Abstract
We have previously demonstrated T cell-independent antitumor effects of a combination of anti-CD40 mAb and CpG oligodeoxynucleotides (CpG) which involved macrophages. As some immunotherapeutic treatments can be potentiated by chemotherapy, we tested if cyclophosphamide (CY) would enhance the antitumor effect of anti-CD40 mAb + CpG. Treatment of B16 melanoma-bearing mice with CY and anti-CD40 mAb + CpG resulted in a significant reduction in tumor growth in immunocompetent mice compared to either CY alone or anti-CD40 mAb with CpG. This enhanced antitumor effect was maintained in SCID mice, as measured by both tumor growth and overall survival. NK cells were not required for this antitumor effect as it was also observed in SCID/beige mice. Moreover, while CY treatment of immunocompetent mice suppressed NK cell activity, it did not negatively affect the antitumor activity of their macrophages when assayed in vitro. Depletion of macrophages in vivo reduced the antitumor effect of CY and anti-CD40 mAb + CpG. These results suggest that therapeutic strategies to activate macrophages may have potential for clinical application in cancer patients receiving chemotherapy.
Keywords: Cyclophosphamide, anti-CD40 mAb, CpG, immunotherapy, macrophages
Introduction
CY is an alkylating chemotherapeutic agent which inhibits tumor cell division by cross-linking DNA. CY has been widely used as a chemotherapeutic agent, alone or in combination, for treatment of human malignancies (1-3). Unfortunately, while chemotherapy alone may provide antitumor effects for many types of cancer, often it is not curative, as tumor cells become drug-resistant and cancer recurs or progresses. This provides a rationale for combining chemotherapy with other antitumor strategies, such as certain forms of immunotherapy. CY when used in combination with immunotherapy has shown efficacy against several tumors in mice, including B16 melanoma (4,5). While high doses of CY cause immunosuppression, lower doses of CY can facilitate immunotherapy either by causing vascular disruption that gives larger molecules better access to the tumor (6), or by depleting T regulatory cells (7,8). Efforts to exploit the immunofacilitating effects of chemotherapy have been pursued through the use of chemotherapy with vaccines, monoclonal antibodies (mAbs) and recombinant cytokines (IL-2, TNF-α, IL-12) in mice (5, 9-11) and in humans (12,13).
Chemotherapeutic agents are not equally immunosuppressive to all components of the immune system. Although chemotherapy in breast cancer patients suppressed both CD4+ and CD8+ T cells, the number of CD14+ monocytes/macrophages (Mϕ) recovered rapidly (13). In mice, CY treatment can limit tumor growth without affecting the accumulation of Mϕ in ascitic tumors (14), and induce an antitumor effect that involves activated Mϕ as effector cells when combined with IL-12 (15). The presence of tumor-infiltrating Mϕ in patients treated with chemotherapy was shown to be an independent predictor of survival (16). Therefore it appears that immunotherapy designed to activate Mϕ may be beneficial for patients undergoing chemotherapy.
Both CD40 ligation (with an agonistic mAb or a soluble ligand) and CpG can enhance antitumor immunity and have been tested in clinical trials as separate modalities (17-23). Our recent mouse studies showed that combining anti-CD40 mAb (αCD40) and CpG in vivo resulted in synergistic activation of Mϕ and induction of potent antitumor effects even in the absence of T- and NK-cells (24). Thus, we hypothesized that activation of Mϕ by αCD40+CpG and the resulting antitumor effects may be enhanced by chemotherapy, even when T cell immunity is suppressed or absent.
Material and Methods
Mice and Cell Lines
Female C57BL/6 mice 7 to 10 weeks old were obtained from Harlan-Sprague-Dawley, Madison, WI, or Taconic, Germantown, NY. Female or male CB-17 SCID and SCID/beige mice were obtained from Taconic. Mice were housed, cared for and used in accordance with the Guide for Care and Use of Laboratory Animals (NIH publication 86-23, National Institutes of Health, Bethesda, MD, 1985). The murine B16 melanoma tumor cell line was grown in RPMI 1640 complete medium supplemented with 10% FCS (Sigma Chemicals, St. Louis, MO), 2 mM L-glutamine and 100 U/ml of penicillin/streptomycin (all from Life Technologies, Inc., Grand Island, NY) at 37°C in a humidified 5% CO2 atmosphere.
Antibodies and reagents
The FGK 45.5 hybridoma cell line producing an agonistic αCD40 was a gift from Dr. Fritz Melchers (Basel Institute for Immunology, Basel, Switzerland). The antibody, enriched for IgG by ammonium sulfate precipitation, was obtained from ascites of nude mice injected i.p. with the hybridoma cells. Endotoxin-free CpG1826 (TCCATGACGTTCCTGACGTT) was purchased from Coley Pharmaceuticals Group, Wellesley, MA. Mice were injected i.p. with 50 μg of CpG in 0.5 ml PBS. In previous experiments, we observed no difference in Mϕ• stimulatory capacity of control non-CpG-ODN 1982 and PBS (diluent); therefore PBS was used as control for CpG1826 (henceforth referred to as CpG). CY was manufactured by Baxter Healthcare Corporation, Deerfield, IL. Bacterial LPS was purchased from Sigma Chemical, St. Louis, MO. Mouse recombinant IFNγ was purchased from eBioscience, San Diego, CA.
