Abstract
8-Aminoadenosine (8-NH2-Ado), a ribosyl nucleoside analog, in preclinical models of multiple myeloma inhibits phosphorylation of proteins in multiple growth and survival pathways, including Akt. Given that Akt controls the activity of mammalian target of rapamycin (mTOR), we hypothesized that 8-NH2-Ado would be active in mantle cell lymphoma (MCL), a hematological malignancy clinically responsive to mTOR inhibitors. In the current study, the preclinical efficacy of 8-NH2-Ado and its resulting effects on Akt/mTOR and extracellular-signal–regulated kinase signaling were evaluated using 4 MCL cell lines, primary MCL cells, and normal lymphocytes from healthy donors. For all MCL cell lines, 8-NH2-Ado inhibited growth and promoted cell death as shown by reduction of thymidine incorporation, loss of mitochondrial membrane potential, and poly (adenosine diphosphate-ribose) polymerase cleavage. The efficacy of 8-NH2-Ado was highly associated with intracellular accumulation of 8-NH2-adenosine triphosphate (ATP) and loss of endogenous ATP. Formation of 8-NH2-ATP was also associated with inhibition of transcription and translation accompanied by loss of phosphorylated (p-)Akt, p-mTOR, p-Erk1/2, p-phosphoprotein (p)38, p-S6, and p-4E-binding protein 1. While normal lymphocytes accumulated 8-NH2-ATP but maintained their viability with 8-NH2-Ado treatment, primary lymphoma cells accumulated higher concentrations of 8-NH2-ATP, had increased loss of ATP, and underwent apoptosis. We conclude that 8-NH2-Ado is efficacious in preclinical models of MCL and inhibits signaling of Akt/mTOR and Erk pathways.
Introduction
Mantle cell lymphoma (MCL) is an incurable hematological malignancy characterized cytogenetically by the t(11;14)(q13;32) resulting in overexpression of cyclin D1.1,2 In addition to elevated cyclin D1 levels, MCL has multiple deregulated or dysfunctional survival and growth pathways including DNA repair, apoptosis, and phosphatidyl-inositol-3 kinase (PI3K)/Akt signaling.3,4 Specifically, Akt and downstream mammalian target of rapamycin (mTOR) activation have been associated with an aggressive blastoid phenotype and consequently may be important in the pathogenesis of MCL.5,6 Indeed, the Akt/mTOR pathway is activated in MCL by amplification of the PI3K catalytic subunit alpha7 or the inactivating hypermethylation of the negative regulator phosphatase and tensin homolog.6 mTOR inhibitors such as temsirolimus8–10 and deforolimus11 as single agents have shown clinically relevant activity in trials for relapsed or refractory MCL, producing 22% to 41% overall response rates. In combination, mTOR inhibitors are postulated to be synergistic with current MCL therapies.12 Because mTOR inhibitors can indirectly activate Akt, PI3K/Akt inhibitors in combination or as single agents may be more effective in the treatment of MCL than mTOR inhibitors alone.6
Given that MCL is sensitive to mTOR and PI3K/Akt inhibitors, we hypothesized that MCL would be responsive to 8-Aminoadenosine (8-NH2-Ado), an adenosine analog previously observed to reduce phosphorylation of Akt in multiple myeloma cell lines.13 In addition to or perhaps as a result of its effects on the Akt pathway, 8-NH2-Ado targets cellular energetics, most notably the reduction of glucose uptake and intracellular adenosine triphosphate (ATP).14–17 This analog accumulates intracellularly as its triphosphate, 8-NH2-ATP, to millimolar concentrations in tumor cells.13 In its triphosphate form, 8-NH2-Ado may competitively inhibit kinases and other enzymes that use ATP as a substrate including RNA polymerases.16 For RNA polymerase II, 8-NH2-ATP is also incorporated into mRNA as a chain terminator and inhibits global transcription.16 Similarly, as demonstrated using yeast18 and bovine19 enzymes, 8-NH2-ATP is a substrate of poly(A) polymerase and incorporates into the polyA tail of transcripts thereby also impacting global transcription. In sum, 8-NH2-Ado has pleiotropic effects on cellular bioenergetics, signaling, and gene expression that may be beneficial for the treatment of hematological malignancies.
In the current study, we evaluated 8-NH2-Ado as a therapeutic agent in MCL. In addition to measuring its effects on transcription and ATP loss, we also evaluated the Akt/mTOR and extracellular–signal–regulated kinase (Erk) signaling pathways. In 4 different MCL cell lines, 8-NH2-Ado accumulated as 8-NH2-ATP, promoted apoptosis, and inhibited key survival and proliferation signaling pathways including Akt/mTOR, phosphoprotein (p)38, and Erk1/2. Consistent with the cell line studies, rates of global protein translation after 8-NH2-Ado treatment were also compromised in primary MCL cells. In contrast with its effects on cell lines and patient lymphoma cells, 8-NH2-Ado treatment of normal lymphocytes did not modulate the phosphorylation status of Akt/mTOR, p38, and Erk1/2 pathways. Inhibition of Akt and Erk pathways may explain, at least in part, the selective ability of 8-NH2-Ado to decrease proliferation and promote cell death of MCL cells.
Methods
Cell line culture
Granta 519, JeKo, Mino, and SP-53 MCL cell lines were a gift from Dr Hesham Amin (M. D. Anderson Cancer Center) and were maintained as described previously.20 All cells were routinely tested for Mycoplasma infection using a MycoTect Kit (Invitrogen). The identities of all cell lines were verified using AmpF/STR Identifier kit (Applied Biosystems). Database information was unavailable to verify the identity of SP-53, but its profile did not match any known cell line.
Patients and primary cell culture
Informed consent was obtained for all patients according to the Declaration of Helsinki, and the protocols were approved by the Institutional Review Boards at M. D. Anderson Cancer Center and Northwestern University. The patient characteristics are listed in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). A separate protocol, which was also Institutional Review Board–approved, allowed us to obtain peripheral blood mononuclear cell (PBMC) samples from healthy volunteers. Unless indicated otherwise, normal and malignant lymphocytes from peripheral blood or from apheresis samples were isolated by density gradient centrifugation over Ficoll Paque (GE Healthcare) and were incubated in RPMI 1640 medium containing 10% heat-inactivated human serum (Sigma-Aldrich).
