Abstract
SH2 domain-containing leukocyte phosphoprotein of 76 kDa (SLP-76) nucleates a signaling complex critical for T-cell receptor (TCR) signal propagation. Mutations in the tyrosines of SLP-76 result in graded defects in TCR-induced signals depending on the tyrosine(s) affected. Here we use 2 strains of genomic knock-in mice expressing tyrosine to phenylalanine mutations to examine the role of TCR signals in the differentiation of effector and memory CD8+ T cells in response to infection in vivo. Our data support a model in which altered TCR signals can determine the rate of memory versus effector cell differentiation independent of initial T-cell expansion. Furthermore, we show that TCR signals sufficient to promote CD8+ T-cell differentiation are different from those required to elicit inflammatory cytokine production.
Introduction
Pathogen clearance requires that CD8+ effector T cells produce inflammatory cytokines and develop cytolytic activity against infected target cells, after which a small number of memory cells survive that rapidly regain effector function in the event of rechallenge. During this process, a relatively homogeneous pool of naive CD8+ T cells differentiates into heterogeneous pools of effector and memory CD8+ T cells.1 To initiate this differentiation program, naive T cells integrate signals from peptide:major histocompatibility complex (MHC) complexes, costimulatory molecules and cytokines. Manipulation of these signals influences the quality and kinetics of CD8+ T-cell memory differentiation.1–4 The molecular signals that orchestrate the function and diversification of effector and memory CD8+ T-cell subsets downstream of these receptors are beginning to be elucidated and how T-cell receptor (TCR) signals affect CD8+ T-cell effector function and fate choices is not fully understood.5–14
The CD8+ effector T-cell pool can be divided into terminally differentiated, short-lived KLRG-1hiIL-7rαlo effector cells (SLECs) and less differentiated KLRG-1loIL-7rαhi memory precursor cells (MPECs).3,15–17 SLECs typically produce the effector cytokine interferonγ (IFNγ) and may also coproduce tumor necrosis factorα (TNFα) in response to antigen but only memory precursors can produce interleukin-2 (IL-2) in addition to IFNγ and TNFα.17,18 The memory pool is a heterogeneous population that is commonly divided into effector memory (Tem) and central memory (Tcm) defined by surface markers including CD62L, which is expressed at higher levels on Tcm.19,20 After contraction, the ratio of CD62Lhi to CD62Llo memory cells increases, although the number of memory cells in the blood and spleen remains constant. While it is not clear whether this transition is the result of direct conversion of Tem to Tcm cells or preferential outgrowth of Tcm cells, it is clear that the rate of transition can be influenced by the strength and duration of initial antigenic stimulus.1,8,21 Furthermore, the memory pool undergoes functional maturation, gaining enhanced proliferative capabilities.22,23 The relative frequencies of memory subsets can vary depending on the nature or persistence of the inciting antigen and understanding how these factors influence memory development and maturation is important for predicting the quality of secondary responses.24–27
While multiple cytokines and transcription factors are known to affect the balance between terminal differentiation and memory formation, the role of TCR signals has been more difficult to ascertain.3,6,7,11,16,28,29 Adjustments in the quantity, quality, or duration of antigen presentation affect the magnitude of the primary response and the kinetics of differentiation, but do not appear to affect the functional quality of the cells.3,5,12,14,21,28,30–33 However, specific TCR signals can differentially affect the long-term fate of CD8+ T cells, as T cells with defective nuclear factor κB activation undergo normal primary expansion and terminal differentiation but not memory development.13
Here we explore the role of TCR signals in the differentiation, heterogeneity, and function of CD8+ effector and memory T cells using 2 strains of genomic knock-in (KI) mice that express mutations within the SH2 domain-containing leukocyte phosphoprotein of 76 kDa (SLP-76) protein.34 SLP-76 is a critical adaptor that links early TCR-induced phosphorylation events to multiple downstream effector molecules.35 We show that T cells from KI mice containing tyrosine to phenylalanine mutations at either residue 145 (Y145F) or residues 112 and 128 (Y112/128F) exhibit defects in proximal signaling downstream of the TCR and that these defects are more pronounced in the Y145F mice compared with the Y112/128F mice. After infection with the Armstrong strain of lymphocytic choriomeningitis virus (LCMV), SLP-76 mutant CD8+ T cells undergo primary expansion to the extent of wild-type (WT) cells despite striking defects in cytokine effector function. Furthermore, the KI CD8+ pools are skewed toward a memory phenotype. Quantitative differences in memory differentiation and cytokine responses between Y145F and Y112/128F mice correlate with differences in TCR signal propagation. These data support a model in which the strength of the initial TCR signals can influence the rate of memory differentiation independent of the magnitude of expansion and that TCR signals required to initiate a program of CD8+ T-cell differentiation are quantitatively and/or qualitatively different from those required to elicit cytokine responses upon restimulation.
Methods
Mice
KI and floxed SLP-76 mice were generated as described.34,36 WT mice were KI littermates or C57B/L6 mice purchased from The Jackson Laboratory. Cre recombinase-human estrogen receptor (CreT2) transgenic mice and the ROSA-enhanced yellow fluorescent protein (YFP) reporter were gifts from E. Brown (University of Pennsylvania)37 and F. Constantini (Columbia University).38 CD45.1 mice were purchased from The Jackson Laboratory. Animal experiments were performed in accordance with approved protocols by the University of Pennsylvania Institutional Animal Care and Use Committee.
Tamoxifen treatment
Mice were treated orally for 5 days with 200 μg/g/day tamoxifen (Sigma-Aldrich) in corn oil.
Flow cytometry
Splenocytes were surface-stained in phosphate-buffered saline containing 2% fetal bovine serum × 0.002% azide for 30 minutes. Intracellular staining was performed using BD Cytofix/Cytoperm kits (BD Biosciences) according to manufacturer's instructions. Peripheral blood was collected into 4% Na citrate; peripheral blood lymphocytes (PBLs) were isolated by density gradient with Ficoll-paque PLUS (GE Healthcare). Samples were collected on a FACSCaliber or LSRII (BD Biosciences) and analyzed using FlowJo software Version 8.8.2 (TreeStar). H2Db tetramers were made as described.21 Antibodies were purchased from BD PharMingen: CD4 (RM4-4), CD4 (RM4-5), CD8α, CD8β, IL-2, IFNγ, CD122; Ebiosciences: KLRG-1, CD27, CD127, CD44, TCRβ; Biolegend: CXCR3, TNFα; and Invitrogen: CD62L.
Biochemistry
Thy1.2 MACs MicroBead (Miltenyi)–purified splenocytes and lymph node cells were stimulated with 5 μg/mL anti-CD3 (500A2; BD Pharmingen) and lysates were prepared as described.34 For conditional mice, B220 MACs MicroBead-depleted splenocytes were sorted for YFP+Thy1.2+ expression using a FACSAria (BD Biosciences). Lysates were probed by immunoblot for phospho-PLCγ1 (Tyr783), phosph p44/42 MAPK (Thr202/Tyr202), phospho-LAT (Tyr191; Cell Signaling Technology), ERK2, and actin (Santa Cruz Biotechnology).