In vivo tumor models and therapy
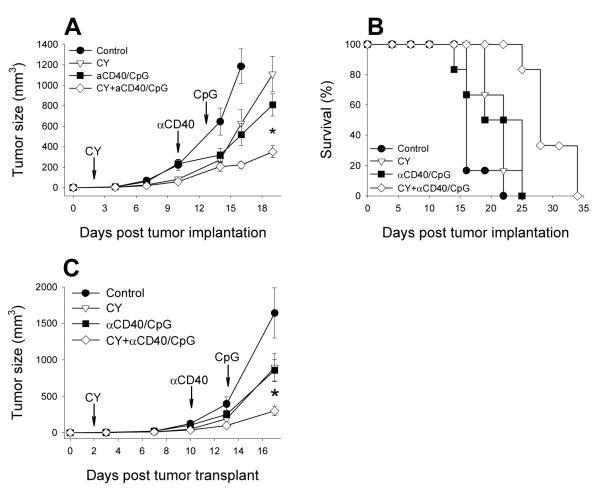
C57BL/6, CB-17 SCID or CB-17 SCID/beige mice were injected subcutaneously (s.c.) with 3×105 (C57BL/6 mice) or 1×105 (SCID or SCID/beige mice) B16 melanoma cells in 0.05 ml PBS (day 0). On day 2 or 7 mice were injected i.p. with CY (2 mg/mouse or ~100 mg/kg) in 0.5 ml PBS. Control mice received PBS. Based on our data indicating that the best Mϕ activation is observed when CpG is given to mice 3 days after αCD40 (24), mice received i.p. 250 μg αCD40 on day 10 after tumor implantation and 50 μg CpG on day 13. Antitumor effects were determined by measuring the perpendicular diameters of s.c. tumors twice weekly. Tumor volumes were calculated according to the formula: (tumor length × tumor width2)/2. In an i.p. tumor model, C57BL/6 mice were implanted i.p. with 1×105 B16 tumor cells on day 0. Mice were treated i.p. with 100 mg/kg CY, 500 μg αCD40 and 50 μg CpG on indicated days. The antitumor effect was estimated either by extended survival of the mice or by a decrease of the number of CD45− B16 cells in PEC using flow cytometry.
Activation of Mϕ
Peritoneal cells (PEC) were obtained via a peritoneal cavity lavage with 5 ml of ice-cold RPMI 1640 complete medium supplemented with 1 IU/ml of heparin (American Pharmaceutical Partners, Inc., Schaumburg, IL). Collected PEC were placed into 96 well flat-bottomed cell culture plates (Corning Inc, Corning, NY) at a concentration of 2.5-3 × 106 cells/ml, 0.1 ml/well. The peritoneal Mϕ population was enriched by adherence to plastic for 1.5-2 hours, followed by removal of non-adherent cells. For in vitro activation, Mϕ were stimulated with IFNγ (10 U/ml) and LPS (10 ng/ml) for 48 hr. For in vivo activation, mice were injected i.p. with 250 μg of αCD40 in 0.5 ml PBS. On day 3, PEC were harvested, enriched as described above, and incubated for 48 hours either in medium alone or in the presence of LPS (10 ng/ml).
Mϕ - mediated tumoristasis in vitro
Tumoristatic activity of Mϕ was determined by the inhibition of DNA synthesis in tumor cells. Briefly, adherent Mϕ were stimulated in vitro as described above and simultaneously co-cultured with B16 or L5178Y tumor cells (1×104/well) for 48 hours. To estimate DNA synthesis, cells were pulsed with 3H-TdR (1 μCi/well) during the last 6 hours of incubation. 3H-TdR-incorporation was determined by β-scintillation of total cells harvested from the cell culture clusters onto glass fiber filters (Packard, Meriden, CT), using the Packard Matrix 9600 Direct β-counter (Packard, Meriden, CT). Results are presented as counts per 5 minutes for triplicate wells ± SE.
NK activity
Spleens from C57BL/6 mice injected i.p. with 100 mg/kg CY or PBS (control) were collected 3 days after the treatment from 3 mice per group and pooled. Spleens were processed to a single cell suspension, red blood cells lysed by hypotonic shock and resultant splenic nucleated cells were tested by flow cytometry for Ly49+ (pan NK cell antigen) cells. The splenocytes were adjusted to 1×105/ml Ly49b+ cells, stimulated with 100 IU rIL-2 for 72 hr and tested for YAC-1 cell lysis in 4-hr 51Cr-release assay as described (25). In another experimental approach, C57BL/6 mice were injected with 100 mg/kg CY on day 0 and 0.5 mg αCD40 mAb on day 3. Splenocytes were obtained on day 6 and tested for cytotoxicity against YAC-1 cells.
Flow cytometric analysis
PEC from treated and control C57BL/6 mice were harvested and stained with anti-F4/80 APC mAb, anti-F4/80 FITC mAb, anti-Gr-1 PE mAb, anti-CD11b APC mAb, anti-CD45-FITC mAb (all from eBioscience, San Diego, CA), anti-CD40 PE mAb, anti-CD80 PE mAb or anti-CD86 PE mAb (all from BDPharmingen, San Diego, CA) for 40 min at 4°C. Isotype-matched irrelevant rat IgG FITC, IgG APC and IgG PE, purchased from eBioscience or BDPharmingen were used as a background control. After washing the cells in ice-cold PBS supplemented with 0.5-2% FCS (flow buffer), the cell pellet was resuspended in 0.3 ml flow buffer and analyzed by flow cytometry using a FACSCalibur flow cytometer and FlowJo software (Ashland, OR). Data were collected for 10,000 events per sample.
In vivo depletion of Mϕ
Peritoneal Mϕ were depleted in vivo with clodronate liposomes as described (26,27). Clodronate was a gift of Roche Diagnostics GmbH, Mannheim, Germany. Liposomes were prepared as described (26) and injected i.p. in tumor-bearing mice on day 9 post tumor cell implantation (0.3 ml) and on day 13 (0.2 ml). Depletion of Mϕ with clodronate liposomes was confirmed in naïve mice by elimination of more than 95% of PEC stained with anti-F4/80 FITC and anti-CD11b APC.
Statistical analysis
A two-tailed Student’s t-test was used to determine significance of differences between experimental and relevant control values.