Reagents
8-NH2-Ado was purchased from R.I. Chemicals Inc [methyl-3H-thymidine], [5,6-3H]-uridine, and [4,5-3H]-L-leucine were purchased from Moravek Biochemicals Inc. Annexin V fluorescein isothiocyanate (FITC) was from BD Biosciences, and 3,3-dihexyloxacarbocyanine iodine (DiOC6) was from Molecular Probes. All other reagents were high-performance liquid chromatography (HPLC) grade and purchased from Sigma-Aldrich.
Nucleotide concentrations
To quantify ATP and uridine triphosphate (UTP) concentrations, neutralized perchloric acid extracts were separated by HPLC and quantified by ultraviolet light detection as described previously.20 For thymidine triphosphate (TTP) quantification, the methanol precipitated extracts were first treated with sodium periodate to oxidize the ribose nucleotides.21 The remaining solution was then immediately analyzed by HPLC as described previously.22
Rates of DNA synthesis, transcription, and translation
Intracellular incorporation of [3H]thymidine, [3H]uridine, and [3H]leucine into DNA, RNA, and proteins was used to quantify rates of DNA synthesis, transcription, and translation, respectively, as described previously.20 Because drug treatment may alter TTP and UTP levels, [3H]thymidine and [3H]uridine uptake was normalized to TTP and UTP specific activity, respectively, to determine the actual rates of DNA synthesis and transcription. TTP and UTP specific activities were determined by HPLC analysis using ultraviolet light and in-line radioscintillation counting.
Apoptosis and cell-cycle assays
Apoptosis was quantified by measuring loss of mitochondrial potential with DiOC6 or annexin-V binding using flow cytometry (FACSCalibur; BD Biosciences) as previously described.20 For cell-cycle analyses, cells were fixed in 70% ethanol, permeabilized, and incubated with propidium iodide at room temperature for 30 minutes. Cells were analyzed by flow cytometry using CellQuest software Version 3.3 (BD Biosciences).
Western blot analysis
Whole cell lysates were prepared and protein concentrations were quantified by Bio-Rad protein assay per the manufacturer's instructions.14 Protein was fractionated on precast Tris-glycine gels (Invitrogen) and transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore), and the membrane was blocked in casein. Primary antibodies were applied and incubated overnight at 4°C. The primary antibodies were purchased from BD Biosciences (glycogen synthase kinase [GSK3β; 610201], poly(adenosine diphosphate-ribose) polymerase [PARP; 556362]); Santa Cruz Biotechnology (Cyclin D1 [sc-819], p38 [sc-535], Mcl-1 [sc-819]); or Cell Signaling (Akt [9272], phosphorylated [p-]Akt [9271S], mTOR [2972], p-mTOR [2971S], p-GSK3β [9316S], S6 [2217], p-S6 [2215], 4E-binding protein 1 [BP1; 9459], p-4E-BP1 Thr70 [9455], Erk1/2[9102], p-Erk1/2[9101], and p-p38 [9211S]). For a protein loading control, the primary antibody for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was obtained from Millipore (MAB374). Secondary antibody was incubated for 1 hour at room temperature using either horseradish peroxidase–linked anti–rabbit antibody (Cell Signaling Technology) or anti–mouse antibody (Amersham Biosciences). Blots were developed using the Enhanced Chemiluminescence Plus Western blotting detection reagent (Amersham Bioscicenes) and visualized by autoradiography using Amersham Hyperfilm HP (GE Healthcare Limited).
Statistical analysis
GraphPad Prism Version 5.0 (GraphPad Software) was used to perform the statistical analyses. P values of < .05 were considered statistically significant.
Results
8-NH2-Ado promotes cell death and growth inhibition with minor changes in cell cycle
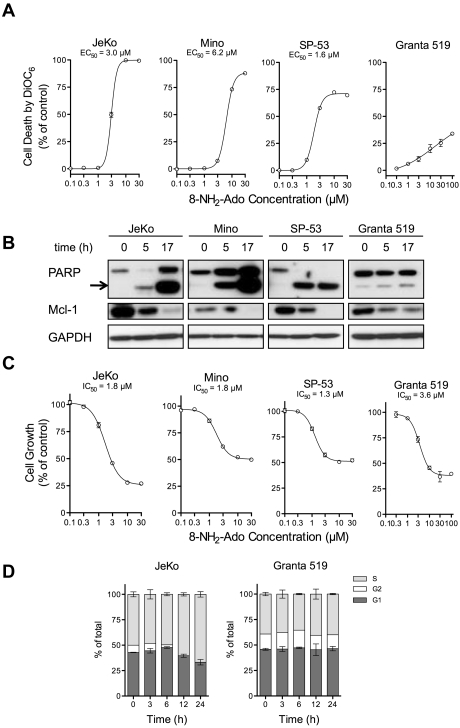
In all 4 MCL cell lines, 8-NH2-Ado induced time and dose-dependent cell death as shown by loss of mitochondrial potential and PARP cleavage (Figure 1A-B). As expected, Mcl-1 protein levels were reduced concurrently with PARP cleavage (Figure 1B) due to caspase activation and subsequent Mcl-1 cleavage. Although Granta 519 cells were the least apoptotic, the proliferation rates of all cell lines were inhibited by 8-NH2-Ado (Figure 1C). For JeKo and Mino cells, the growth IC50 values (Figure 1C) were substantially less than the half maximal effective concentration (EC)50 values (30%-50%, Figure 1A) indicating that 8-NH2-Ado effects on growth inhibition may be reversible at low concentrations. Interestingly, while JeKo cells accumulated in the S-phase with loss of the G2 fraction, growth inhibitory effects of 8-NH2-Ado were independent of cell cycle in Granta 519 cells (Figure 1D).
