Abstract
We hypothesized that longitudinal changes in peak oxygen uptake (V̇O2), ventilatory efficiency, and exercise-induced right-to-left shunting in pulmonary arterial hypertension (PAH) patients would predict outcomes better than baseline measurements alone. Patients with PAH die prematurely. Identifying prognostic markers is critical for managing PAH patients, however longitudinal prognostic information in PAH is limited. We enrolled 103 PAH patients into a long-term, prospective outcome study using serial cardiopulmonary exercise testing to measure peak V̇O2, ventilatory efficiency (ratio of ventilation to CO2 output at the anaerobic threshold), right-to-left shunting, and other factors in patients treated with optimal therapy. Patients were followed for a mean of 4.7 years. During the study period, 20 patients died, and 3 underwent lung transplantation. Baseline peak V̇O2 and ventilatory efficiency were 0.79 L/min and 49 (normal <34), respectively, reflecting severe disease. Poorer ventilatory efficiency and higher New York Heart Association classification were associated with poor outcome both at baseline and at follow-up. In multivariate analysis, the persistence or development of an exercise-induced right-to-left shunt strongly predicted death or transplantation (p <0.0001) independent of hemodynamics and all other exercise measures, including peak V̇O2, and ventilatory efficiency. The absence of a shunt at baseline was associated with a 20% rate of non-survival, which decreased to 7% at follow-up. Poorer ventilatory efficiency appeared to be associated with poor outcome in patients without a shunt. In conclusion, a persistent exercise-induced right-to-left shunt and poor ventilatory efficiency are highly predictive of poor outcome in patients with pulmonary arterial hypertension.
Keywords: hypertension, pulmonary, shunt, exercise, ventilation, prognosis
The use of cardiopulmonary exercise testing (CPET) for diagnosing pulmonary vascular disease [1], to establish PAH severity [2], and to demonstrate response to therapy [3-4] has been well-established. Baseline measurements of aerobic capacity and ventilatory efficiency with CPET have been shown to predict survival in patients with PAH [5]. In addition, creation of a right-to-left shunt via atrial septostomy has been used as a means of reducing right atrial pressure [6] and to “decompress” the right ventricle as an attempt to increase cardiac output and increase systemic oxygen transport [7]. In some series, at least 40% have evidence of right-to-left shunting during exercise with oxygen desaturation during CPET [8]. Because of the progressive nature of PAH, we hypothesized that serial measurements of aerobic capacity and ventilatory efficiency would predict outcome better than a single measurement, and would be useful in the noninvasive serial assessment of patients with PAH. In addition, we hypothesized that the presence of an exercise-induced right-to-left shunt in our patients would predict better outcome.
Methods
We prospectively enrolled 103 patients with idiopathic, familial, and associated PAH (IPAH, FPAH, and APAH) patients, as defined by currently accepted diagnostic WHO Group I criteria [9] into a long-term, noninvasive outcome study evaluating serial CPET measurements. All patients underwent diagnostic right heart catheterization with measurements of right atrial, right ventricular, pulmonary arterial, and pulmonary capillary wedge pressure, and cardiac output. Patients with disorders other than PAH were excluded according to recommended diagnostic guidelines for PAH [10]. Most patients were receiving PAH-specific treatments, including endothelin antagonists, phosphodiesterase-5 inhibitors, or prostacyclin analogs.
Patients underwent CPET twice: first at baseline and second after a median of 0.7 years. CPET was performed using cycle ergometry, as previously described [2]. Patients were exercised using a progressively increasing work rate test to maximum tolerance on an electromagnetically braked cycle ergometer [2]. Gas exchange was measured breath-by-breath using MedGraphics (St. Paul, Minnesota) CPET equipment that recorded heart rate (HR), ventilation (VE), CO2 output (V̇CO2), O2 uptake (V̇O2), work rate (WR), and end-tidal CO2 (PETCO2) and O2 (PETO2) as well as other gas exchange variables [11]. From these data, peak V̇O2, peak HR, peak O2 pulse, respiratory exchange ratio (RER, which equals VCO2/V̇O2), anaerobic threshold (AT), ventilatory equivalent for CO2 at AT (V̇E/V̇CO2@AT), and other parameters as previously described [11-12]. Reproducibility for CPET in our laboratory has been previously reported, with coefficients of variation < 2.2% for peak V̇O2 and most other CPET variables [13].