Results
Antitumor effects of CY and αCD40 + CpG against B16 melanoma in immunocompetent mice
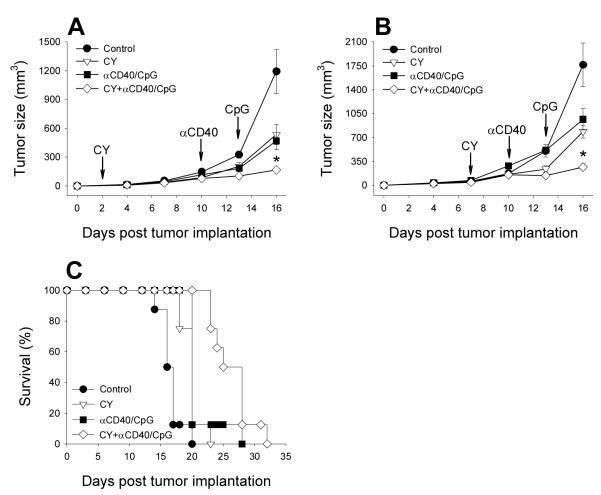
C57BL/6 mice were implanted s.c. with 3×105 B16 tumor cells on day 0 and treated with CY either 2 days (Figure 1A) or 7 days later (Figure 1B). Immunotherapy, given either alone or in combination with CY, consisted of αCD40 injected on day 10 followed by CpG 3 days later as we described previously (24). The results in Figure 1 show that CY and immunotherapy, given alone, induced some level of antitumor effect, but their combination resulted in a greater level of tumor growth reduction (P<0.05 for the CY+ αCD40/CpG group vs. any other group). The enhanced antitumor effect of the combination of CY, αCD40 and CpG was also seen against i.p. B16 tumor (Figure 1C). The mean survival times ± SEM (days) were as follows: control − 16.63 ± 0.60; CY − 19.88 ± 0.55; αCD40/CpG − 21.00 ± 1.00; CY+ αCD40/CpG − 26.38 ± 1.12. Each treatment resulted in increased survival of mice as compared with the control group (p<0.005), and CY + αCD40/CpG treatment induced increased survival as compared with individual treatments (P<0.005).
Figure 1.
CY combined with αCD40/CpG induce enhanced antitumor effect. C57BL/6 mice were implanted s.c. with 3×105 B16 tumor cells on day 0. Mice had no treatment or were treated with 100 mg/kg CY i.p. on day 2 (A) or day 7 (B), αCD40 (250 μg in 0.5 ml) i.p. on day 10 with CpG (50 μg in 0.5ml) i.p. on day 13, or a combination of CY with αCD40 and CpG. Means ± SEM of tumors volumes are presented for 5-6 mice per group. * - P<0.05 for combined group vs. any other group. Arrows indicate treatment schedule. C. C57BL/6 mice were implanted i.p. with 1×105 B16 tumor cells on day 0. Mice were treated with 100 mg/kg CY i.p. on day 1, αCD40 (500 μg in 0.5 ml) i.p. on day 4 and 11 with CpG (50 μg in 0.5ml) i.p. on day 7, or a combination of CY with αCD40 and CpG. Control mice received PBS or rat IgG. Survival of 8 mice per group is presented.
Antitumor effects of CY and αCD40 + CpG against B16 tumor in immunodeficient mice
In many studies that reported the augmenting effects of CY on tumor immunotherapy, these effects were found to be T-cell dependent: depletion of T regulatory cells by CY facilitated T-cell-mediated antitumor responses of immunotherapy (7,8). Therefore, in our T-cell-independent model of immunotherapy using αCD40 and CpG (24), we sought to determine if the augmented effect of CY on αCD40 + CpG is dependent on T cells. T- and B-cell immunodeficient SCID mice were implanted s.c. with B16 cells and treated with CY with and without αCD40 + CpG as described above. The results in Figure 2A show that the enhanced antitumor effect of the combination of CY and αCD40 + CpG observed in immunocompetent mice (Figure 1) was also observed in SCID mice (P<0.05). The reduced rate of tumor growth achieved by the combined therapy led to the extended survival of mice (Figure 2B). The survival days of mice in the groups were as follows (Mean ± SEM): control − 17.0 ± 1.0; CY − 21.5 ± 0.9; αCD40/CpG − 20.6 ± 2.0; CY + αCD40/CpG − 29.5 ± 1.5. The combined treatment resulted in significantly extended survival compared to control mice (p<0.001), mice receiving CY alone (p<0.01), or αCD40/CpG alone (p<0.01). These results indicate that the additive effect of CY and αCD40/CpG immunotherapy is T-cell independent.
Figure 2.
CY combined with αCD40/CpG inhibits tumor growth and extends survival of immunodeficient mice. SCID mice (A, B) or SCID/beige (C) mice were implanted with 1×105 B16 tumor cells s.c. on day 0. Mice had no treatment or were treated with 100 mg/kg CY i.p. on day 2, αCD40 (250 μg) i.p. on day 10 with CpG (50 μg) i.p. on day 13, or a combination of CY with αCD40 and CpG. Arrows indicate treatment schedule. Means ± SEM of tumors volumes (A, C) or percentages of survival (B) of 6-8 mice per group are presented. * - P<0.05 (A) or P<0.025 (C) for combined group vs. any other group.
To determine if NK cells play a role in the observed effect, we injected B16 cells in T-, B- and NK cell-deficient SCID/beige mice and tested CY and αCD40/CpG as described above. Figure 2C shows results similar to those observed in immunocompetent and SCID mice (Figures 1 and 2A,B, respectively), i.e. that a combination of CY and immunotherapy resulted in a greater antitumor effect than the single treatments. The combination of CY and αCD40/CpG was effective in inducing B16 tumor growth suppression in C57BL/6 mice depleted of NK cells with anti-NK1.1 mAb (data not shown). The lack of a role for NK cells in the additive in vivo antitumor effect of CY + immunotherapy was further supported by in vitro testing. CY treatment of naïve immunocompetent mice resulted in substantial reduction of their NK cell cytotoxic function following in vitro activation with IL-2 (Figure 3A) or in vivo activation with αCD40 (Figure 3B).