Figure 1.
8-NH2-Ado promotes cell death and inhibits cell growth but with only minor cell-cycle effects in MCL cell lines. (A) Cell death after 24 hours of continuous incubation was quantified by loss of mitochondrial membrane potential as measured by DiOC6 staining, at various concentrations of 8-NH2-Ado. For each cell line, the dose-response curve was estimated by nonlinear regression using a variable Hill Slope model. The EC50 was defined as the drug concentration required to achieve 50% of the maximum response. The standard errors of the estimated EC50 values for JeKo, Mino, and SP-53 were less than 3%; the EC50 value could not be accurately estimated for Granta 519. (B) Cell death was also evaluated by PARP cleavage. The arrow denotes cleaved PARP. The cells were continuously incubated with 3μM 8-NH2-Ado for 24 hours. (C) The growth inhibition of MCL cells was determined after 24 hours of continuous exposure to multiple concentrations of 8-NH2-Ado. The cell concentrations were quantified using a particle count and size analyzer (Beckman Coulter). The IC50 values were estimated by nonlinear regression using a variable Hill Slope model. The IC50 value was defined as the drug concentration required to achieve 50% of the maximum growth inhibition. The standard errors for the IC50 value estimates were less than 8% for all cell lines. (D) The cell-cycle effects were determined by flow cytometry for JeKo and Granta 519 after continuous treatment with 3μM 8-NH2-Ado at various time points up to 24 hours. Independent experiments were performed in triplicate shown with SD error bars.
8-NH2-ATP accumulation is associated with reduced ATP/UTP levels and cell death
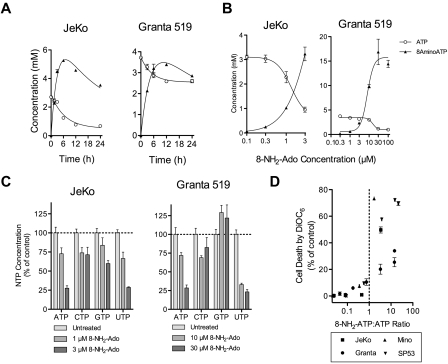
All cell lines, including JeKo and Granta 519, accumulated high concentrations of 8-NH2-ATP, the putative active metabolite (Figure 2A-B). In fact, although the fraction of apoptotic Granta 519 cells was comparatively low (< 30%, Figure 1A), Granta 519 cells accumulated the highest concentration of 8-NH2-ATP reported for any malignancy (15mM), and ATP levels were reduced 70%. Thus, low 8-NH2-ATP accumulation or ATP decline were not the cause of reduced apoptosis in Granta 519 cells. Although we do not know the exact mechanism for resistance to 8-NH2-Ado, Granta 519 also does not undergo apoptosis with 8-chloroadenosine, and its resistance is mainly attributed to high expression of B-cell lymphoma (Bcl)–2.20,23 Consistent with this hypothesis, Bcl-2 inhibitors promote apoptosis of Granta 519.24
Figure 2.
Cell death and growth inhibition with 8-NH2-Ado are associated with accumulation of intracellular 8-NH2-ATP and reduction of ATP and UTP concentrations. (A) Three micromolar 8-NH2-Ado accumulated as 8-NH2-ATP and depleted ATP after continuous treatment for up to 24 hours. (B) 8-NH2-ATP accumulation and depletion of ATP was dose-dependent after 24 hours for MCL cell lines JeKo and Granta 519. (C) ATP and UTP pool concentrations were substantially depleted after 24 hours of continuous 3μM 8-NH2-Ado treatment. For panels A through C, experiments were performed with JeKo and Granta 519 cells. (D) Cell death after 24 hours of 8-NH2-Ado treatment at multiple concentrations was positively associated with 8-NH2-ATP:ATP ratios for the 4 MCL cell lines. Independent experiments were performed in triplicate shown with SD error bars.
Interestingly, while loss of ATP by 8-NH2-Ado treatment has previously been reported, ATP loss in these MCL cell lines was also accompanied by loss of UTP (Figure 2C); for Granta 519 cells, the loss of UTP was even more pronounced than that of ATP. After 24 hours of continuous 8-NH2-Ado exposure, loss of ATP and the accumulation of 8-NH2-ATP were generally associated with cell death (Figure 2D). While the extent of the death varied between cell lines, substantial death (> 15%) was observed when 8-NH2-ATP:ATP ratios were higher than 1.
8-NH2-Ado inhibits global transcription, DNA synthesis, and translation
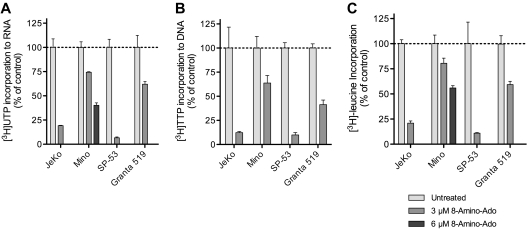
At 3μM 8-NH2-Ado, a concentration less than 2 × EC50 for JeKo and SP-53 (Figure 1A), we quantified the effects of 24 hours of treatment with 8-NH2-Ado on [3H] thymidine, uridine, and leucine incorporation (Figure 3). For Mino, 6μM 8-NH2-Ado, its EC50 value, was also evaluated for its effect on DNA, RNA, and protein synthesis (Figure 1A). For thymidine and uridine (Figure 3A-B), incorporation was normalized to the specific activity of its corresponding triphosphate as determined by HPLC because the total TTP (data not shown) and UTP concentrations (Figure 2C) were reduced by 8-NH2-Ado treatment. As expected, based on the growth inhibition data (Figure 1B) and minimal cell-cycle effects (Figure 1C), 8-NH2-Ado treatment inhibited TTP incorporation into DNA (Figure 3A). The rates of RNA synthesis and protein synthesis were also inhibited in all cell lines (Figure 3B-C). However, JeKo and SP53 responses were overall greater than those of Mino and Granta 519. As shown earlier, global transcription inhibition by 8-NH2-Ado also may have caused loss of Mcl-1, a labile protein encoded by a short half-life transcript (Figure 1B).
