Abstract
A singular feature of the catalytic C-cluster of carbon monoxide dehydrogenase is a sulfide-bridged Ni⋯Fe locus where substrate is bound and transformed in the reversible reaction CO + H2O ⇌ CO2 + 2H+ + 2e−. A similar structure has been sought in this work. Mononuclear planar NiII complexes [Ni(pyN2Me2)L]1− (pyN2Me2 = bis(2,6-dimethylphenyl)-2,6-pyridinedicarboxamidate(2-)) derived from a NNN pincer ligand have been prepared including L = OH− (1) and CN− (7). Complex 1 reacts with ethyl formate and CO2 to form unidentate L = HCO2− (5) and HCO3− (6) products. A binucleating macrocycle was prepared which specifically binds NiII at a NNN pincer site and five-coordinate FeII at a triamine site. The NiII macrocyle forms hydroxo (14) and cyanide complexes (15) analogous to 1 and 7. Reaction of 14 with FeCl2 alone and with ethyl formate and 15 with FeCl2 affords molecules with the NiII-L-FeII bridge unit in which L = µ2:η1-OH− (17) and µ2:η2-HCO2− (18) and -CN− (19). All bridges are non-linear (17, 140.0°; 18, M-O-C 135.9° (Ni), 120.2° (Fe); 19, Ni-C-N 170.3°, Fe-N-C 141.8°) with Ni⋯Fe separations of 3.7–4.8 Å. The NiIIFeII complexes, lacking appropriate Ni-Fe-S cluster structures, are not site analogues but their synthesis and reactivity provide the first demonstration that molecular NiII…FeII sites and bridges can be attained, a necessity in the biomimetic chemistry of C-clusters.
Introduction
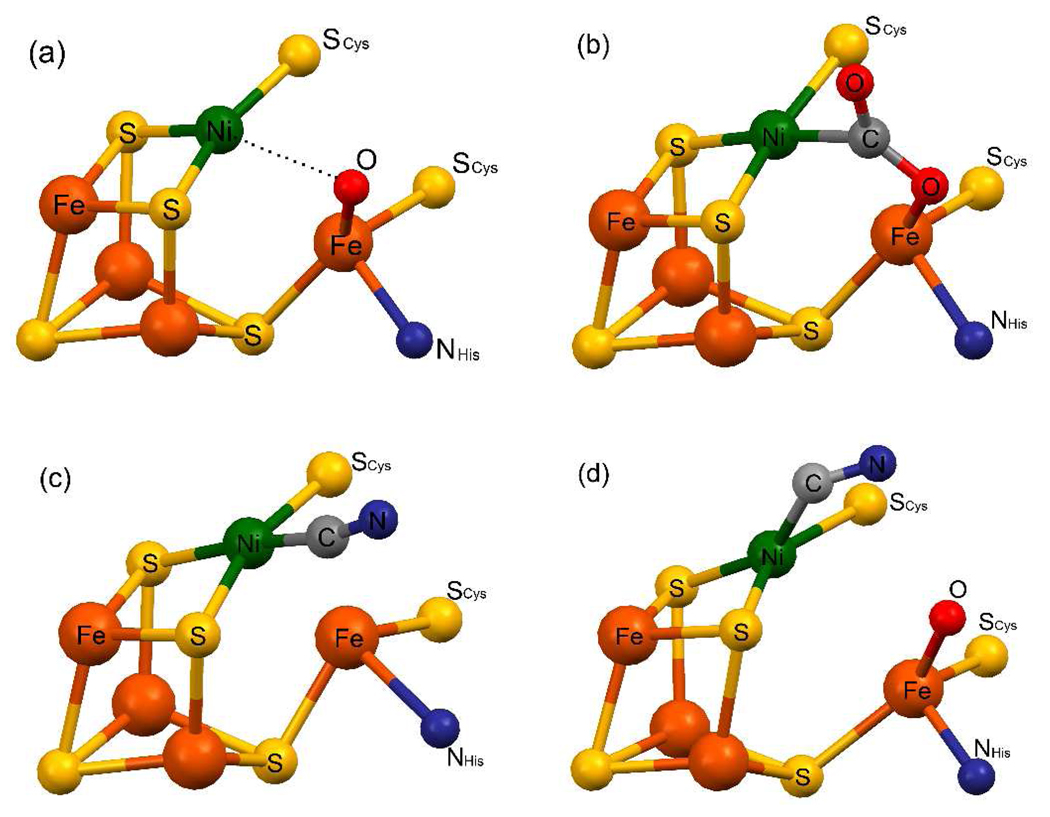
Among the many challenges in the biomimetic chemistry of metal clusters1 is the construction of molecules that approach or achieve the structures of the catalytic sites of carbon monoxide dehydrogenases (CODHs2).3,4 These enzymes contain either a Mo-Cu-S or a Ni-Fe-S active site and catalyze the reversible reaction CO + H2O ⇌ CO2 + 2H+ + 2e−, a process of prime significance in the global carbon cycle. Nickel-containing CODHs5,6 utilize as their catalytic site for CO/CO2 interconversion the structurally complex C-cluster, which has been crystallographically investigated in enzymes from several different anaerobic bacteria.7–9 The most recent results (2007–09) for enzymes from three sources (MtCODH,10 MbCODH/ACS,11 MtCODH/ACS12) point toward a consistent structure based on a NiFe3S4 cluster to which is appended an external (exo) iron atom. As shown in Figure 1(a), the structure is a bridged assembly of core composition NiFe4S4 with an aquo or hydroxo ligand bound to the exo iron with approximate tetrahedral geometry. The consensus structure-based mechanistic proposal is that CO binds at the nickel site, undergoes nucleophilic attack by coordinated hydroxide to form a carbon-bound hydroxycarbonyl bridged intermediate such as (b). This species subsequently liberates CO2 and two electrons upon reaction with water to regenerate (a).
