pylori. Infect Immun. 1992 May;60(5):1946–1951. doi: 10.1128/iai.60.5.1946-1951.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Graham D. Y. Receptor-mediated adherence of Campylobacter pylori to mouse Y-1 adrenal cell monolayers. Infect Immun. 1989 Aug;57(8):2272–2278. doi: 10.1128/iai.57.8.2272-2278.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Karjalainen T. K., Evans D. J., Jr, Graham D. Y., Lee C. H. Cloning, nucleotide sequence, and expression of a gene encoding an adhesin subunit protein of Helicobacter pylori. J Bacteriol. 1993 Feb;175(3):674–683. doi: 10.1128/jb.175.3.674-683.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geis G., Suerbaum S., Forsthoff B., Leying H., Opferkuch W. Ultrastructure and biochemical studies of the flagellar sheath of Helicobacter pylori. J Med Microbiol. 1993 May;38(5):371–377. doi: 10.1099/00222615-38-5-371. [DOI] [PubMed] [Google Scholar]
- Handfield-Jones S. E., Kennedy C. T., Bradfield J. B. Angiosarcoma arising in an angiomatous naevus following irradiation in childhood. Br J Dermatol. 1988 Jan;118(1):109–112. doi: 10.1111/j.1365-2133.1988.tb01758.x. [DOI] [PubMed] [Google Scholar]
- Harris L. A., Logan S. M., Guerry P., Trust T. J. Antigenic variation of Campylobacter flagella. J Bacteriol. 1987 Nov;169(11):5066–5071. doi: 10.1128/jb.169.11.5066-5071.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L. T., Mobley H. L. Purification and N-terminal analysis of urease from Helicobacter pylori. Infect Immun. 1990 Apr;58(4):992–998. doi: 10.1128/iai.58.4.992-998.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Keeling P. W., Smyth C. J. Identification of erythrocyte-binding antigens in Helicobacter pylori. J Gen Microbiol. 1992 Jul;138(7):1503–1513. doi: 10.1099/00221287-138-7-1503. [DOI] [PubMed] [Google Scholar]
- Hussell T., Isaacson P. G., Crabtree J. E., Spencer J. The response of cells from low-grade B-cell gastric lymphomas of mucosa-associated lymphoid tissue to Helicobacter pylori. Lancet. 1993 Sep 4;342(8871):571–574. doi: 10.1016/0140-6736(93)91408-e. [DOI] [PubMed] [Google Scholar]
- Illingworth D. S., Walter K. S., Griffiths P. L., Barclay R. Siderophore production and iron-regulated envelope proteins of Helicobacter pylori. Zentralbl Bakteriol. 1993 Sep;280(1-2):113–119. [PubMed] [Google Scholar]
- Kennell W. L., Egli C., Hancock R. E., Holt S. C. Pore-forming ability of major outer membrane proteins from Wolinella recta ATCC 33238. Infect Immun. 1992 Feb;60(2):380–384. doi: 10.1128/iai.60.2.380-384.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostrzynska M., Betts J. D., Austin J. W., Trust T. J. Identification, characterization, and spatial localization of two flagellin species in Helicobacter pylori flagella. J Bacteriol. 1991 Feb;173(3):937–946. doi: 10.1128/jb.173.3.937-946.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee A., Fox J., Hazell S. Pathogenicity of Helicobacter pylori: a perspective. Infect Immun. 1993 May;61(5):1601–1610. doi: 10.1128/iai.61.5.1601-1610.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan S. M., Trust T. J. Molecular identification of surface protein antigens of Campylobacter jejuni. Infect Immun. 1983 Nov;42(2):675–682. doi: 10.1128/iai.42.2.675-682.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan S. M., Trust T. J. Outer membrane characteristics of Campylobacter jejuni. Infect Immun. 1982 Dec;38(3):898–906. doi: 10.1128/iai.38.3.898-906.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills S. D., Kurjanczyk L. A., Penner J. L. Antigenicity of Helicobacter pylori lipopolysaccharides. J Clin Microbiol. 1992 Dec;30(12):3175–3180. doi: 10.1128/jcm.30.12.3175-3180.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley H. L., Cortesia M. J., Rosenthal L. E., Jones B. D. Characterization of urease from Campylobacter pylori. J Clin Microbiol. 1988 May;26(5):831–836. doi: 10.1128/jcm.26.5.831-836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran A. P., Helander I. M., Kosunen T. U. Compositional analysis of Helicobacter pylori rough-form lipopolysaccharides. J Bacteriol. 1992 Feb;174(4):1370–1377. doi: 10.1128/jb.174.4.1370-1377.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell D. G. Identification of the outer membrane proteins of Campylobacter pyloridis and antigenic cross-reactivity between C. pyloridis and C. jejuni. J Gen Microbiol. 1987 Jan;133(1):163–170. doi: 10.1099/00221287-133-1-163. [DOI] [PubMed] [Google Scholar]
- O'Toole P. W., Austin J. W., Trust T. J. Identification and molecular characterization of a major ring-forming surface protein from the gastric pathogen Helicobacter mustelae. Mol Microbiol. 1994 Jan;11(2):349–361. doi: 10.1111/j.1365-2958.1994.tb00315.x. [DOI] [PubMed] [Google Scholar]
- O'Toole P. W., Logan S. M., Kostrzynska M., Wadström T., Trust T. J. Isolation and biochemical and molecular analyses of a species-specific protein antigen from the gastric pathogen Helicobacter pylori. J Bacteriol. 1991 Jan;173(2):505–513. doi: 10.1128/jb.173.2.505-513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Osborn M. J., Wu H. C. Proteins of the outer membrane of gram-negative bacteria. Annu Rev Microbiol. 1980;34:369–422. doi: 10.1146/annurev.mi.34.100180.002101. [DOI] [PubMed] [Google Scholar]
- Page W. J., Huyer G., Huyer M., Worobec E. A. Characterization of the porins of Campylobacter jejuni and Campylobacter coli and implications for antibiotic susceptibility. Antimicrob Agents Chemother. 1989 Mar;33(3):297–303. doi: 10.1128/aac.33.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page W. J., Taylor D. E. Comparison of methods used to separate the inner and outer membranes of cell envelopes of Campylobacter spp. J Gen Microbiol. 1988 Nov;134(11):2925–2932. doi: 10.1099/00221287-134-11-2925. [DOI] [PubMed] [Google Scholar]
- Pearson T. W., Pinder M., Roelants G. E., Kar S. K., Lundin L. B., Mayor-Withey K. S., Hwett R. S. Methods for derivation and detection of anti-parasite monoclonal antibodies. J Immunol Methods. 1980;34(2):141–154. doi: 10.1016/0022-1759(80)90168-4. [DOI] [PubMed] [Google Scholar]
- Poquet I., Kornacker M. G., Pugsley A. P. The role of the lipoprotein sorting signal (aspartate +2) in pullulanase secretion. Mol Microbiol. 1993 Sep;9(5):1061–1069. doi: 10.1111/j.1365-2958.1993.tb01235.x. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Taylor D. N., Blaser M. J. The epidemiology of Helicobacter pylori infection. Epidemiol Rev. 1991;13:42–59. doi: 10.1093/oxfordjournals.epirev.a036078. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Tufano M. A., Rossano F., Catalanotti P., Liguori G., Capasso C., Ceccarelli M. T., Marinelli P. Immunobiological activities of Helicobacter pylori porins. Infect Immun. 1994 Apr;62(4):1392–1399. doi: 10.1128/iai.62.4.1392-1399.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]