Figure 3.
8-NH2-Ado treatment of MCL cell lines reduces the rates of macromolecule synthesis. 8-NH2-Ado inhibited the rates of (A) transcription as quantified by uridine incorporation, (B) DNA synthesis as quantified by thymidine incorporation, and (C) translation as quantified by leucine incorporation. MCL cell lines were treated continuously with 3μM 8-NH2-Ado for 24 hours. Because 3μM 8-NH2-Ado was below its EC50 (Figure 1), Mino cells were also treated with 6μM 8-NH2-Ado for uridine and leucine incorporation experiments. All sample values were normalized to cell count. For [3H]uridine and [3H]thymidine incorporation values, the total counts of the acid-insoluble pellet were also normalized to the specific activity of UTP and TTP in the acid-soluble fraction at the end of the 45-minute chase. Independent experiments were performed in triplicate shown with SD error bars.
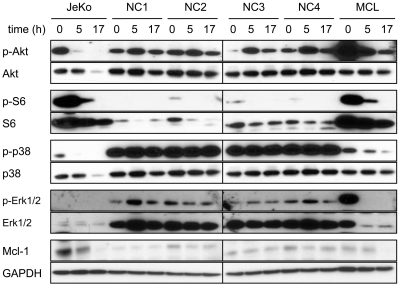
8-NH2-Ado inhibits Akt/mTOR and Erk phosphorylation of MCL cells but not PBMCs
We have previously observed that 8-NH2-Ado can reduce phosphorylation of key growth kinases in myeloma cell lines, specifically p-Akt.13 Thus, we hypothesized that 8-NH2-Ado would reduce the rates of macromolecular synthesis observed in MCL cell lines because of a decrease in activity of the mTOR/Akt pathway.
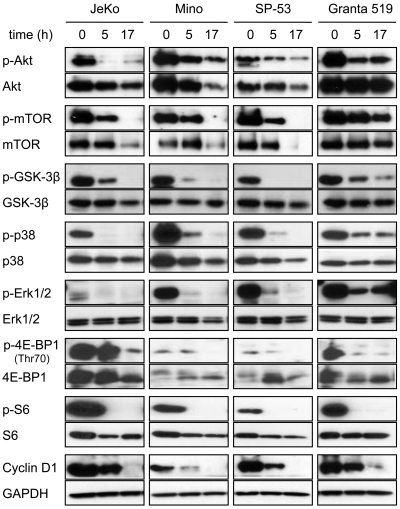
To investigate the effects of 8-NH2-Ado on the mTOR/Akt pathway, we incubated MCL cell lines with 3μM 8-NH2-Ado for 0, 5, and 17 hours and immunoblotted for both phosphoproteins and total proteins for Akt, mTOR, GSK3β, S6, and 4E-BP1 kinases (Figure 4). For the 4 cell lines, all of these proteins were phosphorylated, as previously reported,23 indicative of constitutive Akt/mTOR activation in untreated cells. After 8-NH2-Ado treatment, however, there was a significant decrease in all phospho-proteins. While the total protein was reduced for some kinases at 17 hours, 8-NH2-Ado treatment impaired pathway signaling at 5 hours without reducing total protein levels. Interestingly, the inhibition of Akt/mTOR pathway signaling at 5 hours was concurrent with PARP cleavage in 3 of 4 cell lines (Figure 1B) suggesting that 8-NH2-Ado–mediated apoptosis may be related to signaling. Consistent with inhibition of the Akt/mTOR pathway, 8-NH2-Ado treatment also promoted a time-dependent decrease in cyclin D1 (Figure 4), a protein dependent on 4E-BP1 for translation and GSK3β for stabilization.25,26 Of note, although cyclin D1 levels were dramatically decreased in JeKo and Granta 519 cells after 8-NH2-Ado treatment, the cells did not accumulate in G0/G1 (Figure 1D), the generally expected cellular response to cyclin D1 reduction. However, because reduction of cyclin D1 alone in these cell lines does not affect survival and has minimal impact on proliferation,27 other mechanisms of 8-NH2-Ado are likely responsible for cell death and growth inhibition (Figure 1).
Figure 4.
8-NH2-Ado inhibits Akt and Erk signaling pathways of MCL cell lines. JeKo, Mino, SP-53, and Granta 519 cells were incubated continuously with 3μM 8-NH2-Ado for 0, 5, and 17 hours. Cell lysates (30 μg) were immunoblotted for phospho- and total protein levels as shown. GAPDH was used as a loading control.
Given that mTOR and 4E-BP1 are also regulated by Erk pathways,28,29 we evaluated the effects of 8-NH2-Ado treatment on Erk1/2 and p38. As reported previously,23 Erk1/2 was constitutively activated in these cell lines. We observed that the 3μM concentration of 8-NH2-Ado significantly decreased the amount of p-p38 and p-Erk1/2 at 5 hours of incubation, with minor or no change in the total protein levels. At 17 hours, p-p38 and p-Erk1/2 were undetectable in Jeko, Mino, and SP-53 cells and substantially reduced in Granta cells.
To assess the selectivity of 8-NH2-Ado on the mTOR/Akt pathway, we evaluated the effect of 8-NH2-Ado in PBMCs isolated from healthy volunteers and compared this effect to those in lymphocytes isolated from an MCL patient with blastic and leukemic disease. For comparison, extracts from JeKo cells were reanalyzed. We first examined the effect of 8-NH2-Ado on the phosphorylation of Akt, S6, p38, and Erk1/2 and found that while 8-NH2-Ado treatment decreased the phosphorylation of all these proteins in the JeKo cells and the lymphocytes isolated from the MCL patient, there was no effect on their phosphorylation status in the PBMCs from healthy volunteers (Figure 5). In addition, while the expression of Mcl-1 in PBMC samples was scarcely detectable and unaffected by 8-NH2-Ado treatment, in JeKo cells and MCL patient lymphoma cells, Mcl-1 was expressed at elevated levels in untreated cells and decreased after exposure to 8-NH2-Ado. These data indicate that while 8-NH2-Ado had a significant effect on the mTOR/Akt and Erk pathways of MCL cell lines and the MCL patient lymphoma, the PBMCs of healthy volunteers were relatively unaffected by 8-NH2-Ado treatment.