The presence of exercise-induced right-to-left shunting was determined by an investigator evaluating the CPET studies as previously described in a similar cohort of PAH patients [8]. The investigator was blinded with respect to CPET sequence and the patient’s treatment and clinical course. Each 9-panel CPET plot was reviewed to identify an exercise-induced right-to-left shunt at the start of unloaded cycling exercise or during exercise, using the following criteria:
an abrupt and sustained increase in PETO2, with
a simultaneous, sustained decrease in PETCO2.
an abrupt and sustained increase in RER
an abrupt and sustained increase in V̇E/V̇O2
an abrupt and sustained increase in V̇E/V̇CO2, and
an associated decline in pulse oximetry (SpO2)
Each of the above criteria was weighted equally, with 4 or more criteria identifying an exercise-induced shunt in each CPET. These criteria were the same (and were evaluated in the same manner) as those used by one of the authors of the reference methodology [8] who was also an investigator in this study (JEH).
All subjects were managed with conventional and PAH-specific therapies as needed during their participation in the study. New York Heart Association (NYHA) classification was assigned without knowledge of the CPET results.
The primary outcome was survival time, defined as time from the first CPET to death by any cause or to urgent lung or heart/lung transplantation, and it was analyzed using a pre-specified sequence of survival analyses implemented with the non-parametric Cox proportional hazards regression model. Proportionality was tested and verified by assessing significance of interactions between survival predictors and survival time. First. the potential effect on survival of each of 12 prespecified CPET measures, weight, body mass index (BMI), NYHA classification (2 vs. > 2), and shunt status at baseline were first examined in individual models. Next, the potential effect of change per month from the baseline CPET to the second CPET on survival time was examined in individual models, and the additional potential effect of CPET change independent of the current (second visit) value for the corresponding CPET measure was assessed. The set of significant (p <0.05) characteristics from these analyses and also peak VO2 and VE/VCO2 @ AT (which were a-priori selected for inclusion due to clinical relevance and regardless of statistical significance) were then included in multivariate analyses of survival from visit 2 using data current at that time.
All available data for the measures included in any particular analysis were used and no imputation of missing data was performed. Several secondary sensitivity analyses were conducted in order to assess the robustness of the conclusions from the primary analyses. Those analyses included (1) survival from the second visit ignoring all baseline visit information, and (2) restriction to the subgroup of subjects with NYHA classification > 2. A p value of <0.05 was considered evidence of statistical significance and no mathematical correction was made for multiple statistical testing.
This study was conducted in accordance with Good Clinical Practices, the current version of the Declaration of Helsinki, and with local institutional regulations. The local ethics review committee (Human Subjects Committee) approved the protocol, and written informed consent was obtained for all patients. All authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
All patients survived at least until the time of their second CPET. Patients were followed for a mean of 4.7 years after the first CPET. Table 1 shows the baseline patient demographics, CPET findings, and invasive hemodynamics. Our population was mostly female, consistent with previously reported cohorts from our institution [2]. The baseline peak V̇O2 and V̇E/V̇CO2@AT, were 0.79 ± 0.26L/min and 49 ±13 (normal <34) respectively, and the pulmonary vascular resistance was 12 ± 6 Wood units, reflecting severe pulmonary vascular disease.
Table 1.