Ca2+ flux
Lymph node cells were loaded with Indo-1 (Molecular Probes), stained with biotinylated anti-CD3 (2C11), anti-CD4 (RM4-4), and anti-CD8β, fluorescein isothiocyanate anti-CD44, phycoerythrin anti-CD8α and PercypCy5.5 anti-CD4 (RM4-5; BD Biosciences) with 4mM probenecid at 30°C for 30 minutes. Cells were stimulated with 12.5 μg/mL streptavidin (Moleclular Probes) or ionomycin (1 μg) as indicated. Ca2+ release was measured as described (BD Biosciences).34
CD69 up-regulation
Splenocytes were incubated at 37°C overnight in the presence of 0.1 μg/mL anti-CD3 (2C11) and analyzed by flow cytometry.
CFSE/BRSE proliferation assays
Splenocytes were loaded with 10μM carboxyfluorescein succinimidyl ester (CFSE) or 2.5μM bodipy Red succinimdyl ester (BRSE; both from Molecular Probes), incubated at 37°C for 72 hours in the presence of 0.01 μg/mL anti-CD3 (2C11) and analyzed by flow cytometry.
Infections
Mice were infected intravenously with 2 × 105 colony-forming units LCMV Armstrong. For rechallenge, splenocytes were pooled by genotype and CD8+ purified using MACS CD8+ T-Cell Isolation kits. Purified cells were equilibrated such that 7500 H2Db:GP33 cells were transferred intravenously into each congenic host. The following day, congenic hosts were infected with 4 × 105 colony-forming units LM:GP33 intravenously.
Restimulation assays
Splenocytes (1 × 106) were left unstimulated or stimulated with 200 ng/mL GP33, GP276, or NP396 peptide or 500 ng/mL ionomycin and 50 ng/mL phorbol-12-myristate-13-acetate (PMA) in the presence of 1000 μg/mL brefeldin A for 5 hours and analyzed by intracellular cytokine staining.
Statistical analyses
P values were determined by a 2-tailed unpaired Student t test using Prism Version 4 software (GraphPad). Graphs show average ± SEM. Slopes and corresponding P values for CD62L and IL-7rα expression were determined using nonlinear regression modeling using Prism.
Results
T cells expressing SLP-76 tyrosine mutations are hyporesponsive to TCR stimulation
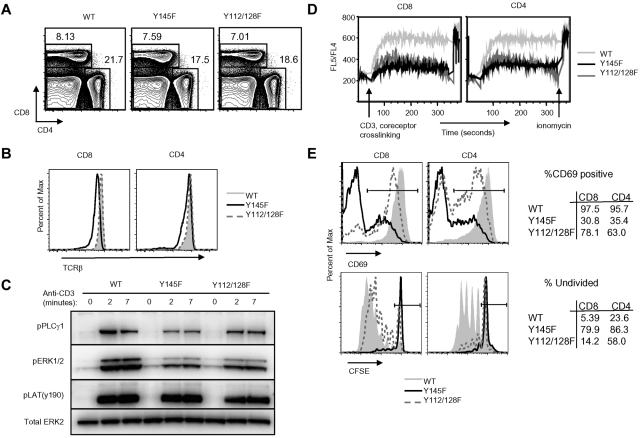
We previously showed that SLP-76 Y145F and Y112/128F KI mice generate thymocytes that exhibit defects in TCR signaling.34 Here we show that despite thymic selection defects, Y145F and Y112/128F mice generate CD4+ and CD8+ T cells in normal ratios (Figure 1A) and numbers (data not shown) in the spleen. TCRβ expression was slightly decreased on CD8+ splenocytes from Y145F mice and an increased frequency of CD4+ cells expressed slightly lower TCRβ levels compared with WT mice (Figure 1B). Both Y145F and Y112/128F cells showed decreased TCR-induced phosphorylation of PLCγ1 and ERK1/2, with the defect more pronounced in Y145F T cells (Figure 1C) but LAT phosphorylation was normal (Figure 1C). Ca2+ flux following TCR and CD4/CD8 co-cross-linking was examined by flow cytometry and was found to be severely impaired in both Y145F and Y112/128F T cells (Figure 1D).
Figure 1.
SLP-76 KI T cells show defects in TCR-induced proximal signal transduction and functional responses in vitro. (A) Contour plots show surface expression of CD4 and CD8 on lymphocytes from WT, Y145F, and Y112/128F spleens (n > 20). (B) Surface expression of TCRβ on CD4+ and CD8+ gated splenocytes from WT, Y145F, and Y112/128F mice (n = 6). (C) Spleen and lymph node cells from WT and KI mice were stimulated with anti-CD3 for the indicated times, lysed, and probed by Western blot with the indicated antibodies. Anti-Erk2 was used for a loading control (n = 3). (D) Ca2+ flux was measured in WT, Y145F, and Y112/128F CD4+ and CD8+ gated lymph node cells by flow cytometry following CD3, CD4, and CD8+ cross-linking (n = 4). (E) Splenocytes from WT, Y145F, and Y112/128F mice were incubated overnight in the presence of 0.1 μg of anti-CD3 or for 72 hours in the presence of 0.01 μg of anti-CD3 then measured for CD69 up-regulation (top panel; n > 5) and CFSE dilution (bottom panel; n > 5), respectively. Numbers in the tables represent frequency of cells within the gates depicted in the histograms.
To determine whether TCR-induced signaling defects observed in SLP-76 KI T cells translate into functional defects, we examined functional responses of KI T cells to TCR stimulation in vitro. After TCR stimulation, Y145F and, to a lesser extent, Y112/128F T cells did not up-regulate CD69, an early activation marker, to the level of WT T cells (Figure 1E top panel). This hierarchical requirement for the SLP-76 tyrosines was also observed during TCR-induced proliferation (Figure 1E bottom panel). Thus, signals transmitted from the TCR in Y112/128F T cells are weaker than those in WT T cells and those in Y145F T cells are weaker still.
Y145F conventional and innate-like lymphocytes have defects in TCR signaling
Our previous studies demonstrated that the majority of CD8 single positive thymocytes generated by Y145F KI mice are innate-like lymphocytes (ILLs).34 ILLs express high levels of the cell surface markers CD122 and CD44 and are poised to produce IFNγ.39 Here we show that CD122hiCD44hiCD8+ ILLs represent the majority of CD8+ T cells in the periphery of Y145F KI but not Y112/128F KI mice (supplemental Figure 1A top panel, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Y145F mice also have an abundance of peripheral CD122hiCD44hiCD4+ cells (supplemental Figure 1A bottom panel). Both CD44hi CD4+ and CD8+ cells in the Y145F mice produce IFNγ when stimulated with PMA and ionomycin ex vivo (supplemental Figure 1B); however, CD44hi T cells from SLP-76 KI mice show defects in TCR-induced Ca2+ flux similar to those observed in their CD44lo counterparts (supplemental Figure 1C). Although KI ILLs have the potential to produce IFNγ, WT SLP-76 expression is still required for coupling the TCR to early signaling events.