Figure 5.
Actions of 8-NH2-Ado on Akt and Erk signaling pathways of peripheral blood monocytes. Freshly isolated PBMCs in 10% fetal bovine serum (FBS), RPMI were incubated continuously with 3μM 8-NH2-Ado for 0, 5, and 17 hours. Lymphoma cells from a lymphoma patient (MCL) were collected from the peripheral blood and allowed to proliferate in culture (20% FBS in Dulbecco modified Eagle medium). Once sufficient cell counts were obtained, the lymphoma cells were incubated continuously with 3μM 8-NH2-Ado for 0, 5, and 17 hours. JeKo cell lysates (Figure 4) were used for comparison. Cell lysates (30 μg) were immunoblotted for phospho- and total protein levels as shown. GAPDH was used as a loading control.
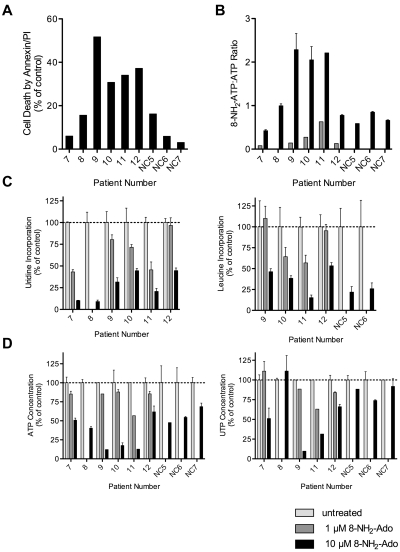
8-NH2-Ado promotes apoptosis, inhibits macromolecule synthesis, and inhibits bioenergetics of primary lymphoma cells
We evaluated the effects of 8-NH2-Ado treatment on primary lymphoma cells isolated from peripheral blood of MCL (N = 4) and other B-cell lymphoma patients (N = 2) with leukemic disease, one with cytologic and cytogenetic evidence of transformation characterized by larger cells and t(8;14)(q24;q32). After continuous exposure to 10μM 8-NH2-Ado, the majority of these lymphoma patient cells did undergo apoptosis including cells from patients 9, 10, 11, and 12 (Figure 6A). For the 2 patients (7 and 8) with lymphoma cells that were most resistant to 8-NH2-Ado treatment, patient 7 had a complex karyotype, and patient 8 had received 4 prior therapies (supplemental Table 1); thus, these 2 patient tumors may have developed resistance mechanisms. 8-NH2-Ado treatment promoted cell death concurrently with reduced rates of transcription and translation (Figure 6C) and loss of ATP (Figure 6D). However, In comparison with 8-NH2-Ado effects on UTP in cell lines, patient cell UTP levels were reduced less consistently and to a lesser degree (Figure 6D). A measure of 8-NH2-ATP intracellular accumulation and activity, 8-NH2-ATP:ATP ratios were more highly associated with cell death (Figure 6B, r = 0.78, P = .02) than to percent loss of ATP alone (Figure 6D, r = 0.68, P = .06). Although 8-NH2-Ado treatment did inhibit transcription and translation at concentrations as low as 1μM, this concentration was not sufficient to promote cell death (Figure 6C) suggesting that other factors, such as Bcl-2 expression, may be more determinant for cell death.
Figure 6.
Differential effects of 8-NH2-Ado on primary lymphoma cells and normal PBMCs. Freshly isolated cells were exposed to 1μM (gray bars) or 10μM (black bars) 8-NH2-Ado continuously for up to 48 hours. PBMC data from healthy donors are presented as controls. (A) Cell death by Annexin-V staining was normalized to untreated control cells. (B) The combined relative 8-NH2-Ado accumulation and ATP loss were represented by 8-NH2-ATP:ATP ratios. (C) Inhibition of transcription (left) and translation (right) was quantified by [3H]uridine and [3H]leucine incorporation. (D) The reduction in ATP (left) and UTP (right) levels were determined by HPLC analyses. When cell numbers were sufficient, independent experiments were performed in triplicate shown with SD error bars. All cells were incubated with 1 or 10μM 8-NH2-Ado for 48 hours in 10% human serum/RPMI with the following exceptions: patient 9 cells were incubated for 24 hours in 10% FBS/RPMI, and patient 12 cells were incubated for 24 hours. Patient numbers 7, 8, 9, and 12 were diagnosed with MCL. Patient 10 was diagnosed with marginal zone B-cell lymphoma, and patient 11 was diagnosed with splenic marginal zone B-cell lymphoma in transformation.
Although 2 MCL patient samples (7 and 8) were relatively resistant to 8-NH2-Ado treatment (Figure 6A), lymphoma cells were more sensitive to apoptosis after 10μM 8-NH2-Ado treatment compared with normal PBMCs (P = .04). The major difference in biochemical response between the PBMCs and lymphoma cells was primarily the accumulation of 8-NH2-ATP and subsequent loss of ATP. Interestingly, while the Akt/mTOR and Erk pathways of PBMCs were not inhibited during short incubation experiments with 3μM 8-NH2-Ado, translation was inhibited after 48 hours of 10μM 8-NH2-Ado treatment (Figure 6). However, even under these more extreme treatment conditions, PBMCs were resistant to apoptosis with 8-NH2-Ado.