Figure 3.
CY treatment inhibits antitumor activity of NK cells (A, B) but not Mϕ (C, D). A. Splenocytes from C57BL/6 mice injected i.p. with 100 mg/kg CY or PBS (control) were collected 3 days after the treatment from 3 mice per group and pooled. They were adjusted to 1×105/ml Ly49b+ cells, stimulated with 100 IU rIL-2 for 72 hr, and tested for YAC-1 cell lysis in 4-hr 51Cr-release assay. Results are presented as % of cytotoxicity. B. C57BL/6 mice were injected with 100 mg/kg CY on day 0 and 0.5 mg αCD40 mAb on day 3. Splenocytes were obtained on day 6, adjusted to 1×105/ml Ly49b+ cells and tested for YAC-1 cell lysis in 4-hr 51Cr-release assay. C. Naïve C57BL/6 mice (2 mice per group) were injected i.p. with 100 mg/kg CY on day -14, -7, or -3. On day 0, PEC from treated and naïve mice were collected, adhered to plastic and stimulated with IFNγ (10 U/ml) and LPS (10 ng/ml) for 48 hr. A Mϕ tumoristatic assay against L5178Y tumor cells was performed as described in Materials and Methods. Mϕ - mediated antitumor effect is reflected by the reduced proliferation of tumor cells. D. C57BL/6 mice (3 mice per group) were implanted s.c. with 3×105 B16 tumor cells (day 0). Mice were treated i.p. with 100 mg/kg CY on day 2 and/or 250 μg αCD40 mAb on day 10 plus 50 μg CpG on day 13. On day 14, PEC from treated and naïve mice were collected, adhered to plastic and incubated with media or LPS (10 ng/ml) for 48 hr. A Mϕ tumoristatic assay against B16 tumor cells was performed as described in Materials and Methods.
CY treatment does not inhibit antitumor activity of Mϕ
As Mϕ were found to be the main antitumor effector cells following αCD40/CpG therapy (24), we hypothesized that CY treatment does not suppress the antitumor activity of Mϕ. To determine the effect of CY on Mϕ antitumor function, we used two separate approaches. First, C57BL/6 mice were injected with CY on day -14, -7 or -3. On day 0, peritoneal Mϕ from treated and naïve mice were stimulated in vitro with IFNγ and LPS, and their antitumor activity was determined by the ability to inhibit 3H-incorporation into tumor cells. The results in Figure 3C show that CY treatments did not affect the ability of activated Mϕ to mediate suppression of tumor cell proliferation (grey bars). Moreover, CY treatment 14 days before the test resulted in the ability of Mϕ to suppress tumor cell proliferation in the absence of IFNγ and LPS (black bars, P<0.05). In the second approach, to approximate our in vivo treatment protocol of tumor-bearing mice, C57BL/6 mice were implanted s.c. with 3×105 B16 tumor cells (day 0) and treated with CY on day 2 followed by αCD40 on day 10 and CpG on day 13. On day 14, peritoneal Mϕ from treated and control mice were incubated with media or LPS in the presence of B16 target cells in vitro. The results in Figure 3D show that CY treatment did not suppress the tumoristatic activity of Mϕ from αCD40/CpG - treated mice and even induced some activation of Mϕ in response to in vitro LPS in mice that did not receive αCD40/CpG. The unchanged antitumor activity of Mϕ following CY treatment in these two approaches correlated with the unchanged or slightly increased ability of Mϕ to produce NO in response to αCD40±CpG/LPS stimulation (data not shown). These experiments were repeated at various times following CY administration and the ability of Mϕ to secrete NO or suppress tumor cell proliferation in vitro were not reduced, although not always stimulated. Together, these data show that Mϕ antitumor functions are not suppressed by CY treatment, suggesting that activated Mϕ are the effector cells in tumor-bearing mice treated with the combination of CY and αCD40/CpG.
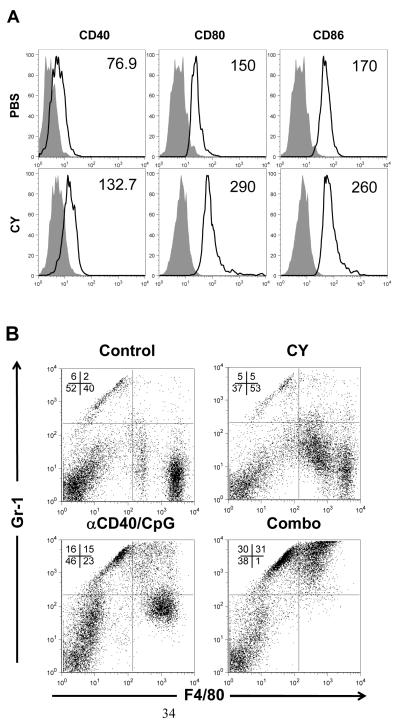
In addition to determining the antitumor activity of Mϕ, we tested the effect of CY treatment on the expression of Mϕ surface molecules known to be involved in immunostimulatory functions. Figure 4A shows that CY treatment upregulated expression of CD40, CD80 and CD86 on Mϕ. These data indicate that Mϕ are activated following CY treatment. In addition, we analyzed the phenotype of PEC from mice treated with CY, αCD40/CpG and their combination. Figure 4B shows that whereas F4/80+ cells in control mice consisted mainly of F4/80bright cells, more than half of F4/80+ cells in CY-treated mice were F4/80dim, a feature of monocytes or activated M□ (28). The percentage of F4/80+ Mϕ among PEC was increased 7 days following CY treatment as compared with PBS-treated mice (63.97 ± 4.26% vs. 43.92 ± 2.85%, n=4 mice per group, p<0.01), whereas total number of F4/80+ Mϕ did not change (2.84 ×106 ± 0.37 vs. 2.73 ×106 ± 0.4). In CY + αCD40/CpG − treated mice, PEC contained more than 60% Gr-1+ cells, half of them F4/80dim and half F4/80− cells (Figure 4B). Virtually all these Gr-1+ cells were CD11b+ (data not shown).