SLP-76 mutant CD8+ T cells show a normal magnitude of primary expansion during acute LCMV infection
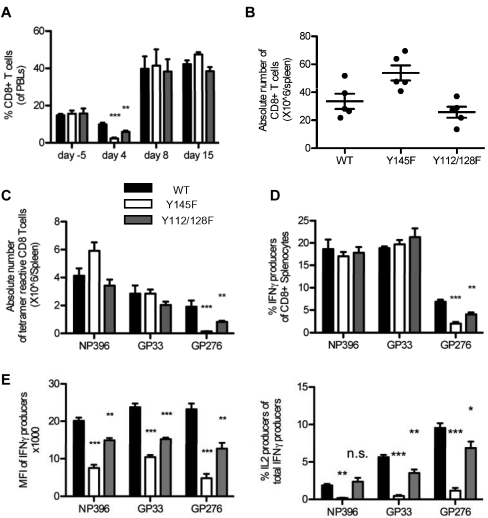
To determine the impact of weakened TCR signaling on the primary CD8+ T-cell response to infection, WT, Y145F, and Y112/128F mice were infected with LCMV Armstrong. Analysis of PBLs 5 days before infection showed that CD8+ T cells were present at similar frequencies in the 3 different strains of mice (Figure 2A). In WT mice at day 4 postinfection, the relative percentage of CD8+ PBLs was decreased from baseline, consistent with previous observations.40 It is unclear why this decrease was more dramatic in KI mice, but it could reflect altered expansion or tissue distribution of responding lymphocyte populations early in the immune response (Figure 2A). Further studies will be needed to define the very early kinetics of the CD8+ response in KI mice. Nevertheless, at the peak of CD8+ T-cell expansion at day 8 postinfection and later at day 15 postinfection, the frequency of circulating CD8+ T cells was similar between the 3 strains of mice (Figure 2A). Furthermore, absolute numbers of CD8+ splenocytes from day 8 postinfection spleens confirmed a normal magnitude of CD8+ T-cell expansion in the KI mice (Figure 2B). Thus, although both Y112/128F and Y145F T cells demonstrate a significant proliferative defect in vitro in response to CD3 cross-linking, in vivo viral challenge can be sufficient to induce WT levels of T-cell expansion. This difference may be due to differences in CD3 versus peptide:MHC stimulation or multiple factors present in vivo that are not accounted for in the in vitro studies including cytokine and coreceptor signals.
Figure 2.
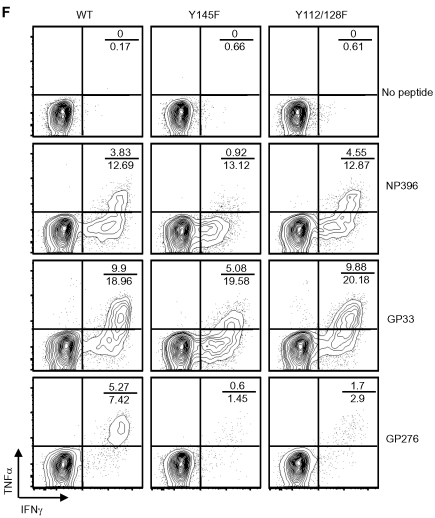
SLP-76 KI mice have intact CD8+ T-cell expansion but defective effector function in response to acute LCMV infection. (A) PBLs were isolated from serial bleeds from WT and KI mice at the indicated time points before and after LCMV infection and were analyzed by flow cytometry. CD8+ T cells (average ± SEM) are represented as a percent of the live lymphocyte gate (data are representative of 3 independent experiments each with 3-5 mice per group). Absolute numbers of (B) CD8+ (average ± SEM) and (C) tetramer-reactive (average ± SEM) splenocytes were calculated from day 8 infected WT and KI mice (representative of 2 independent experiments each with 5 mice per group). (D-F) Splenocytes from day 8 infected WT, Y145F, and Y112/128F KI mice were cultured in vitro with the indicated stimuli and analyzed by flow cytometry (representative of 2 independent experiments each with 5 mice per group). (D) Percent of CD8+ cells that produced IFNγ was calculated (average ± SEM). (E) The MFI of IFNγ staining in all CD8+ T cells producing IFNγ is shown (average ± SEM; left panel) and the percentage of total IFNγ producers that also produced IL-2 is shown (right panel). (F) Representative contour plots show IFNγ and TNFα expression in CD8+ splenocytes. Numbers indicate the percentage of CD8+, TNFα IFNγ double producers over the percentage of total CD8+ IFNγ producers (representative of 2 independent experiments each with 5 mice per group). Significant P values, when present, comparing KI to WT are indicated by asterisks: ***P < .001, **P = .001-.01, *P = .01-.05.
We next examined the quality of the T-cell response to LCMV in WT and KI mice. Expansion of T cells reactive to specific LCMV epitopes were examined using 3 different H2Db:peptide tetramers: NP396, GP33, and GP276 (Figure 2C). While expansion of CD8+ T cells reactive to the more dominant epitopes NP396 and GP33 was intact in both strains of KI mice, few cells reactive to the subdominant epitope, GP276, were observed in the Y145F KI mice. Y112/128F KI mice showed a lesser but consistent decrease in the numbers of CD8+ splenocytes reactive to GP276. The decrease of GP276-reactive T cells observed in the SLP-76 KI mice could be due to decreased responsiveness and expansion of cells specific for less dominant epitiopes or altered selection of cells specific for this epitope during development, or a combination of these possibilities.
LCMV-specific SLP-76 mutant T cells are functionally defective
To determine whether the LCMV-specific KI T cells were functional in response to TCR ligation, CD8+ splenocytes from day 8 postinfection WT and SLP-76 KI mice were analyzed ex vivo for their ability to produce cytokines following incubation with NP396, GP33, and GP276 peptides. Consistent with tetramer reactivity, fewer SLP-76 KI T cells produced cytokines in response to GP276 peptide compared with WT cells (Figure 2D). The amount of IFNγ produced per cell, as measured by the mean fluorescence intensity (MFI), was lower in T cells from both KI strains in response to all 3 peptides (Figure 2E left panel). While similar frequencies of CD8+ cells from these mice produced IFNγ in response to NP396 and GP33 (Figure 2D), the frequency of cells that also produced TNFα was lower in the Y145F mice but normal in the Y112/128F mice (Figure 2F). The frequency of IL-2 producing T cells was diminished in Y145F mice in response to all 3 peptides, while IL-2 production in Y112/128F T cells was decreased in response to 2 (GP33 and GP276) of the 3 LCMV-derived peptides tested (Figure 2E right panel). Despite the weaker effector cytokine responses, all mice cleared virus from serum and liver by day 8 (data not shown).