Patient Charcteristics
| Measure | |
|---|---|
| Age (years) | 49 ± 13 |
| Female:Male | 91:12 |
| Idiopathic pulmonary arterial hypertension:Familial pulmonary arterial hypertension: Associated pulmonary arterial hypertension | 63:2:38 |
| New York Heart Association Functional Class 2: 3: 4 | 14:85:4 |
| Weight (kg) | 73 ± 21 |
| CPET | |
| Peak oxygen consumption (V̇O2) (L/min) | 0.79 ± 0.26 |
| Ventilatory equivalent for CO2 at the anaerobic threshold (V̇E/V̇CO2@AT) | 49 ± 13 |
| End-tidal CO2 pressure at the anaerobic threshold (PETCO2@AT) (mmHg) | 28 ± 6 |
| Anaerobic threshold (L/min) | 0.6 ± 0.2 |
| Peak Work Rate (Watts) | 53 ± 26 |
| Peak Heart Rate (bpm) | 132 ± 20 |
| Rest Systolic Blood Pressure (mmHg) | 123 ± 17 |
| Hemodynamics | |
| Right Atrial Pressure (mmHg) | 10 ± 5 |
| Mean Pulmonary Arterial Pressure (mmHg) | 54 ± 16 |
| Cardiac Output (L/min) | 3.7 ± 1.4 |
| Pulmonary Vascular Resistance (dyne*sec)/cm5) | 12 ± 6 |
During the study period, 20 patients died, and 3 underwent lung transplantation, one of whom died subsequently. The overall survival for the entire patient population was 81% at 5 years (95% CI: 0.70 to 0.88). Table 2 demonstrates survival status related to exercise values at the baseline visit. Higher V̇E/V̇CO2@AT, lower AT, lower PETCO2@AT, higher NYHA classification, and marginally lower peakV̇O2 increased the hazard of non-survival. At baseline, neither systolic blood pressure at rest nor at peak exercise was significantly different between survivors and non-survivors. None of the patients with NYHA II classification at baseline died. Restricting analyses for all of the other factors to only subjects with NYHA>2 gives similar results to those in Table 2 (data not shown). After a median of 0.7 years (range: 0.2 to 5.9), the repeat CPET showed similar associations between V̇E/V̇CO2@AT, AT, PETCO2@AT, and NYHA classification (data not shown). For the 17 patients with APAH in this study, survival was similar to that of patients with IPAH. There was also no difference in survival for the patients already receiving PAH-specific treatments at study entry vs. those that were not.
Table 2.
Measures at first visit (2) and univariate survival analysis from first visit
| Measure | Units | SD (n103) | All | Surviving | Not Surviving | Cox Hazard Ratio(1) and 95% CI | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | N | Mean | N | Mean | Cox Hazard Ratio | Lower | Upper | p-value | |||
| Peak oxygen consumption | L/min | 0.26 | 103 | 80 | 0.82 | 23 | 0.71 | 0.67 | 0.43 | 1.05 | 0.08 |
| Peak oxygen consumption | ml/min/kg | 2.84 | 103 | 80 | 11.0 | 23 | 11.0 | 0.95 | 0.62 | 1.45 | 0.80 |
| Ventilatory equivalent for CO2 at the anaerobic threshold | - | 12.6 | 103 | 80 | 47.0 | 23 | 54.2 | 1.48 | 1.05 | 2.09 | 0.03 |
| Peak Work Rate | Watts | 25.9 | 96 | 75 | 54.6 | 21 | 47.8 | 0.78 | 0.49 | 1.24 | 0.30 |
| Rest Systolic Blood Pressure | mmHg | 17.4 | 96 | 74 | 123 | 22 | 121 | 0.94 | 0.60 | 1.47 | 0.79 |
| Peak Systolic Blood Pressure | mmHg | 27.3 | 97 | 75 | 159 | 22 | 152 | 0.80 | 0.53 | 1.21 | 0.29 |
| Rest Diastolic Blood Pressure | mmHg | 12.4 | 91 | 70 | 76.9 | 21 | 76.4 | 1.04 | 0.68 | 1.58 | 0.86 |
| Peak Diastolic Blood Pressure | mmHg | 17.2 | 91 | 70 | 87 | 21 | 82 | 0.94 | 0.60 | 1.48 | 0.80 |
| Anaerobic threshold | L/min | 0.19 | 90 | 68 | 0.61 | 22 | 0.51 | 0.53 | 0.32 | 0.90 | 0.02 |
| End-tidal CO2 pressure at the anaerobic threshold | mmHg | 6.2 | 96 | 77 | 28.5 | 19 | 25.0 | 0.62 | 0.39 | 0.97 | 0.04 |
| Peak Heart Rate | bpm | 19.5 | 94 | 73 | 132 | 21 | 135 | 1.09 | 0.70 | 1.71 | 0.69 |
| Weight | kg | 20.6 | 103 | 80 | 75.4 | 23 | 67.1 | 0.72 | 0.46 | 1.13 | 0.16 |
| Non-Survival Among: | |||||||||||
| NYHA II | NYHA 3-4 | ||||||||||
| New York Heart Association Functional Class | 3-4 vs. 2 | 103 | 0/14 =0% | 23/89 =25.8% | 0.03 | ||||||
| No Shunt | Shunt | ||||||||||
| Visit 1 | Yes / No | 103 | 13/64 =20.3% | 10/39 = 25.6% | 1.71 | 0.74 | 3.97 | 0.21 | |||
| Visit 2 | Yes / No | 102 | 5/70 = 7.1% | 18/32 = 56.2% | 10.6 | 3.87 | 28.8 | <0.0001 | |||
Hazard ratio for non-survival corresponding to a 1 SD increase in the measure.