Figure 4.
Phenotype of peritoneal Mϕ after CY treatment. A. C57BL/6 mice were injected i.p. with 100 mg/kg CY or PBS (control). Three days later, PEC were harvested, and F4/80+ Mϕ were analyzed by flow cytometry for expression of CD40, CD80, and CD86. The results are presented as histograms where the isotype control antibody is shown in gray, and the black line histogram represents the specific mAb. The numbers included in each histogram represent the mean fluorescence intensity values for specific staining. This figure is representative of 3 similar experiments. B. C57BL/6 mice were injected i.p. with 100 mg/kg CY or PBS (control) on day 0. On day 3, some mice received 0.5 mg anti-CD40 mAb or rat IgG (control) i.p. Three days later, the mice received 50 mcg CpG or PBS (control) i.p. On day 7, PEC were collected and analyzed for F4/80 and Gr-1 expression by flow cytometry. The numbers show the percentage of cells in each quadrant. The data representative of 3 mice per group are shown. This figure is representative of 3 similar experiments.
We also used the peritoneal B16 tumor model to analyze Mϕ within peritoneal cavity (tumor-associated Mϕ). PEC from mice injected with B16 cells i.p. 15 days earlier contained 34.4 ± 5.2% of Gr-1+ F4/80− cells and 4.3 ± 0.5% of Gr1+ F4/80+ cells (Mean ± SEM of 6 mice per group). PEC of tumor-bearing mice treated with Cy (day 8), αCD40 (day 11) and CpG (day 14) contained similar percentage of Gr-1+ F4/80− cells (40.6 ± 3.6) and increased (p<0.001) percentage of Gr1+ F4/80+ cells (27.7 ± 2.2, 5 mice per group). Peritoneal CD11c+ dendritic cells were not increased following the treatment and comprised < 3%. Together, these results show increase of Gr1+ F4/80+ cells in CY + αCD40/CpG − treated mice.
Antitumor effect of CY in combination with αCD40 and CpG involves Mϕ
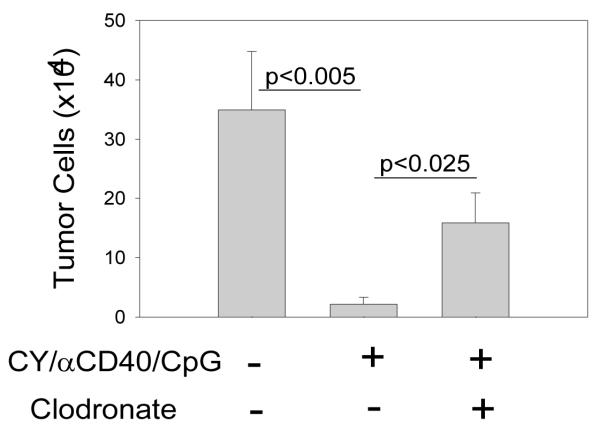
Based on our data showing that the combinatorial treatment of mice with CY, αCD40 and CpG induced antitumor effect in SCID/beige mice (Figure 2C), and that Mϕ antitumor function, as measured in vitro, was not suppressed in CY-treated mice (Figure 3C, D), we hypothesized that Mϕ were the antitumor effector cells in vivo in this experimental model. To test this hypothesis, we depleted Mϕ by injecting tumor-bearing mice with clodronate liposomes and treated them with CY, αCD40 and CpG. We used the i.p. B16 model we developed in which the number of CD45− B16 tumor cells was found to be substantially reduced in the peritoneal cavity of tumor-bearing mice as soon as 4 days after the treatment with αCD40 (all cells in PEC from naïve mice were CD45+, data not shown). The results in Figure 5 show that CY, αCD40 and CpG therapy induced a substantial reduction of tumor cell load in mice (P<0.05). Treatment with clodronate liposomes significantly reduced this antitumor effect (P<0.025). While i.p. injection of clodronate liposomes resulted in elimination of virtually all CD11b+ F4/80+ (“bright”) Mϕ in naïve mice (data not shown), in CY, αCD40 and CpG – treated tumor-bearing mice clodronate liposomes depleted F4/80bright cells, but F4/80dim cells were still present. Together, these in vivo results confirm Mϕ involvement in the antitumor effect of CY, αCD40 and CpG.
Figure 5.
Antitumor effect of αCD40/CpG involves Mϕ. C57BL/6 mice were injected i.p. with 1×105 B16 cells (day 0). Mice were treated with 100 mg/kg CY i.p. on day 8, 0.5 mg anti-CD40 mAb on day 11 and 0.05 mg CpG on day 14 or left untreated. Mϕ were depleted by giving the mice i.p. clodronate liposomes on day 9 and 13. On day 15, peritoneal cells were removed, counted and the percentage of CD45− tumor cells was determined by flow cytometry. The results are presented as Mean ± SEM of B16 cell number (total PEC number × % CD45− cells) of 7-9 mice per group from two independent experiments.
Discussion
There is a body of evidence that CY can induce antitumor effects not only by directly affecting dividing cancer cells, but also indirectly by augmenting T-cell concomitant antitumor immune responses (29). The immunomodulating effects of CY can be attributed, at least in part, to the ability of CY to eliminate tumor-induced T regulatory/suppressor cells (7,8). Several studies have reported the beneficial effects of CY administered prior to adoptive transfer of T cells (30), dendritic cell vaccines (31) or activated NK cells (5). To our knowledge, the results presented here show for the first time that combining CY and immunotherapy can result in enhanced antitumor effects without the involvement of T cells or NK cells.