Conditional KI T cells show defects in TCR signaling
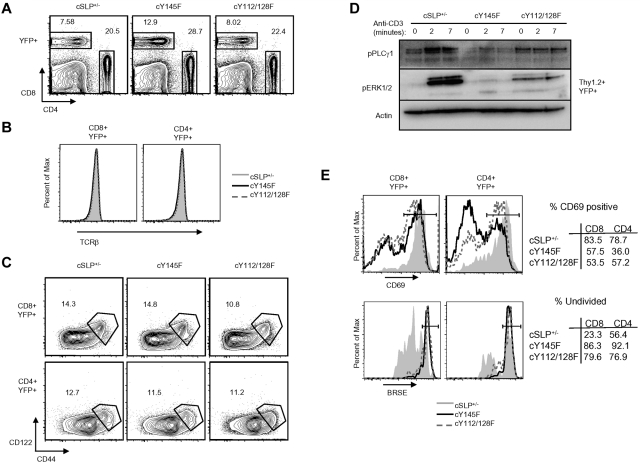
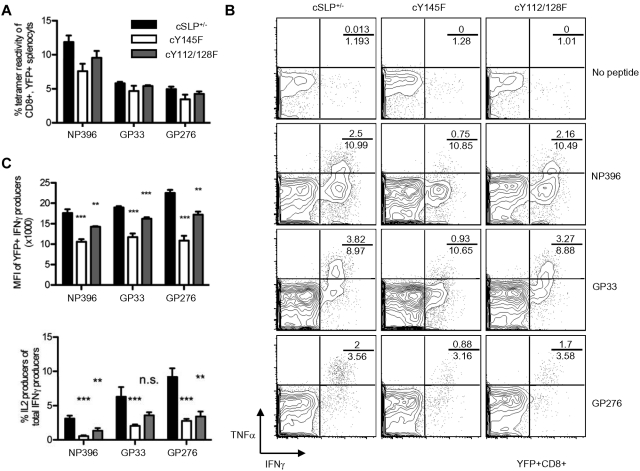
Due to demonstrated defects in thymocyte selection, TCR usage and the T-cell repertoire differ in SLP-76 KI strains compared with WT animals.34 To determine whether differences in the CD8+ T-cell repertoire, including the abundance of ILLs, or thymic selection contribute to the altered LCMV cytokine responses, we utilized a previously described strategy41 to generate conditional KI (cKI) mice. T cells in these mice are heterozygous for mutant SLP-76 and WT SLP-76 and are allowed to develop normally before WT SLP-76 is excised using drug-inducible cre recombinase activity. Conditional mice have 1 WT SLP-76 allele that is flanked by lox-p sites and the other allele is either Y145F SLP-76 (cY145F), Y112/128F SLP-76 (cY112/128F), or WT SLP-76 (cSLP-76+/−). These mice also express Cre-ERT2 under the ubiquitin promoter allowing for cre activity only in the presence of the estrogen analog tamoxifen. YFP knocked into the Rosa-26 locus is preceded by a lox-p-flanked stop codon such that YFP identifies those cells with Cre activity.
Here we show that following tamoxifen treatment, YFP+ CD4+ and CD8+ cKI T are generated and display levels of TCRβ on their surface similar to that of YFP+ cSLP-76+/− T cells (Figures 3A-B). Moreover, cKI and cSLP-76+/− mice have similar frequencies of peripheral CD122hiCD44hi T cells (Figure 3C).
Figure 3.
Conditional SLP-76 KI T cells show defects in TCR induced proximal signal transduction and functional responses in vitro. (A) Contour plots display CD4 and CD8 surface expression on YFP+ splenocytes from tamoxifen-treated cSLP-76+/−, cY145F, and cY112/128F mice (n > 5). (B) Surface expression of TCRβ on CD4+YFP+ and CD8+YFP+ gated splenocytes from cSLP-76+/−, cY145F, and cY112/128F mice (n = 5-7). (C) Contour plots show surface expression of CD44 and CD122 on CD4+YFP+ and CD8+YFP+ splenocytes from cSLP-76+/−, cY145, and cY112/128F mice. Numbers represent the percentage of cells in each gate. (D) YFP+ T cells from cSLP-76+/−, cY145, and cY112/128F mice were stimulated with anti-CD3 for the indicated times, lysed, and probed by Western blot with the indicated antibodies (n = 2). (E) CD69 up-regulation (top panel, n = 5-7) and BRSE dilution (bottom panel, n = 3-5) in splenocytes from cSLP+/−, cY145F, and cY112/128F mice were measured by flow cytometry following overnight and 72 hours stimulation with anti-CD3, respectively. Histograms are gated on CD8+YFP+ and CD4+YFP+ lymphocytes. Numbers in the tables represent frequency of cells within the gates depicted in the histograms. Significant P values, when present comparing KI to WT are indicated by asterisks: ***P < .001, **P = .001-.01, *P = .01-.05.
Deletion of WT SLP-76 in peripheral T cells expressing mutant SLP-76 confers similar TCR signaling abnormalities to those observed in T cells that develop only in the presence of the mutant SLP-76 proteins. After TCR ligation, YFP+ cKI T cells demonstrate defective PLCγ1 and ERK1/2 phosphorylation (Figure 3D), reduced up-regulation of CD69, and diminished proliferation (Figure 3E). These data suggest that SLP-76 tyrosines are required for TCR signal transduction and function, independent of their role in thymic selection.
Conditional SLP-76 KI T cells show defects in their response to LCMV challenge
To determine whether the defective LCMV response seen in the KI mice were the result of altered thymic development, tamoxifen-treated cKI and cSLP-76+/− mice were infected with LCMV. At day 8 postinfection the frequency of YFP+CD8+ T cells reactive to 3 LCMV tetramers was not significantly different in either cKI mouse strain, compared with cSLP-76+/− mice (Figure 4A). This is in contrast to the near absence of GP276 tetramer-reactive T cells observed in Y145F KI mice (Figure 2). Despite normal frequencies of IFNγ producers, when challenged with LCMV peptides, cY145F, but not cY112/128 T cells, failed to produce WT levels of TNFα similar to what was observed in the KI mice (Figure 4B). The MFI of IFNγ in peptide-responsive cKI T cells was less than that in cSLP+/− T cells, and this difference was more pronounced in the Y145F cKI cells compared with the Y112/128F cKI cells (Figure 4C top panel). The frequency of IFNγ producers that produced IL-2 was less among cells from cKI mice compared with cSLP-76+/− mice, similar to observations in nonconditional KI mice (Figure 4C bottom panel). These experiments indicate that altered thymic development in SLP-76 KI mice is not responsible for the diminished ability of T cells from these mice to respond to LCMV peptide restimulation. However, the contribution of GP276-reactive T cells to an LCMV response in Y145F mice was impacted by developmental events. Moreover, these experiments indicate that ILLs neither diminished nor augmented anti-LCMV responses measured in the nonconditional Y145F mice.
Figure 4.
LCMV-infected cSLP-76 KI T cells show defective effector responses in vitro. Mice were infected with LCMV Armstrong 7-10 days following tamoxifen treatment. (A) Tetramer reactivity among splenocytes from day 8 infected cSLP+/−, cY145F, and cY112/128F mice is represented as a percent of CD8+YFP+ lymphocytes (representative of 2 independent experiments each with 5 mice per group). (B-C) Splenocytes from day 8 infected cSLP+/−, cY145F, and cY112/128F mice were incubated in vitro with the indicated peptides and analyzed by flow cytometry. (B) Representative contour plots depict IFNγ and TNFα in YFP+CD8+ lymphocytes (representative of 2 independent experiments each with 5 mice per group). Numbers indicate the percentage of CD8+, TNFα IFNγ double producers over the percentage of total CD8+ IFNγ producers. (C) MFI of IFNγ (top panel) and IL-2 coproduction (bottom panel) were determined among CD8+YFP+ IFNγ producing splenocytes (representative of 2 independent experiments each with 5 mice per group). Bar graphs show averages ± SEM. Significant P values, when present comparing KI to WT are indicated by asterisks: ***P < .001, **P = .001-.01, *P = .01-.05.