Discussion
In the current report, we demonstrate that 8-NH2-Ado is efficacious in preclinical models of MCL, consisting of 4 cell lines and primary lymphoma cells without affecting normal peripheral blood cells. Importantly, 8-NH2-Ado actions on lymphoma cells were not only antiproliferative but cytotoxic. The cytotoxic actions of 8-NH2-Ado, an adenosine analog, can be attributed to its effects on the many cellular processes that use ATP, for instance, transcription.13–17 Here, we report that 8-NH2-Ado actions also include the inhibition of the Akt/mTOR and Erk pathways that are critical to the pathogenesis, proliferation, and survival of MCL.5,12,30,31
For the MCL cell lines in this study, 8-NH2-Ado treatment was able to dramatically reduce phosphorylation of kinases in the Akt/mTOR and Erk pathways after relatively short incubations (5 hours, Figures 4–5). We demonstrated consistent inhibition of these pathways by measuring downstream loss of phosphorylated 4E-BP1, S6, GSK3β, loss of cyclin D1, and reduced rates of global translation as quantified by [3H]leucine incorporation (Figure 3C). 8-NH2-Ado may be therapeutically advantageous compared with other mTOR inhibitors because 8-NH2-Ado inactivates the Akt/mTOR pathway without apparent feedback regulation. While it is clear that inhibition of the mTOR pathway is a clinically relevant approach for the treatment of patients with MCL,8,9,11 mTOR inhibitors as a single agents are cytostatic and do not consistently reduce cyclin D1 levels.6,23 Others have demonstrated the importance of the Akt and the Erk pathways as mechanisms of resistance to apoptosis.6,23 Thus, we conclude that 8-NH2-Ado as a therapeutic agent in MCL warrants further investigation because its actions may target the same pathways but overcome the limitations of other mTOR inhibitors.
Interestingly, the inhibition of Akt/mTOR and Erk pathways is characteristic of 8-NH2-Ado and does not appear to be a universal mechanism of action for the drug class. Specifically, 8-chloroadenosine has cytotoxic actions and inhibits global transcription of these same MCL cell lines but without consistent loss of cyclin D1 or translation inhibition.20 8-Cl-ado also requires p38 activation in leukemia cells to promote apoptosis and inhibit proliferation.32 Furthermore, in comparative studies, only 8-NH2-Ado and not 8-Cl-Ado or other deoxynucleoside analogs decreased phosphorylation of Akt/Erk pathway proteins. Thus, 8-NH2-Ado actions are distinct from currently characterized ribose and deoxyribose nucleoside analogs and require separate study.
While the exact mechanisms for Akt/mTOR and Erk pathway inhibition by 8-NH2-Ado are unknown at this time, we hypothesize that 8-NH2-Ado accumulation as an intracellular triphosphate will competitively inhibit kinases. This explanation is more likely than a reduction of global ATP levels because ATP levels, while substantially reduced in these cell lines after treatment (70% loss, Figure 2B), are generally not rate-limiting in kinase reactions.33 Consistent with this hypothesis, as previously reported for myeloma cells,13 a comparable decrease in intracellular ATP achieved by inhibiting glycolysis did not affect the phosphorylation of Akt or Erk proteins. Interestingly, although ATP levels were reduced by 8-NH2-Ado treatment, the phosphorylation of adenosine monophosphate (AMP)–protein kinase (AMPK, T172) was unaffected indicating that 8-NH2-Ado effects on the mTOR pathway are likely independent of AMPK activation (data not shown). Overall, these results provide justification for further mechanistic, in vitro studies of 8-NH2-ATP as a kinase inhibitor.
To further evaluate the selectivity of 8-NH2-Ado for lymphoma cells, the efficacy of 8-NH2-Ado was determined using PBMCs from healthy donors (Figures 5 and 6). 8-NH2-Ado did not inhibit the Akt/mTOR and Erk pathways in these PBMCs. Even with higher concentrations and prolonged exposure, the PBMCs did not undergo substantial apoptosis (< 20% death at 48 hours). While the number of lymphoma patient samples was limited regarding kinase inhibitory effects, the results were consistent with the data in 4 cell lines and were in stark contrast to the observations in normal PBMCs.
Although we cannot eliminate other cell type–specific events, the lack of PBMC response may have been due to reduced accumulation of 8-NH2-ATP in PBMCs compared with lymphoma cells (Figure 6). Although the number of primary lymphoma patient samples was low in this study (N = 6), we generally observed higher accumulation of 8-NH2-ATP in lymphoma cells. In support of this hypothesis, accumulation of 8-NH2-ATP and the corresponding level of ATP expressed as a ratio (8-NH2-ATP:ATP) were associated with cell death (P = .03). Thus, we conclude that 8-NH2-Ado actions are at least in part dependent on accumulation of 8-NH2-ATP, and accumulation may be dependent on cell type.
The variability in the accumulation of 8-NH2-ATP and cytotoxicity we observed in patient MCL cells may be explained in part by variability in the expression of adenosine kinase (Figure 6). To be activated and accumulate in the cells, 8-NH2-Ado like other 8-substituted adenosine analogs, must first be phosphorylated by adenosine kinase.15 Interestingly, patient MCL cells expressed variable levels of adenosine kinase mRNA (15-fold range), and high expression was associated with worse clinical outcomes (2.1 versus 8.0 years overall survival, P = .004, not shown, data from Rosenwald et al34). These data suggest that 8-NH2-Ado may selectively accumulate as 8-NH2-ATP in MCL cells with a more aggressive phenotype.
In addition to variability in 8-NH2-ATP accumulation and subsequent ATP loss, there may be other factors that promote resistance to 8-NH2-Ado treatment. For instance, Granta 519 cells were able to accumulate extraordinarily high concentrations of 8-NH2-ATP (15mM) after treatment with 8-NH2-Ado, but the cells did not undergo apoptosis presumably because of Bcl-2 overexpression (Figure 1). For the patient lymphoma cells, the most resistant cells either had a complex karyotype (patient 7) or were from a patient with multiple prior treatments (patient 8; Figure 6 and supplemental Table 1). Thus, these MCL cells may have acquired multiple mechanisms of resistance to therapeutics. In addition, this study did not evaluate other possible mechanisms of resistance to 8-NH2-Ado such as those induced by lymph node microenvironment or by bone marrow stromal cells. These are of particular importance as microenvironment factors activate Akt and Erk pathways.35 We are pursuing these investigations by co-culturing lymphoma cell lines with bone marrow stromal cells.