We have previously shown that αCD40 and CpG, given separately or in combination, can induce T cell-independent antitumor effects involving Mϕ (24,32-34). The results of this study confirm that Mϕ are activated following αCD40/CpG treatment, and show that in vivo CY treatment did not inhibit the ability of Mϕ to be activated. In contrast to numerous T cell-based approaches, a relatively limited number of approaches aimed at Mϕ activation have been tested for cancer therapy experimentally and in clinical trials. One of them, using muramyl tripeptide phosphatidylethanolamine (MTP-PE), has shown promise in both experimental systems and clinical trials (35,36). However, combining MTP-PE with chemotherapy (cisplatin) in mice and dogs did not lead to enhanced antitumor effects (37,38). Clinical testing in children with osteosarcoma suggested that the chemotherapy regimen used might influence the potential benefit of MTP-PE treatment and thus improve overall survival (39). The preclinical data reported here show that αCD40/CpG immunotherapy in combination with CY leads to enhanced antitumor effects. In addition to CY, we have also seen additive or synergistic antitumor effects of αCD40/CpG when combined with the CHO chemotherapeutic regimen consisting of CY, doxorubicin and vincristine (Buhtoiarov et al., in press).
The exact mechanism of the enhanced antitumor activity of the combination of CY and αCD40/CpG is not clear. Our experiments suggest that in vivo CY treatment may render Mϕ more responsive to the subsequent activation with αCD40/CpG (Figure 3D). This may be due to the increased percentage of Mϕ in the peritoneal cavity, possibly caused by selective reduction of other cells including CD40+ B cells, consequently exposing Mϕ to more αCD40/CpG. However, while some analyses we performed demonstrated enhanced activation of Mϕ with a combination of CY and αCD40/CpG compared to αCD40/CpG without CY, this enhancement was not reproducible (data not shown). It is also possible that CY aids αCD40/CpG therapy by activating cells other than Mϕ. For example, CY has been shown to induce early myeloid cells with the CD11b+Gr-1+ phenotype of myeloid-derived suppressor cells (MDSC), both in mice (40-42) and cancer patients (43). In response to IFNγ and CD40 ligation, CY-induced MDSC produce NO, which has antitumor activity (41). It was suggested that induction of these cells by CY was dependent on the presence of tumor-specific T cells producing IFNγ (39,41). However, we have previously shown that IFNγ can be induced in Mϕ in response to CD40 ligation (24,33,44), suggesting that T cells may not need to be involved. Although adoptive transfer of CY-induced MDSC inhibited T cell-dependent antitumor effects of αCD40 (42), in our studies the additive effect of CY and immunotherapy was observed in T cell-deficient mice. Our data show an increased percentage of cells with MDSC phenotype (CD11b+Gr-1+) in PEC of CY, αCD40 and CpG – treated mice, suggesting that they can contribute to the antitumor effects observed in vivo. The inability of anti-Gr-1 mAb to deplete all Gr-1+ cells or reduce the antitumor effect of CY + αCD40/CpG in our preliminary experiments (data not shown) could be due to the recently described resistance of certain MDSC to anti-Gr-1 mAb – mediated depletion in vivo (45). Therefore, the role of MDSC in the observed antitumor effects warrants further investigation.
Our results demonstrate that immunological approaches directed toward activating Mϕ can be combined with chemotherapy to achieve better antitumor effects in immunocompromised mice, and suggest that such strategies might be considered for immunosuppressed cancer patients. This concept might sound counterintuitive, given that tumor-infiltrating Mϕ have been viewed as tumor-promoting rather than antitumor in their effects: their number usually correlates with poor prognosis (46). However, it also has been reported that chemotherapy combined with immunotherapy (rituximab) resulted in a high content of tumor-associated Mϕ which correlated with favorable outcome in follicular lymphoma patients (47). Moreover, it has been shown that pro-tumor Mϕ (M2 type) can be converted into tumor-infiltrating antitumor Mϕ (M1 type) by immunotherapy containing CpG (48). Our data indicate that activation of Mϕ with αCD40 and CpG, especially when combined with chemotherapy, results in antitumor effects in mice, suggesting that this approach should be considered for clinical development and testing.
Acknowledgements
We thank Drs Jacquelyn A. Hank and Jacek Gan for helpful discussions of the results of this study and Julie A. Waisbren for technical assistance. Dr. Ilia N. Buhtoiarov is a UICC fellow.
This work was supported by NIH-NCI grants CA87025 and CA32685, a grant from the Midwest Athletes Against Childhood Cancer (MACC) Fund, and support from The Crawdaddy Foundation.
Abbreviations
- CY
cyclophosphamide
- mAb
monoclonal antibody
- Mϕ
macrophages
- IFN-γ
interferon gamma
- PEC
peritoneal cells.