Shunt (2) refers to shunt status at second visit and survival from second visit.
The absence of a shunt during the baseline CPET was associated with a 20% likelihood of death or transplantation, which decreased to 7% at the second visit (Table 2). This was due to the new development of shunting in 8 patients, of whom only 2 survived (75% nonsurvival) as well as a disappearance of shunting in 17 patients, of whom 16 survived (6% nonsurvival). After the second CPET, only 7% without an exercise-induced shunt were non-survivors, whereas 56% with an exercise-induced shunt were non-survivors (p < 0.0001).
Multivariate assessment evaluating potential confounding between shunt status and other CPET measures showed that, corrected for shunt status at visit 2, CPET measures did not add significantly to prognosis. Three distinct prognostic groups can thus be summarized (Table 3):
Table 3.
Survival prognosis
| NYHA Classification | Mortality | 5-Year Survival Probability and 95% CI | |
|---|---|---|---|
| II | 0/14 = 0% | 1.00 | --- |
| III–IV without shunt | 5/58 = 9% | 0.89 | 0.71 – 0.96 |
| III–IV with shunt | 18/30 = 60% | 0.35 | 0.16 – 0.54 |
patients with NYHA class II symptoms;
patients with greater than NYHA class II symptoms and no shunt at visit 2; and
patients with greater than NYHA class II symptoms and shunt present at visit 2.
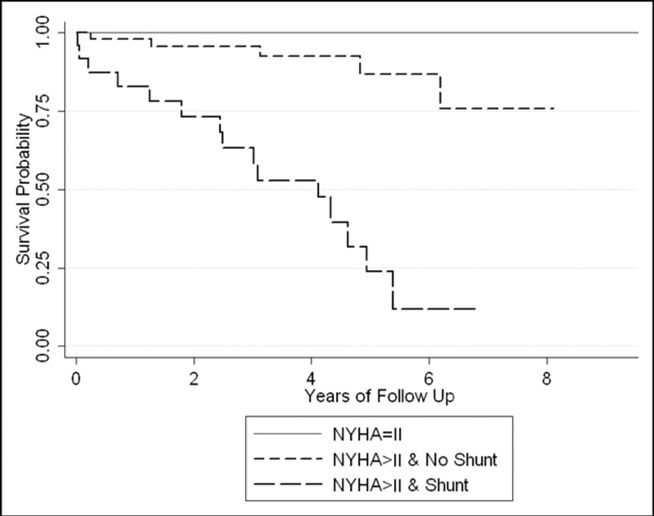
Of the 58 patients with greater than NYHA class II without a shunt at visit 2 (group 2), 5 (9%) died (5-year survival probability 0.89, 95% confidence interval 0.71 to 0.96). In contrast, 18 (60%) of 30 patients with greater than NYHA class II with a shunt at visit 2 (group 3) died (5-year survival probability 0.35, 95% confidence interval 0.16 to 0.54). Figure 1 shows the Kaplan-Meier survival estimates for the three prognostic groups.
Figure 1.
Kaplan-Meier plot of survival for 103 patients, separated by NYHA classification and shunt status at Visit 2.
NYHA = New York Heart Association
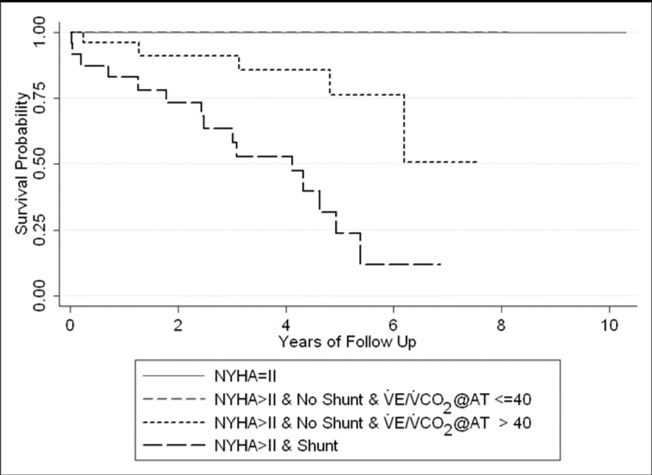
In a post-hoc analysis, none of the resting invasive hemodynamic measurements at initial presentation significantly improved prognosis beyond the study’s conclusions (p>0.11), although on univariate analysis, CO and PVR were associated with prognosis (p<0.01). In a 2nd exploratory post-hoc analysis of CPET measures within the >NYHA II group without shunting at visit 2, higher V̇E/V̇CO2@AT was significantly associated with increased mortality. Specifically, all of the 5 deaths in that subgroup had a V̇E/V̇CO2@AT above the median (>40). The difference in survival within these groups is shown in the Kaplan-Meier plot in Figure 2.