Cytokine defects in KI T cells are the result of defective responses to TCR restimulation and not altered differentiation
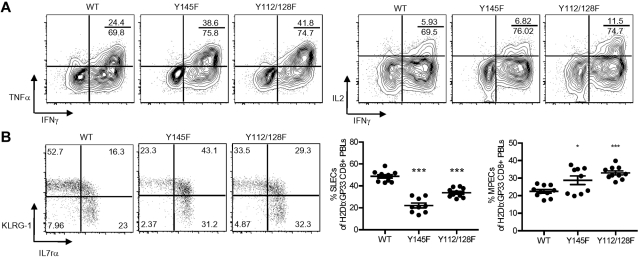
Terminally differentiated cells lose cytokine polyfunctionality, while cells that retain polyfunctionality, in particular IL-2 production, are more likely to survive and differentiate into memory cells.16–18 To test whether the poor polyfunctionality of KI T cells reflects enhanced terminal differentiation or defective TCR responses to subsequent stimulation, day 8 postinfection splenocytes were stimulated with PMA plus ionomycin. PMA/ionomycin stimulation mimics TCR-induced signals downstream of SLP-76 thereby eliminating the need for intact proximal signaling events and revealing the differentiation status of the cells. PMA/ionomycin stimulation showed that KI effector CD8+ T cells with TNFα and IL-2 potential were present in frequencies similar to or greater than those observed in WT splenocytes, suggesting that the KI effector cells were not skewed toward terminal differentiation (Figure 5A). These data also suggest that the previously observed cytokine defects were the result of abnormal TCR restimulation. Effector cell surface marker expression can be used to distinguish terminally differentiated cells from those with memory potential. IL-7rα re-expression and low levels of KLRG-1 expression are associated with MPECs, while SLECs express low levels of IL-7rα and high levels of KLRG-1.15–17 Analysis of peripheral blood on day15 postinfection revealed that the frequency of SLECs among H2Db:GP33-reactive KI T cells was significantly less than that of the WT T-cell population whereas the frequency of MPECs was greater (Figure 5B). However, SLEC and MPEC designations do not take into account the increased frequency of cells expressing high levels of both KLRG-1 and IL-7rα in KI mice (Figure 5B). These cells are present in WT mice, but their contribution to memory is not clear.
Figure 5.
Poor polyfunctionality in KI mice is the result of defective responses to TCR restimulation. (A) Splenocytes from day 8 postinfection WT, Y145F, and Y112/128F mice were stimulated with PMA plus ionomycin. Representative contour plots show IFNγ and TNFα (left panel) and IFNγ and IL-2 (right panel) expression in CD8+ cells. Numbers indicate the percentage of CD8+, IFNγ, TNFα (left panel), or IFNγ, IL-2 (right panel) double producers over the percentage of total CD8+ IFNγ producers. (B) Dot plots show IL-7rα and KLRG-1 surface expression on H2Db:GP33-reactive CD8+ PBLs from WT, Y145F, and Y112/128F mice 15 days postinfection (left panel). Numbers indicate the percentage of CD8+ H2Db:GP33-reactive lymphocytes within each gate. Top left and bottom right gates from top panel were used to quantify the percentage of SLECs (middle panel) and MPECs (right panel), respectively. For graphs each point represents an individual mouse; bars represent average ± SEM. Data are representative of 3 independent experiments with 5-10 mice per group. Significant P values, when present, comparing KI to WT are indicated by asterisks: ***P < .001, **P = .001-.01, *P = .01-.05.
SLP-76 KI mice generate long-lived antigen-specific T cells
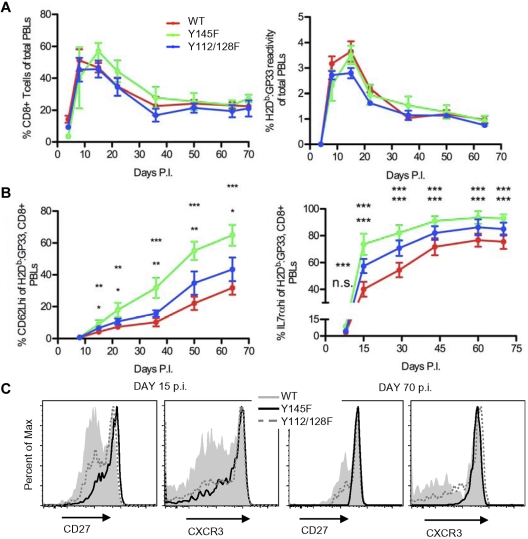
To determine whether virus-specific KI T cells can differentiate into long-lived memory cells, we monitored KI and WT mice for the persistence of H2Db:GP33-reactive CD8+ T cells in the blood of infected animals. The kinetics of expansion and contraction of total CD8+ PBLs and H2Db:GP33-reactive CD8+ PBLs was similar between KI and WT mice (Figure 6A). After 5 weeks, the frequency of CD8+ and H2Db:GP33-reactive CD8+ cells in all mice remained stable for the duration of the experiments up to 130 days (Figure 6A and data not shown). We next examined the H2Db:GP33-reactive CD8+ population for acquisition of CD62L, an indicator of Tcm cells. CD62L up-regulation within the population occurred at a significantly faster rate in both KI mice, (Figure 6B left panel). In addition, the KI LCMV-specific population contained more IL-7rαhi cells consistent with skewed memory differentiation (Figure 6B right panel). These differences were more pronounced in Y145F versus Y112/128F mice. Throughout the immune response, circulating LCMV-specific KI CD8+ T cells expressed higher levels of CD27 and CXCR3 (Figure 6C), 2 markers associated with functional maturation in memory cells.23,42 Compared with WT controls, the GP33-specific KI T-cell population showed accelerated acquisition of a Tcm surface phenotype and an increased frequency of cells expressing a functionally mature phenotype.
Figure 6.
SLP-76 KI mice generate long-lived memory T cells with accelerated acquisition of a “mature” phenotype. WT, Y145F, and Y112/128F mice were infected with LCMV Armstrong and monitored for 60-130 days. Data are representative of 3 independent experiments with 5-10 mice per group. (A) The percentage of CD8+ T cells (left panel) and H2Db:GP33-reactive CD8+ T cells (right panel) among PBLs from WT, Y145F, and Y112/128F mice at indicated days postinfection. (B) The percentage of CD62Lhi cells (left panel) and IL-7rαhi cells (right panel) among H2Db:GP33-reactive CD8+ PBLs from WT, Y145F, and Y112/128F mice at indicated days postinfection. Average percentage of cells ± SEM is shown. (C) CD27 and CXCR3 surface expression on WT, Y145F, and Y112/128F H2Db:GP33-reactive, CD8+ PBLs from day 15 (left panel) and day 70 (right panel) postinfection. Time points with significant differences between Y145F KI and WT (top asterisks) and Y112/128F KI and WT (bottom asterisks) are depicted: ***P < .001, **P = .001-.01, *P = .01-.05. Slopes of the graphic representation of CD62L expression over time were compared between WT and Y145F and WT and Y112/128F by nonlinear regression; P < .0001 for both comparisons. Slopes of the graphic representation of IL-7rα expression over time were compared between WT and Y145F and WT and Y112/128F by nonlinear regression and were not significantly different.
Long-lived (> day 70 postinfection) H2Db:GP33-reactive CD8+ T cells were found in similar numbers in the spleens of Y112/128F and WT mice but were slightly increased in the spleens of Y145F mice (supplemental Figure 2A). Compared with WT controls and similar to our observations in the PBL populations, the splenic, long-lived, LCMV-specific KI population was more skewed toward a Tcm phenotype and expressed markers indicative of a functionally mature memory population (supplemental Figure 2B). Furthermore, the long-lived KI splenic T cells had defective cytokine production in response to peptide that was similar to or worse than Day 8 postinfection KI T cells (supplemental Figure 2C-D).