References
- 1.Assouline S, Sylvestre MP, Carriere P, et al. Comparison of peripheral blood progenitor cell yield from standard chemotherapy used in the treatment of lymphoid malignancies and high-dose cyclophosphamide: a retrospective review of 141 patients. Transfusion. 2006;2:174–179. doi: 10.1111/j.1537-2995.2006.00695.x. [DOI] [PubMed] [Google Scholar]
- 2.Dang CT. Drug treatments for adjuvant chemotherapy in breast cancer: recent trials and future directions Expert Rev. Anticancer Ther. 2006;3:427–436. doi: 10.1586/14737140.6.3.427. Review. [DOI] [PubMed] [Google Scholar]
- 3.Rosti G, Carminati O, Monti M, et al. Chemotherapy advances in small cell lung cancer. Ann Oncol. 2006;(Suppl 5):v99–102. doi: 10.1093/annonc/mdj961. Review. [DOI] [PubMed] [Google Scholar]
- 4.Borovansky J. Experimental chemotherapy of murine melanomas: Is there a discrepancy compared to clinical experience? Neoplasma. 1997;44:277–281. [PubMed] [Google Scholar]
- 5.Goldfarb RH, Ohashi M, Brunson KW, et al. Augmentation of IL-2 activated natural killer cell adoptive immunotherapy with cyclophosphamide. Anticancer Res. 1998;18:1441–1446. [PubMed] [Google Scholar]
- 6.Holden SA, Lan Y, Pardo AM, et al. Augmentation of antitumor activity of an antibody-interleukin 2 immunocytokine with chemotherapeutic agents. Clinical Cancer Res. 2001;7:2862–2869. [PubMed] [Google Scholar]
- 7.North RJ. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med. 1982;155:1063–1074. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lutsiak ME, Semnani RT, De PR, et al. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–2868. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 9.Yu B, Kusmartsev S, Cheng FD, et al. Effective combination of chemotherapy and dendritic cell administration for the treatment of advanced-stage experimental breast cancer. Clinical Cancer Res. 2003;9:285–294. [PubMed] [Google Scholar]
- 10.Sredni B, Weil M, Khomenok G, et al. Ammonium trichloro(dioxoethylene-o,o ’)tellurate (AS101) sensitizes tumors to chemotherapy by inhibiting the tumor interleukin 10 autocrine loop. Cancer Res. 2004;64:1843–1852. doi: 10.1158/0008-5472.can-03-3179. [DOI] [PubMed] [Google Scholar]
- 11.Eralp Y, Wang XY, Wang JP, et al. Doxorubicin and paclitaxel enhance the antitumor efficacy of vaccines directed against HER 2/neu in a murine mammary carcinoma model. Breast Cancer Res. 2004;6:R275–R283. doi: 10.1186/bcr787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell MS. Combinations of anticancer drugs and immunotherapy. Cancer Immunol Immunother. 2003;52:686–692. doi: 10.1007/s00262-003-0427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solomayer EF, Feuerer M, Bai L, et al. Influence of adjuvant hormone therapy and chemotherapy on the immune system analysed in the bone marrow of patients with breast cancer. Clin Cancer Res. 2003;9:174–180. [PubMed] [Google Scholar]
- 14.Dye ES, North RJ. Macrophage Accumulation in Murine Ascites Tumors.1. Cytoxan-Induced Dominance of Macrophages over Tumor-Cells and the Antitumor Effect of Endotoxin. J Immunol. 1980;125:1650–1657. [PubMed] [Google Scholar]
- 15.Tsung K, Dolan JP, Tsung YL, et al. Macrophages as effector cells in interleukin 12-induced T cell-dependent tumor rejection. Cancer Res. 2002;62:5069–5075. [PubMed] [Google Scholar]
- 16.Kawai O, Ishii G, Kubota K, et al. Predominant infiltration of macrophages and CD8(+) T Cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer. Cancer. 2008;113:1387–1395. doi: 10.1002/cncr.23712. [DOI] [PubMed] [Google Scholar]
- 17.Weiner GJ. The immunobiology and clinical potential of immunostimulatory CpG oligodeoxynucleotides. J Leukoc Biol. 2000;68:455–463. [PubMed] [Google Scholar]
- 18.Friedberg JW, Kim H, McCauley M, et al. Combination immunotherapy with a CpG oligonucleotide (1018 ISS) and rituximab in patients with non-Hodgkin lymphoma: increased interferon-alpha/beta-inducible gene expression, without significant toxicity. Blood. 2005;105:489–495. doi: 10.1182/blood-2004-06-2156. [DOI] [PubMed] [Google Scholar]
- 19.Law CL, Gordon KA, Collier J, et al. Preclinical antilymphoma activity of a humanized anti-CD40 monoclonal antibody, SGN-40. Cancer Res. 2005;18:8331–8338. doi: 10.1158/0008-5472.CAN-05-0095. [DOI] [PubMed] [Google Scholar]
- 20.Leonard JP, Link BK, Emmanouilides C, et al. Phase I trial of toll-like receptor 9 agonist PF-3512676 with and following rituximab in patients with recurrent indolent and aggressive non Hodgkin’s lymphoma. Clin Cancer Res. 2007;13:6168–6174. doi: 10.1158/1078-0432.CCR-07-0815. [DOI] [PubMed] [Google Scholar]
- 21.Vonderheide RH, Flaherty KT, Khalil M, et al. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol. 2007;25:876–883. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]
- 22.Murad YM, Clay TM, Lyerly HK, et al. CPG-7909 (PF-3512676, ProMune): toll-like receptor-9 agonist in cancer therapy. Expert Opin Biol Ther. 2007;7:1257–1266. doi: 10.1517/14712598.7.8.1257. Review. [DOI] [PubMed] [Google Scholar]
- 23.Molenkamp BG, Sluijter BJ, van Leeuwen PA, et al. Local administration of PF-3512676 CpG-B instigates tumor-specific CD8+ T-cell reactivity in melanoma patients. Clin Cancer Res. 2008;14:4532–4542. doi: 10.1158/1078-0432.CCR-07-4711. [DOI] [PubMed] [Google Scholar]
- 24.Buhtoiarov IN, Lum HD, Berke G, et al. Synergistic Activation of Macrophages via CD40 and TLR9 results in T cell independent antitumor effects. J Immunol. 2006;176:309–318. doi: 10.4049/jimmunol.176.1.309. [DOI] [PubMed] [Google Scholar]
- 25.Imboden M, Murphy KR, Rakhmilevich AL, et al. The Level of MHC Class I Expression on Murine Adenocarcinoma Can Change the Anti-Tumor Effector Mechanism of Immunocytokine Therapy. Cancer Res. 2001;61:1500–1507. [PubMed] [Google Scholar]