Figure 2.
Kaplan-Meier plot of survival for 103 patients, separated by NYHA classification, shunt status, and V̇E/V̇CO2@AT at Visit 2. (see text).
NYHA = New York Heart Association; V̇E/V̇CO2@AT = ratio of ventilation to CO2 output at the anaerobic threshold
Discussion
While prior studies have demonstrated the prognostic utility of CPET in PAH patients [5], our study is the first to evaluate the prognostic value of repeated CPET measurements. In addition, our study is the first to show that an exercise-induced right-to-left shunt predicts a poorer outcome in PAH patients. Moreover, we have shown that, beyond the traditional measurements of exercise capacity and ventilatory efficiency, assessing shunt status is useful for predicting outcome in these patients.
Right-to-left shunting during exercise in PAH is due to an abnormally high pulmonary vascular resistance leading to right atrial pressure exceeding left atrial pressure, forcing systemic venous blood through a patent foramen ovale (PFO) directly into the systemic arterial circulation. When such shunting occurs, it is usually manifest at the onset of exercise, but occasionally becomes evident only in late exercise.
The likelihood of an exercise-induced shunt in PAH patients can be detected noninvasively with CPET [8], with no added patient effort required during the CPET. However, it is important for the investigator to recognize the characteristic gas exchange patterns that are typical of an exercise-induced shunt, because these patterns and the changes in these patterns during exercise are predictive of poor outcome.
The association of an exercise-induced shunt with poor outcome was not initially intuitive, since atrial septostomy in patients without an exercise-induced shunt has been used as a means of reducing right atrial pressure [6] and to “decompress” the right ventricle in order to attempt to increase cardiac output and increase systemic oxygen transport [7]. Older observational reports have suggested that a PFO may in fact be associated with improved survival, presumably by permitting right ventricular unloading [14]. Although patients with exercise-induced shunting might derive benefit from their ability to decompress their right ventricles, in our patients the development or persistence of an exercise-induced shunt was more likely a manifestation of very severe pulmonary hypertension. Thus, we believe that although a PFO may be protective for a particular PAH patient, the development or persistence of an exercise-induced shunt during the course of therapy is a marker of their PAH severity, and denotes a poorer prognosis.
A small study evaluating 34 patients with PAH found that oxygen desaturation during a 6-minute walk (6MW) test predicted mortality in IPAH patients [15]. However, these patients were untreated and testing was not repeated over time. Also, the investigators did not comment specifically on the nature or mechanism of the oxygen desaturation. Although it is likely that some of their subjects manifested desaturation as the result of an exercise-induced shunt, pulse oximetry does not always accurately measure concurrent arterial oxygen saturation during exercise [16] and thus the pulse oximetry may not accurately detect the presence or absence of an exercise-induced shunt. Furthermore, in that study, all 34 patients had idiopathic PAH, and survival was stratified only by a single pretreatment 6MW test. Our study included patients with both idiopathic PAH and associated PAH, and, importantly, our patients were evaluated with CPET at a second visit in order to determine the patient’s clinical and physiological course over time. Fortuitously, exercise gas exchange measurements provided a mechanistic understanding of the development of an exercise-induced shunt and worsening of exercise-induced arterial hypoxemia, and allowed for further stratification of shunt patients by degree of ventilatory efficiency.
Groepenhoff, et al. [17] evaluated 115 patients with PAH, of which 18 died (none were transplanted). They found that exercise capacity measured with 6MW testing, as a surrogate for peak V̇O2, predicted survival as well as nearly all CPET measures. In their study, 6MW distance correlated with peak V̇O2 (r=0.63); this correlation was similar to that seen in heart failure patients (r=0.65) [18] and more recently seen in PAH patients (r=0.63) [19]. However, Groepenhoff, et al. did not report on the presence of an exercise-induced shunt, since it cannot be detected with 6MW testing.