SLP-76 KI memory cells can expand in response to rechallenge in vivo
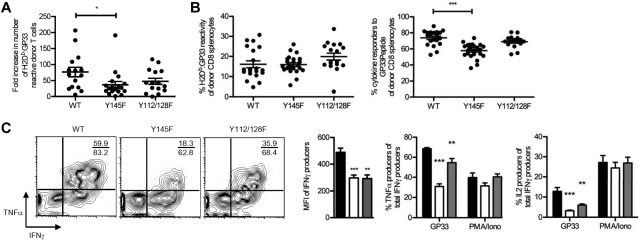
To determine whether memory KI T cells can function in vivo, CD8+ purified splenocytes from day 70 or greater postinfection KI and WT mice were transferred into WT congenic hosts, which were then infected with a strain of Listeria monocytogenes that expresses GP33 (LM:GP33). Seven to 8 days postinfection, host spleens were analyzed for the presence and function of donor H2Db:GP33-reactive T cells. Despite the increased frequencies of more mature memory phenotype cells in the KI memory pools, their response to rechallenge was not enhanced. Indeed, pooled data from all day 7 postinfection experiments suggests that a mild proliferative defect may be present in the Y145F KI memory cells (Figure 7A) as in some experiments, Y145F KI cells failed to expand to the extent of WT cells. The frequency of donor Y145F KI T cells, but not Y112/128F KI T cells, that responded to GP33 peptide (as measured by their ability to produce IFNγ) was less than that of WT donor cells despite similar frequencies of H2Db:GP33-reactive T cells (Figure 7B). These results suggest that fewer secondary effector GP33-specific Y145FKI T cells responded to peptide. The GP33 epitope also contains a peptide capable of presentation by H2Kb.43 Therefore, the diminished response observed in GP33-stimulated Y145F cultures might also reflect defective H2Kb responsiveness. Consistent with our observations in primary effectors and long-lived memory cells, the KI effector T cells following secondary challenge showed altered TNFα and IL-2 production and decreased MFI of IFNγ compared with donor WT T cells following GP33 peptide stimulation (Figure 7C). Furthermore, these defects were not observed when the donor cells were stimulated with PMA and ionomycin (Figure 7C). These data demonstrate that while KI memory cells show an accelerated acquisition of a mature memory phenotype and can expand upon in vivo rechallenge, the resultant KI secondary effectors are defective in their ability to liberate cytokine responses downstream of the TCR.
Figure 7.
SLP-76 KI memory cells expand in response to rechallenge in vivo. Equal numbers of CD8+ T cells from LCMV immune WT, Y145F, and Y112/128F mice were transferred into congenic hosts. Host mice were subsequently infected with LM:GP33 and analyzed 7-8 days later. (A) Fold increase of donor-derived H2Db:GP33-reactive cells in spleens from host mice that received WT, Y145F, and Y112/128F donor cells after 7 days postinfection. Each point represents an individual mouse; bars indicate average ± SEM. Data are pooled from 3 independent experiments each with 2-7 recipients per group. (B) Percentage H2Db:GP33-reactive cells of donor CD8+ T cells from spleens of day 7-8 postrechallenge mice (average ± SEM; left panel). Percentage of donor CD8+ T cells that produced IFNγ in response to GP33 peptide stimulation is graphed (average ± SEM; right panel). Data are pooled from 4 independent experiments, each with 2-7 recipients per group. (C) Splenocytes from recipient mice receiving WT, Y145F, and Y112/128F on day 7 following LM:GP33 infection were stimulated with GP33 or PMA plus ionomycin. Representative contour plots depict TNFα and IFNγ expression in CD45.2+CD8+ lymphocytes that were stimulated with GP33 peptide (left panel). MFI of IFNγ in response to GP33 is shown (left-most graph). Numbers indicate the percentage TNFα IFNγ double producers over the percentage of total IFNγ producers within the CD45.2+CD8+ gate. Peptide- or PMA/ionomycin-responsive splenocytes were quantified as of the frequency of CD45.2+CD8+ IFNγ, producers that coproduced TNFα (center graph), or IL-2 (right-most graph; representative of 4 independent experiments each with 2-7 recipients per group). Significant P values, when present, comparing KI to WT are indicated by asterisks: ***P < .001, **P = .001-.01, *P = .01-.05.
Discussion
Using SLP-76 KI mice and LCMV as a model of CD8+ T-cell–dependent immunity, we asked how dampened TCR signals affect CD8+ T-cell differentiation. We show that while the magnitude of the CD8+ T-cell–mediated immune response is preserved in mice in which SLP-76 tyrosines are mutated, differentiation and function of the responsive T cells are altered. Consistent with previously described models of memory formation, our data suggest that graded diminishment in proximal TCR signaling results in graded enhancement of memory precursor development.3 Unlike previous studies, here we show that dampened TCR signals can alter the balance between terminal differentiation and memory potential independent of the magnitude of the response. Moreover, this study demonstrates that the intracellular signals that drive expansion, differentiation and memory maintenance can be separated from those that regulate cytokine production.
SLP-76 N-terminal tyrosines are essential to enable a SLP-76-nucleated multimolecular signaling complex to optimally transduce TCR signals into divergent downstream signaling pathways, the best characterized of which involve intracellular Ca2+ mobilization and activation of mitogen-activated protein kinases.35 Using KI mice, we show that in vivo tyrosine to phenylalanine mutations at residues 145 or 112 and 128 together confer defects in peripheral TCR signal transduction that result in activation and proliferation defects following in vitro TCR stimulation. Signaling and functional defects are more severe in the Y145F compared with the Y112/128F KI T cells. As shown using cKI mice, these TCR signaling defects are independent of the role of SLP-76 tyrosines in T-cell thymic development.34
Having defined the nature of signaling defects in SLP-76 KI T cells, we used this system to address how specific mutations in proximal TCR signaling machinery impact effector versus memory differentiation, establishment, and maturation of memory populations and the functional capacities of primary and secondary effectors. The role of TCR signal strength in these processes has largely been tested indirectly by manipulating the immunogen or by comparing responses to different epitopes.2,5,8,12,14,21,24,31,33 These studies have shown that weak externally supplied signals diminish the magnitude of expansion, hasten the onset T-cell contraction and increase the rate of acquisition of Tcm cells. More recently, infection of mice with defects in nuclear factor κB activation resulted in intact expansion and effector differentiation but loss of memory formation, thus, providing evidence that distinct TCR signals can direct effector versus memory fates.13
Here, mice with biochemical defects in TCR-induced Ca2+ flux and PLCγ1 and ERK1/2 activation have a surprisingly normal magnitude of CD8+ T-cell expansion in response to acute LCMV infection despite profound in vitro proliferative defects. This observation is in contrast to previous studies that have shown decreased expansion in the context of diminished signals from the TCR, including studies in mice deficient for Itk, a kinase whose function is dependent on the tyrosines of SLP-76.12,44,45 It is possible that Itk has kinase- or SLP-76–independent functions that are important for proliferative responses in vivo. It is also possible that the overall strength of the TCR signal in SLP-76 KI T cells falls above the threshold for optimal proliferation and that the strength of TCR signal in Itk-deficient cells falls below this threshold.