In conclusion, 8-NH2-Ado is a cytotoxic agent in preclinical models of MCL. The efficacy of 8-NH2-Ado is dependent on the accumulation of 8-NH2-ATP that is selective for lymphoma cells. Inhibition of the mTOR/Akt and Erk pathways is unique to 8-NH2-Ado, and given the importance of these pathways in the pathogenesis and progression of cancer, its specific mechanism as a kinase inhibitor warrants further investigation.
Supplementary Material
Acknowledgments
We thank Dr Walter Pagel for critically editing the manuscript.
This work was supported by grants from the National Cancer Institute: R01CA 85915 (V.G. and S.T.R.) and Lymphoma Specialized Program of Research Excellence CA136411 (V.G., L.J.M., and S.S.N.). J.B.D. is supported by a GlaxoSmithKline Translational Research in Multidisciplinary Program (TRIUMPH) postdoctoral fellowship at the M. D. Anderson Cancer Center. The primary tumor samples were provided by the University of Texas M. D. Anderson Cancer Center Lymphoma Tissue Bank.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.B.D. and M.S. designed and performed the research, analyzed data, and wrote the manuscript; M.L.A. and J.Q. performed the research; N.L.K. and S.T.R. designed the research and assisted in writing the manuscript; L.J.M. and S.S.N. identified patients and performed apheresis to obtain primary cells; and V.G. conceptualized and designed the research and assisted in writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Varsha Gandhi, Department of Experimental Therapeutics, 1515 Holcombe Blvd, Unit 71, The University of Texas M. D. Anderson Cancer Center, Houston, TX 77030; e-mail: vgandhi@mdanderson.org.
References
- 1.Medeiros LJ, Van Krieken JH, Jaffe ES, Raffeld M. Association of Bcl-1 rearrangements with lymphocytic lymphoma of intermediate differentiation. Blood. 1990;76(10):2086–2090. [PubMed] [Google Scholar]
- 2.Motokura T, Bloom T, Kim HG, et al. A novel cyclin encoded by a Bcl1-linked candidate oncogene. Nature. 1991;350(6318):512–515. doi: 10.1038/350512a0. [DOI] [PubMed] [Google Scholar]
- 3.Stilgenbauer S, Winkler D, Ott G, et al. Molecular characterization of 11q deletions points to a pathogenic role of the ATM gene in mantle cell lymphoma. Blood. 1999;94(9):3262–3264. [PubMed] [Google Scholar]
- 4.Rizzatti EG, Falcao RP, Panepucci RA, et al. Gene expression profiling of mantle cell lymphoma cells reveals aberrant expression of genes from the PI3K-AKT, WNT and TGFbeta; signalling pathways. Br J Haematol. 2005;130(4):516–526. doi: 10.1111/j.1365-2141.2005.05630.x. [DOI] [PubMed] [Google Scholar]
- 5.Rudelius M, Pittaluga S, Nishizuka S, et al. Constitutive activation of Akt contributes to the pathogenesis and survival of mantle cell lymphoma. Blood. 2006;108(5):1668–1676. doi: 10.1182/blood-2006-04-015586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dal Col J, Zancai P, Terrin L, et al. Distinct functional significance of Akt and mTOR constitutive activation in mantle cell lymphoma. Blood. 2008;111(10):5142–5151. doi: 10.1182/blood-2007-07-103481. [DOI] [PubMed] [Google Scholar]
- 7.Psyrri A, Papageorgiou S, Liakata E, et al. Phosphatidylinositol 3′-kinase catalytic subunit alpha gene amplification contributes to the pathogenesis of mantle cell lymphoma. Clin Cancer Res. 2009;15(18):5724–5732. doi: 10.1158/1078-0432.CCR-08-3215. [DOI] [PubMed] [Google Scholar]
- 8.Witzig TE, Geyer SM, Ghobrial I, et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol. 2005;23(23):5347–5356. doi: 10.1200/JCO.2005.13.466. [DOI] [PubMed] [Google Scholar]
- 9.Hess G, Herbrecht R, Romaguera J, et al. Phase III study to evaluate temsirolimus compared with investigator's choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2009;27(23):3822–3829. doi: 10.1200/JCO.2008.20.7977. [DOI] [PubMed] [Google Scholar]
- 10.Ansell SM, Inwards DJ, Rowland KM, Jr, et al. Low-dose, single-agent temsirolimus for relapsed mantle cell lymphoma. Cancer. 2008;113(3):508–514. doi: 10.1002/cncr.23580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rizzieri DA, Feldman E, DiPersio JF, et al. A phase 2 clinical trial of deforolimus (AP23573, MK-8669), a novel mammalian target of rapamycin inhibitor, in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res. 2008;14(9):2756–2762. doi: 10.1158/1078-0432.CCR-07-1372. [DOI] [PubMed] [Google Scholar]
- 12.Haritunians T, Mori A, O'Kelly J, Luong QT, Giles FJ, Koeffler HP. Antiproliferative activity of RAD001 (everolimus) as a single agent and combined with other agents in mantle cell lymphoma. Leukemia. 2007;21(2):333–339. doi: 10.1038/sj.leu.2404471. [DOI] [PubMed] [Google Scholar]
- 13.Ghias K, Ma C, Gandhi V, Platanias LC, Krett NL, Rosen ST. 8-Amino-adenosine induces loss of phosphorylation of p38 mitogen-activated protein kinase, extracellular signal-regulated kinase 1/2, and Akt kinase: role in induction of apoptosis in multiple myeloma. Mol Cancer Ther. 2005;4(4):569–577. doi: 10.1158/1535-7163.MCT-04-0303. [DOI] [PubMed] [Google Scholar]