Wensel, et al showed that peak systolic blood pressure (SBP) correlated positively with survival in a cohort of 70 patients with IPAH undergoing CPET [5]. We did not find a significant relationship between rest or exercise blood pressure and survival in our study. The mean resting and peak SBP in our patients was higher than in the study by Wensel, et al. (123 and 159 mmHg vs. 113 and 129 mmHg, respectively). In addition, overall survival in the Wensel study was 68% at 1 year while 85% of our patients survived for 5 years, suggesting that our patients were less ill. In this era of effective PAH-specific treatments, our data may be more representative of currently diagnosed PAH populations (including patients with APAH ), and thus provide further evidence of the discriminating nature of our CPET measurements.
Reduced aerobic capacity, reduced ventilatory efficiency, hypoxemia, and hypocapnia have all been associated with increased mortality in PAH patients. This study adds the importance of specifically evaluating exercise-induced right-to-left shunting. The strength of an exercise-induced right-to-left shunt for predicting outcome in our study was robust. In our multivariate analysis, the presence of an exercise-induced shunt predicted outcome independently from any other CPET measurement. Interestingly, in our post-hoc analysis exploring the group of patients without a shunt, V̇E/V̇CO2@AT predicted outcome in the absence of an exercise-induced shunt, whereas peak V̇O2 did not. This further suggests that aerobic capacity alone (peak V̇O2 and/or 6MW distance) may not provide optimal discrimination between survival and non-survival. We believe that NYHA class predicted outcome in this study because it is a discrete variable, and none of the NYHA class 2 patients died.
Our study has several limitations, including a more heterogeneous study population than seen in some prior studies, enrolling both IPAH and APAH patients in our study, as well as the use of varied PAH treatments. This “all-comer” approach perhaps better reflects a real-world pulmonary hypertension clinic. However it does not address the potential influence of the PAH diagnosis itself upon outcome because, although there was no significant difference in survival between treatment groups, between IPAH/FPAH and APAH groups, or within the APAH groups, this could have been due to the small sample sizes of each subgroup. We did not confirm the presence of a resting shunt using echocardiography or catheterization. However, in our previous report of CPET describing detection of exercise-induced shunts in a similar cohort of patients using the same methodology as in the present study, the overall accuracy of CPET as compared to resting echocardiography for detecting a shunt was high (94%) [8]. Invasive hemodynamic evaluation was not repeated over time in this study, making an evaluation of the incremental value of CPET compared to hemodynamics impossible. However, this study sought to evaluate a noninvasive measure of PAH severity, and evaluated the physiologic response to exercise rather than measurements made at rest. It is possible that exercise hemodynamics might have yielded useful prognostic information, however this was beyond the scope of our study. Due to practical issues, the follow-up period for the 2nd CPET visit was variable for some patients in this study, which may have introduced a bias. However, the majority of the patients returned for their 2nd visit within 6 months of their targeted 1 year period, allowing for reasonable comparisons among the subjects analyzed in this clinical study. Finally, we enrolled few patients with NYHA 4 symptoms in this study. This may have led to a survival bias, as evidenced by the lower mortality rate in our study relative to previously reported studies. Nevertheless, even in these patients with less advanced PAH, the presence of a right-toleft shunt robustly distinguished survivors vs. non-survivors.
Acknowledgments
We are grateful to Joy Beckmann, RN and Daisy Camanga, RN for their tireless efforts in managing patients and to Chase Kissling, Kevin Chang, and Daniel Dumitrescu, MD for their help in database organization.