- 26.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Meth. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 27.Fink K, Ng C, Nkenfou C, et al. Depletion of macrophages in mice results in higher dengue virus titers and highlights the role of macrophages for virus control. Eur J Immunol. 2009;39:2809–2821. doi: 10.1002/eji.200939389. [DOI] [PubMed] [Google Scholar]
- 28.Gordon S, Lawson L, Rabinowitz S, et al. Antigen markers of macrophage differentiation in murine tissues. Curr Top Microbiol Immunol. 1992;181:1–37. doi: 10.1007/978-3-642-77377-8_1. [DOI] [PubMed] [Google Scholar]
- 29.Turk MJ, Guevara-Patiño JA, Rizzuto GA, et al. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200:771–782. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Awwad M, North RJ. Cyclophosphamide (Cy)-facilitated adoptive immunotherapy of a Cy-resistant tumour. Evidence that Cy permits the expression of adoptive T-cell mediated immunity by removing suppressor T cells rather than by reducing tumour burden. Immunol. 1988;65:87–92. [PMC free article] [PubMed] [Google Scholar]
- 31.Liu JY, Wu Y, Zhang XS, et al. Single administration of low dose cyclophosphamide augments the antitumor effect of dendritic cell vaccine. Cancer Immunol Immunother. 2007;56:1597–1604. doi: 10.1007/s00262-007-0305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turner JG, Rakhmilevich AL, Burdelya L, et al. Anti-CD40 Antibody Induces Antitumor and Anti-metastatic Effects: Role of Natural Killer Cells. J Immunol. 2001;166:89–94. doi: 10.4049/jimmunol.166.1.89. [DOI] [PubMed] [Google Scholar]
- 33.Buhtoiarov IN, Lum H, Berke G, et al. CD40 ligation induces antitumor reactivity of murine macrophages via an IFN gamma-dependent mechanism. J Immunol. 2005;174:6013–6022. doi: 10.4049/jimmunol.174.10.6013. [DOI] [PubMed] [Google Scholar]
- 34.Buhtoiarov IN, Sondel PM, Eickhoff JC, et al. Macrophages are essential for antitumor effects against weakly immunogenic murine tumors induced by class B CpG-ODN. Immunol. 2007;120:412–423. doi: 10.1111/j.1365-2567.2006.02517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacEwen EG, Kurzman ID, Vail DM, et al. Adjuvant therapy for melanoma in dogs: results of randomized clinical trials using surgery, liposome-encapsulated muramyl tripeptide, and granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 1999;5:4249–4258. [PubMed] [Google Scholar]
- 36.Nardin A, Lefebvre ML, Labroquère K, et al. Liposomal muramyl tripeptide phosphatidylethanolamine: Targeting and activating macrophages for adjuvant treatment of osteosarcoma. Curr Cancer Drug Targets. 2006;6:123–133. doi: 10.2174/156800906776056473. Review. [DOI] [PubMed] [Google Scholar]
- 37.Kurzman ID, MacEwen EG, Rosenthal RC, et al. Adjuvant therapy for osteosarcoma in dogs: results of randomized clinical trials using combined liposome-encapsulated muramyl tripeptide and cisplatin. Clin Cancer Res. 1995;1:1595–1601. [PubMed] [Google Scholar]
- 38.Klimp AH, De Vries EG, Scherphof GL, et al. Chemo-immunotherapy of ovarian cancer in a murine tumour model. Anticancer Res. 2000;20:2585–2592. [PubMed] [Google Scholar]
- 39.Meyers PA, Schwartz CL, Krailo MD, et al. Children’s Oncology Group.Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival--a report from the Children’s Oncology Group. J Clin Oncol. 2008;26:633–638. doi: 10.1200/JCO.2008.14.0095. [DOI] [PubMed] [Google Scholar]
- 40.Angulo I, de las Heras FG, Garcia-Bustos JF, et al. Nitric oxide-producing CD11b(+)Ly-6G(Gr-1)(+)CD31(ER-MP12)(+) cells in the spleen of cyclophosphamide-treated mice: implications for T-cell responses in immunosuppressed mice. Blood. 2000;95:212–220. [PubMed] [Google Scholar]
- 41.Pelaez B, Campillo JA, Lopez-Asenjo JA, et al. Cyclophosphamide induces the development of early myeloid cells suppressing tumor cell growth by a nitric oxide-dependent mechanism. J Immunol. 2001;166:6608–6615. doi: 10.4049/jimmunol.166.11.6608. [DOI] [PubMed] [Google Scholar]
- 42.Honeychurch J, Glennie MJ, Illidge TM. Cyclophosphamide inhibition of anti-CD40 monoclonal antibody-based therapy of B cell lymphoma is dependent on CD11b+ cells. Cancer Res. 2005;65:7493–7501. doi: 10.1158/0008-5472.CAN-04-3808. [DOI] [PubMed] [Google Scholar]
- 43.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, et al. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lum HD, Buhtoiarov IN, Berke G, et al. In vivo CD40 ligation can induce T cell-independent antitumor effects that involve macrophages. J Leuk Biol. 2006;79:1181–1192. doi: 10.1189/jlb.0405191. [DOI] [PubMed] [Google Scholar]
- 45.Ribechini E, Leenen PJ, Lutz MB. Gr-1 antibody induces STAT signaling, macrophage marker expression and abrogation of myeloid-derived suppressor cell activity in BM cells. Eur J Immunol. 2009;39:3538–3551. doi: 10.1002/eji.200939530. [DOI] [PubMed] [Google Scholar]
- 46.Mantovani A, Schioppa T, Porta C, et al. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006;25:315–322. doi: 10.1007/s10555-006-9001-7. Review. [DOI] [PubMed] [Google Scholar]
- 47.Taskinen M, Karjalainen-Lindsberg ML, Nyman H, et al. A high tumor-associated macrophage content predicts favorable outcome in follicular lymphoma patients treated with rituximab and cyclophosphamide-doxorubicin-vincristine-prednisone. Clin Cancer Res. 2007;13:5784–2789. doi: 10.1158/1078-0432.CCR-07-0778. [DOI] [PubMed] [Google Scholar]
- 48.Guiducci C, Vicari AP, Sangaletti S, et al. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 2005;65:3437–3446. doi: 10.1158/0008-5472.CAN-04-4262. [DOI] [PubMed] [Google Scholar]