Dampened TCR signaling in KI T cells correlated with an increased number and rate of accumulation of GP33-specific CD62Lhi cells. Our data are consistent with previous studies suggesting that the transition from a Tem- to a Tcm-dominant CD8+ T-cell memory pool occurs faster in the face of weak initial antigen stimuli.8,21 Furthermore, the KI memory pool is enriched for cells expressing high levels of CD27 and CXCR3, 2 activation markers associated with increased functional avidity.22,23,46 Although we have not ruled out a role for CD4+ T-cell help in our model, the phenotype of LCMV-specific KI CD8+ T cells does not resemble that of previously described CD8+ T cells that differentiate in the absence of proper CD4+ T-cell help.47 Similar to our observations involving dampened SLP-76–dependent signaling, complete deletion of SLP-76 during the contraction phase or after CD8+ T-cell memory formation triggers an accelerated transition to a Tcm-dominant memory pool.48 While these data suggest a late role for SLP-76 signals in memory differentiation they do not preclude an early role for SLP-76 in programming differentiation patterns. Taken together, these data suggest that the kinetics of the transition of the memory pool to a more mature and Tcm-dominant phenotype is influenced by TCR signals and that strong TCR signals are required for terminal differentiation.
Both direct and indirect mechanisms may play roles in how TCR signal strength can regulate CD8+ T-cell differentiation. Dampened TCR signals could indirectly result in the false perception of abbreviated antigen exposure, such that even if the kinetics of antigen clearance are similar in KI and WT mice, KI cells may not “see” low levels of antigen during later phases of the response. T-cell memory differentiation is hastened if the level of antigen or the duration of antigen exposure is decreased.21 Specific signals downstream of SLP-76 may play a direct role in the altered differentiation of KI T cells. For example, through its association with Itk, Y145 of SLP-76 has been implicated in the down-regulation of T-bet, a key regulator of CD8+ T-cell fate choices6,49 (J. Chang and S. Reiner, unpublished data, University of Pennsylvania, Philadelphia, PA, 2009).
SLP-76 KI CD8+ T cells of various differential stages show striking disconnects between surface phenotype and functional responses to subsequent TCR stimulation. This is consistent with recent studies showing that deletion of SLP-76 in CD4+ T cells following priming results in the generation of phenotypic effector and memory cells that cannot produce cytokine in response to TCR ligation.50 While the surface phenotype of KI T-cell populations may suggest a memory bias, the failure of KI T cells to produce IL-2 following MHC:peptide stimulation suggests otherwise. However, when primary or secondary effector cells were stimulated with PMA plus ionomycin to bypass the proximal TCR signaling machinery, KI cells were fully capable of IL-2 production, suggesting that their differentiation was intact. The ability to undergo TCR-dependent differentiation but failure to produce cytokine in response to subsequent TCR stimulation is reminiscent of defects long observed in the T-helper 2 responses of ITK–/– and ITK/RLK–/– CD4+ T cells and more recently observed in the Y145F and Y112/128F KI CD4+ T cells51 (J.E.S.-G., unpublished data, 2009). The SLP-76 KI mice therefore reveal that the TCR signals required for CD8+ T-cell differentiation are either quantitatively or qualitatively different from the TCR signals required for the generation of effector cytokines. In addition, both the quality of differentiation and of cytokine production are linked to TCR signal strength, as both cytokine recall and memory differentiation are less affected in the Y112/128F KI mice compared with the Y145F KI mice.
By manipulating a key intracellular signaling molecule, we have demonstrated that the differentiation fate of CD8+ T cells engaged in an immune response can be affected by the strength of signaling downstream of the TCR. Strong TCR signals permit balanced development of terminally differentiated effector cells and long-lived memory CD8+ T cells, whereas weak or dampened signals favor the development of memory cells at the expense of terminal effectors. Dampened TCR signaling can be sufficient for primary clonal expansion and for development of a complete immune response but still result in skewed differentiation and defective effector function. Thus, the ability to acquire effector function and the ability to exert effector function are separable and are differentially regulated through the TCR.
Supplementary Material
Acknowledgments
We thank Haina Shin for technical assistance, Matthew Riese and Scott Lieberman for critically reading the manuscript, and Justina Stadanlick for editorial assistance.
This work was supported by grants from the Arthritis Foundation (to M.S.J.) and the National Institutes of Health (K01AR52802 to M.S.J., R37GM053256 to G.A.K., and T32-RR007063-10 to J.E.S.-G.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.E.S.-G. wrote the paper, designed experiments, performed most research, and analyzed data; J.C.B. performed experiments; M.G. performed experiments; T.Z. performed experiments; J.S.K. performed experiments; J.S.M. designed the experiments and generated critical reagents; E.J.W. designed experiments and generated critical reagents; G.A.K. wrote the paper, designed experiments, and supervised the project; and M.S.J. wrote the paper, designed experiments, performed research, analyzed data, and supervised the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Martha S. Jordan, Department of Pathology and Laboratory Medicine, University of Pennsylvania, 413 BRB II/III, 421 Curie Blvd, Philadelphia, PA 19104; e-mail: jordanm@mail.med.upenn.edu.
References
- 1.Obar JJ, Lefrancois L. Memory CD8+ T cell differentiation. Ann N Y Acad Sci. 2010;1183:251–266. doi: 10.1111/j.1749-6632.2009.05126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2(5):415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joshi NS, Kaech SM. Effector CD8 T cell development: a balancing act between memory cell potential and terminal differentiation. J Immunol. 2008;180(3):1309–1315. doi: 10.4049/jimmunol.180.3.1309. [DOI] [PubMed] [Google Scholar]
- 4.Sandau MM, Kohlmeier JE, Woodland DL, Jameson SC. IL-15 regulates both quantitative and qualitative features of the memory CD8 T cell pool. J Immunol. 2010;184(1):35–44. doi: 10.4049/jimmunol.0803355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lefrancois L, Marzo A, Williams K. Sustained response initiation is required for T cell clonal expansion but not for effector or memory development in vivo. J Immunol. 2003;171(6):2832–2839. doi: 10.4049/jimmunol.171.6.2832. [DOI] [PubMed] [Google Scholar]
- 6.Intlekofer AM, Takemoto N, Wherry EJ, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6(12):1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 7.Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting Edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J Immunol. 2006;177(11):7515–7519. doi: 10.4049/jimmunol.177.11.7515. [DOI] [PubMed] [Google Scholar]
- 8.Sarkar S, Teichgraber V, Kalia V, et al. Strength of stimulus and clonal competition impact the rate of memory CD8 T cell differentiation. J Immunol. 2007;179(10):6704–6714. doi: 10.4049/jimmunol.179.10.6704. [DOI] [PubMed] [Google Scholar]
- 9.Kotturi MF, Scott I, Wolfe T, et al. Naive precursor frequencies and MHC binding rather than the degree of epitope diversity shape CD8+ T cell immunodominance. J Immunol. 2008;181(3):2124–2133. doi: 10.4049/jimmunol.181.3.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munitic I, Decaluwe H, Evaristo C, et al. Epitope specificity and relative clonal abundance do not affect CD8 differentiation patterns during lymphocytic choriomeningitis virus infection. J Virol. 2009;83(22):11795–11807. doi: 10.1128/JVI.01402-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rutishauser RL, Martins GA, Kalachikov S, et al. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31(2):296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458(7235):211–214. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teixeiro E, Daniels MA, Hamilton SE, et al. Different T cell receptor signals determine CD8+ memory versus effector development. Science. 2009;323(5913):502–505. doi: 10.1126/science.1163612. [DOI] [PubMed] [Google Scholar]