Grant Support This research was supported by NIH grant # 1 K23 RR17596-01, NIH/Harbor-UCLA General Clinical Research Center (GCRC) Grant # M01-RR00425, and by American Heart Association Beginning Grant-in-Aid # 0160126Y
Footnotes
Relevant Relationships with Industry: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Markowitz DH, Systrom DM. Diagnosis of pulmonary vascular limit to exercise by cardiopulmonary exercise testing. J Heart Lung Transplant. 2004;23:88–95. doi: 10.1016/s1053-2498(03)00064-0. [DOI] [PubMed] [Google Scholar]
- 2.Sun XG, Hansen JE, Oudiz RJ, Wasserman K. Exercise pathophysiology in patients with primary pulmonary hypertension. Circulation. 2001;104:429–435. doi: 10.1161/hc2901.093198. [DOI] [PubMed] [Google Scholar]
- 3.Opitz CF, Wensel R, Winkler J, Halank M, Bruch L, Kleber FX, Höffken G, Anker SD, Negassa A, Felix SB, Hetzer R, Ewert R. Clinical efficacy and survival with first-line inhaled iloprost therapy in patients with idiopathic pulmonary arterial hypertension. Eur Heart J. 2005;26:1895–1902. doi: 10.1093/eurheartj/ehi283. [DOI] [PubMed] [Google Scholar]
- 4.Oudiz RJ, Roveran G, Sun X-G, Hansen JE, Wasserman K. Cardiopulmonary Responses to Sildenafil in Pulmonary Arterial Hypertension. Eur J Heart Fail. 2007;9:917–921. doi: 10.1016/j.ejheart.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wensel R, Opitz CF, Anker SD, Winkler J, Höffken G, Kleber FX, Sharma R, Hummel M, Hetzer R, Ewert R. Assessment of survival in patients with primary pulmonary hypertension: importance of cardiopulmonary exercise testing. Circulation. 2002;106:319–324. doi: 10.1161/01.cir.0000022687.18568.2a. [DOI] [PubMed] [Google Scholar]
- 6.Rich S, Lam W. Atrial septostomy as palliative therapy for refractory primary pulmonary hypertension. Am J Cardiol. 1983;51:1560–1561. doi: 10.1016/0002-9149(83)90678-1. [DOI] [PubMed] [Google Scholar]
- 7.Barst RJ. Role of atrial septostomy in the treatment of pulmonary vascular disease. Thorax. 2000;55:95–96. doi: 10.1136/thorax.55.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun XG, Hansen JE, Oudiz RJ, Wasserman K. Gas exchange detection of exercise-induced right-toleft shunt in patients with primary pulmonary hypertension. Circulation. 2002;105:54–60. doi: 10.1161/hc0102.101509. [DOI] [PubMed] [Google Scholar]
- 9.Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin MT, Jing ZC, Krowka MJ, Langleben D, Nakanishi N, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–S54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 10.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53:1573–1619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Wasserman K, Hansen JE, Sue DY, Stringer WW, Whipp BJ. Principles of Exercise Testing and Interpretation. 4. Philadelphia: Lippincott, Williams & Wilkins; 2005. p. 568. [Google Scholar]
- 12.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting the anaerobic threshold by gas exchange. J Appl Physiol. 1986;60:2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 13.Hansen JE, Sun XG, Yasunobu Y, Garafano RP, Gates G, Barst RJ, Wasserman K. Reproducibility of cardiopulmonary exercise measurements in patients with pulmonary arterial hypertension. Chest. 2004;126:816–824. doi: 10.1378/chest.126.3.816. [DOI] [PubMed] [Google Scholar]
- 14.Rozkovec A, Montanes P, Oakley CM. Factors that influence the outcome of primary pulmonary hypertension. Br Heart J. 1986;55:449–458. doi: 10.1136/hrt.55.5.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paciocco G, Martinez FJ, Bossone E, Pielsticker E, Gillespie B, Rubenfire M. Oxygen desaturation on the six-minute walk test and mortality in untreated primary pulmonary hypertension. Eur Respir J. 2001;17:647–652. doi: 10.1183/09031936.01.17406470. [DOI] [PubMed] [Google Scholar]
- 16.Hansen JE, Casaburi R. Validity of ear oximetry in clinical exercise testing. Chest. 1987;91:333–337. doi: 10.1378/chest.91.3.333. [DOI] [PubMed] [Google Scholar]
- 17.Groepenhoff H, Vonk-Noordegraaf A, Boonstra A, Spreeuwenberg MD, Postmus PE, Bogaard HJ. Exercise testing to estimate survival in pulmonary hypertension. Med Sci Sports Exerc. 2008;40:1725–1732. doi: 10.1249/MSS.0b013e31817c92c0. [DOI] [PubMed] [Google Scholar]
- 18.Guyatt GH, Sullivan MJ, Thompson PJ. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132:919–923. [PMC free article] [PubMed] [Google Scholar]
- 19.Oudiz RJ, Barst RJ, Hansen JE, Sun XG, Garofano R, Wu X, Wasserman K. Cardiopulmonary exercise testing and six-minute walk correlations in pulmonary arterial hypertension. Am J Cardiol. 2006;97:123–126. doi: 10.1016/j.amjcard.2005.07.129. [DOI] [PubMed] [Google Scholar]