- 14.Shanmugam M, McBrayer SK, Qian J, et al. Targeting glucose consumption and autophagy in myeloma with the novel nucleoside analogue 8-Aminoadenosine. J Biol Chem. 2009;284(39):26816–26830. doi: 10.1074/jbc.M109.000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krett NL, Davies KM, Ayres M, et al. 8-Amino-adenosine is a potential therapeutic agent for multiple myeloma. Mol Cancer Ther. 2004;3(11):1411–1420. [PubMed] [Google Scholar]
- 16.Frey JA, Gandhi V. 8-Amino-adenosine inhibits multiple mechanisms of transcription. Mol Cancer Ther. 2010;9(1):236–245. doi: 10.1158/1535-7163.MCT-09-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balakrishnan K, Wierda WG, Keating MJ, Gandhi V. Mechanisms of cell death of chronic lymphocytic leukemia lymphocytes by RNA-directed agent, 8-NH2-adenosine. Clin Cancer Res. 2005;11(18):6745–6752. doi: 10.1158/1078-0432.CCR-05-0553. [DOI] [PubMed] [Google Scholar]
- 18.Chen LS, Sheppard TL. Chain termination and inhibition of Saccharomyces cerevisiae poly(A) polymerase by C-8-modified ATP analogs. J Biol Chem. 2004;279(39):40405–40411. doi: 10.1074/jbc.M401752200. [DOI] [PubMed] [Google Scholar]
- 19.Chen LS, Du-Cuny L, Vethantham V, et al. Chain termination and inhibition of mammalian poly(A) polymerase by modified ATP analogues. Biochem Pharmacol. 2010;79(5):669–677. doi: 10.1016/j.bcp.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dennison JB, Balakrishnan K, Gandhi V. Preclinical activity of 8-chloroadenosine with mantle cell lymphoma: roles of energy depletion and inhibition of DNA and RNA synthesis. Br J Haematol. 2009;147(3):297–307. doi: 10.1111/j.1365-2141.2009.07850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neu HC, Heppel LA. Nucleotide sequence analysis of polyribonucleotides by means of periodate oxidation followed by cleavage with an amine. J Biol Chem. 1964;239(9):2927–2934. [PubMed] [Google Scholar]
- 22.Harris BA, Plunkett W. Biochemical basis for the cytotoxicity of 9-beta-D-xylofuranosyladenine in Chinese hamster ovary cells. Cancer Res. 1981;41(3):1039–1044. [PubMed] [Google Scholar]
- 23.Yazbeck VY, Buglio D, Georgakis GV, et al. Temsirolimus downregulates p21 without altering cyclin D1 expression and induces autophagy and synergizes with vorinostat in mantle cell lymphoma. Exp Hematol. 2008;36(4):443–450. doi: 10.1016/j.exphem.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Paoluzzi L, Gonen M, Gardner JR, et al. Targeting Bcl-2 family members with the BH3 mimetic AT-101 markedly enhances the therapeutic effects of chemotherapeutic agents in in vitro and in vivo models of B-cell lymphoma. Blood. 2008;111(11):5350–5358. doi: 10.1182/blood-2007-12-129833. [DOI] [PubMed] [Google Scholar]
- 25.Rosenwald IB, Kaspar R, Rousseau D, et al. Eukaryotic translation initiation factor 4E regulates expression of cyclin D1 at transcriptional and post-transcriptional levels. J Biol Chem. 1995;270(36):21176–21180. doi: 10.1074/jbc.270.36.21176. [DOI] [PubMed] [Google Scholar]
- 26.Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12(22):3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klier M, Anastasov N, Hermann A, et al. Specific lentiviral shRNA-mediated knockdown of cyclin D1 in mantle cell lymphoma has minimal effects on cell survival and reveals a regulatory circuit with cyclin D2. Leukemia. 2008;22(11):2097–2105. doi: 10.1038/leu.2008.213. [DOI] [PubMed] [Google Scholar]
- 28.Waskiewicz AJ, Flynn A, Proud CG, Cooper JA. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J. 1997;16(8):1909–1920. doi: 10.1093/emboj/16.8.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional Inactivation of TSC2 by Erk: Implications for tuberous sclerosisand cancer pathogenesis. Cell. 2005;121(2):179–193. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 30.Peponi E, Drakos E, Reyes G, Leventaki V, Rassidakis GZ, Medeiros LJ. Activation of mammalian target of rapamycin signaling promotes cell cycle progression and protects cells from apoptosis in mantle cell lymphoma. Am J Pathol. 2006;169(6):2171–2180. doi: 10.2353/ajpath.2006.051078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hipp S, Ringshausen I, Oelsner M, Bogner C, Peschel C, Decker T. Inhibition of the mammalian target of rapamycin and the induction of cell cycle arrest in mantle cell lymphoma cells. Haematologica. 2005;90(10):1433–1434. [PubMed] [Google Scholar]
- 32.Ahn Y-H, Jung JM, Hong SH. 8-Chloro-cyclic AMP-induced growth inhibition and apoptosis Is mediated by p38 mitogen-activated protein kinase activation in HL60 cells. Cancer Res. 2005;65(11):4896–4901. doi: 10.1158/0008-5472.CAN-04-3122. [DOI] [PubMed] [Google Scholar]
- 33.Koresawa M, Okabe T. High-throughput screening with quantitation of ATP consumption: a universal non-radioisotope, homogeneous assay for protein kinase. Assay Drug Dev Technol. 2004;2(2):153–160. doi: 10.1089/154065804323056495. [DOI] [PubMed] [Google Scholar]
- 34.Rosenwald A, Wright G, Wiestner A, et al. The proliferation gene expression signature is a quantitative integrator of oncogenic events that predicts survival in mantle cell lymphoma. Cancer Cell. 2003;3(2):185–197. doi: 10.1016/s1535-6108(03)00028-x. [DOI] [PubMed] [Google Scholar]
- 35.Gandhi V, Balakrishnan K, Chen LS. Mcl-1: the 1 in CLL. Blood. 2008;112(9):3538–3540. doi: 10.1182/blood-2008-07-170241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.