- 14.Ndhlovu ZM, Oelke M, Schneck JP, Griffin DE. Dynamic regulation of functionally distinct virus-specific T cells. Proc Natl Acad Sci U S A. 2010;107(8):3669–3674. doi: 10.1073/pnas.0915168107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4(12):1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 16.Joshi NS, Cui W, Chandele A, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27(2):281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205(3):625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Precopio ML, Betts MR, Parrino J, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J Exp Med. 2007;204(6):1405–1416. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 20.Bachmann MF, Wolint P, Schwarz K, Jager P, Oxenius A. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor α and CD62L. J Immunol. 2005;175(7):4686–4696. doi: 10.4049/jimmunol.175.7.4686. [DOI] [PubMed] [Google Scholar]
- 21.Wherry EJ, Teichgraber V, Becker TC, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4(3):225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 22.Roberts AD, Ely KH, Woodland DL. Differential contributions of central and effector memory T cells to recall responses. J Exp Med. 2005;202(1):123–133. doi: 10.1084/jem.20050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hikono H, Kohlmeier JE, Takamura S, Wittmer ST, Roberts AD, Woodland DL. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J Exp Med. 2007;204(7):1625–1636. doi: 10.1084/jem.20070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77(8):4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Obar JJ, Crist SG, Gondek DC, Usherwood EJ. Different functional capacities of latent and lytic antigen-specific CD8 T cells in murine gammaherpesvirus infection. J Immunol. 2004;172(2):1213–1219. doi: 10.4049/jimmunol.172.2.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obar JJ, Fuse S, Leung EK, Bellfy SC, Usherwood EJ. gammaherpesvirus persistence alters key CD8 T-cell memory characteristics and enhances antiviral protection. J Virol. 2006;80(17):8303–8315. doi: 10.1128/JVI.00237-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheridan BS, Khanna KM, Frank GM, Hendricks RL. Latent virus influences the generation and maintenance of CD8+ T cell memory. J Immunol. 2006;177(12):8356–8364. doi: 10.4049/jimmunol.177.12.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27(3):393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Araki K, Turner AP, Shaffer VO, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460(7251):108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D'Souza WN, Hedrick SM. Cutting edge: latecomer CD8 T cells are imprinted with a unique differentiation program. J Immunol. 2006;177(2):777–781. doi: 10.4049/jimmunol.177.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Badovinac VP, Harty JT. Manipulating the rate of memory CD8+ T cell generation after acute infection. J Immunol. 2007;179(1):53–63. doi: 10.4049/jimmunol.179.1.53. [DOI] [PubMed] [Google Scholar]
- 32.Williams MA, Bevan MJ. Shortening the infectious period does not alter expansion of CD8 T cells but diminishes their capacity to differentiate into memory cells. J Immunol. 2004;173(11):6694–6702. doi: 10.4049/jimmunol.173.11.6694. [DOI] [PubMed] [Google Scholar]
- 33.Wherry EJ, Puorro KA, Porgador A, Eisenlohr LC. The induction of virus-specific CTL as a function of increasing epitope expression: responses rise steadily until excessively high levels of epitope are attained. J Immunol. 1999;163(7):3735–3745. [PubMed] [Google Scholar]
- 34.Jordan MS, Smith JE, Burns JC, et al. Complementation in trans of altered thymocyte development in mice expressing mutant forms of the adaptor molecule SLP76. Immunity. 2008;28(3):359–369. doi: 10.1016/j.immuni.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maltzman JS, Kovoor L, Clements JL, Koretzky GA. Conditional deletion reveals a cell-autonomous requirement of SLP-76 for thymocyte selection. J Exp Med. 2005;202(7):893–900. doi: 10.1084/jem.20051128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruzankina Y, Pinzon-Guzman C, Asare A, et al. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell. 2007;1(1):113–126. doi: 10.1016/j.stem.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Srinivas S, Watanabe T, Lin CS, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berg LJ. Signalling through TEC kinases regulates conventional versus innate CD8(+) T-cell development. Nat Rev Immunol. 2007;7(6):479–485. doi: 10.1038/nri2091. [DOI] [PubMed] [Google Scholar]
- 40.Shiow LR, Rosen DB, Brdickova N, et al. CD69 acts downstream of interferon-α/β to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440(7083):540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 41.Sonnenberg GF, Mangan PR, Bezman NA, et al. Mislocalization of SLP-76 leads to aberrant inflammatory cytokine and autoantibody production. Blood. 2010;115(11):2186–2195. doi: 10.1182/blood-2009-08-237438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slifka MK, Whitton JL. Functional avidity maturation of CD8(+) T cells without selection of higher affinity TCR. Nat Immunol. 2001;2(8):711–717. doi: 10.1038/90650. [DOI] [PubMed] [Google Scholar]
- 43.Hudrisier D, Oldstone MB, Gairin JE. The signal sequence of lymphocytic choriomeningitis virus contains an immunodominant cytotoxic T cell epitope that is restricted by both H-2D(b) and H-2K(b) molecules. Virology. 1997;234(1):62–73. doi: 10.1006/viro.1997.8627. [DOI] [PubMed] [Google Scholar]
- 44.Atherly LO, Brehm MA, Welsh RM, Berg LJ. Tec kinases Itk and Rlk are required for CD8+ T cell responses to virus infection independent of their role in CD4+ T cell help. J Immunol. 2006;176(3):1571–1581. doi: 10.4049/jimmunol.176.3.1571. [DOI] [PubMed] [Google Scholar]
- 45.Bogin Y, Ainey C, Beach D, Yablonski D. SLP-76 mediates and maintains activation of the Tec family kinase ITK via the T cell antigen receptor-induced association between SLP-76 and ITK. Proc Natl Acad Sci U S A. 2007;104(16):6638–6643. doi: 10.1073/pnas.0609771104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roberts AD, Woodland DL. Cutting edge: effector memory CD8+ T cells play a prominent role in recall responses to secondary viral infection in the lung. J Immunol. 2004;172(11):6533–6537. doi: 10.4049/jimmunol.172.11.6533. [DOI] [PubMed] [Google Scholar]
- 47.Intlekofer AM, Takemoto N, Kao C, et al. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J Exp Med. 2007;204(9):2015–2021. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wiehagen KR, Corbo E, Schmidt M, Shin H, Wherry EJ, Maltzman JS. Loss of tonic T-cell receptor signals alters the generation but not the persistence of CD8+ memory T cells. Blood. 2010;116(25):5560–5570. doi: 10.1182/blood-2010-06-292458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller AT, Wilcox HM, Lai Z, Berg LJ. Signaling through Itk promotes T helper 2 differentiation via negative regulation of T-bet. Immunity. 2004;21(1):67–80. doi: 10.1016/j.immuni.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 50.Bushar ND, Corbo E, Schmidt M, Maltzman JS, Farber DL. Ablation of SLP-76 signaling after T cell priming generates memory CD4 T cells impaired in steady-state and cytokine-driven homeostasis. Proc Natl Acad Sci U S A. 2010;107(2):827–831. doi: 10.1073/pnas.0908126107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Au-Yeung BB, Katzman SD, Fowell DJ. Cutting edge: Itk-dependent signals required for CD4+ T cells to exert, but not gain, Th2 effector function. J Immunol. 2006;176(7):3895–3899. doi: 10.4049/jimmunol.176.7.3895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.