Figure 1.
Structures of C-clusters in different reaction states with selected interatomic distances (Å) and angles. (a) ChCODH in the Cred1 or −320 mV (resting) state; Ni-O 2.72, Fe-O 1.95, Ni-Fe 2.85.10 (b) ChCODH in the −600 + CO2 (intermediate) state; Ni-C 1.96, Fe-O 2.05, C-OFe 1.25, Ni-Fe 2.76, Ni-C-O 119°, Fe-O-C 105°.10 (c) ChCODH in the cyanide-bound state: Ni-C 1.79, Ni-Fe 2.56, Ni-C-N 170°.10 (d) MtCODH/ACS in the cyanide-bound state; Ni-C 1.99, Fe-O 2.05, Ni-C-N 114°.12 In (a) and (d), OH−/OH2 is bound to Fe. Terminal cysteinate ligation at three Fe atoms in each structure is omitted.
Previous synthetic work directed toward the C-cluster, some of it prior to the availability of structural information, has resulted in clusters with certain relevant features. Phosphine ligation in the cubanes [(Ph3P)NiFe3S4(SR)3]2− simulates the binding of CO and other π-acid ligands at a tetrahedral nickel site.13–15 Further, the approximately planar Ni sites in intermediate (b) and the cyanide-ligated C-clusters (c) and (d) (Figure 1) are approached in clusters such as [(tdt)NiFe3S4(LS3)]3−. This and related cubanoid clusters are produced by phosphine substitution with a strong in-plane chelating ligand and attendant rupture of the axial Ni-(µ3-S) bond.16,17 However, key attributes of the C-cluster have not been experimentally attained. These include an exo iron site linked to the NiFe3S4 portion through a µ3-S bridge (or any bridge), coordinative unsaturation at the nickel site as manifested in the apparent three-coordinate "T-shaped"10 Ni(µ3-S)2SCys fragment (Figure 1(a)), and the juxtaposition of a terminal OH−/OH2 ligand at a distance too long to represent a bridging interaction to the nickel site (Ni⋯OH 2.7 Å). Multiple attempts to bridge FeII to an NiFe3S4 unit have not succeeded.
Given the current synthetic inaccessibility of a complete C-cluster analogue, we have utilized a different ligand construct with which to pursue reactivity with CO and CO2 by NiII alone and in the presence of proximal FeII. The few FeII-OH complexes known are stabilized by steric factors18 or hydrogen bonding interactions;19,20 reactivity toward CO or CO2 has not been reported. Pincer NiII, PdII, and PtII terminal hydroxo complexes with PCP donor atom sets react with these substrates, in several cases affording C-bound hydroxycarbonyl products21–23 similar to intermediate (b) but bound in the η 1 mode. Consequently, we have utilized pincers with a less-abiological NNN donor set as a ligand for NiII in mononuclear complexes and in a binucleating macrocycle incorporating a triamine (dien) binding site for FeII. This investigation is an initial step toward a binuclear NiII⋯FeII system allowing examination of biological substrate and inhibitor binding at one or both metal centers in terminal or bridge modes. Other than with the enzymes themselves, there is no information on reactivity and structures of binuclear NiIIFeII molecules with any species.
Experimental Section
Preparation of Compounds
All reactions and manipulations were performed under a pure dinitrogen atmosphere using Schlenk techniques or an inert atmosphere box. Volume reduction and drying steps were performed in vacuo. DMF was freshly distilled and dried over 3-Å molecular sieves for 3 d. Other solvents were passed through an Innovative Technology or MBraun solvent purification system. All solvents were degassed before use. Compounds were identified by spectroscopic and/or crystallographic characterization; representative compounds were analyzed (Kolbe Microanalytical Laboratory, Mülheim, Germany). Cation resonances are omitted from NMR data. Compounds with no NMR data were insufficiently soluble for measurement and were identified crystallographically.
In the sections that follow, complexes and ligands are numerically designated according to the Chart.
Chart I.
Abbreviations and Designation of Ligands and Complexes
ACS = acetyl coenzyme A synthase; Ch = carboxydothermus hydrogenoformans; CODH = carbon monoxide dehydrogenase; dien = diethylenetriamine; dme = 1,2-dimethoxyethane; Mb = Methanosarcina barkeri; Me6tren = tris(N,N'-dimethyl-2-aminoethyl)amine; Mt = Moorella thermoacetica; pyN2 = N,N'-bis(phenyl)-2,6-pyridinedicarboxamidate(2-); pdtc = pyridine-2,6-dithiocarboxylate(2-); pyN2iPr2 = N,N'-bis(2,6-diisopropylphenyl)-2,6-pyridinedicarboxamidate(2-); pyN2Me2 = bis(2,6-dimethylphenyl)-2,6-pyridinedicarboxamidate(2-); pyN2dienMe3 = bis(phenyl-3,3'-(2,5,8-tri-N-methylamino-1,9-nonyl))-2,6-pyridinedicarboxamidate(2-); tdt = toluene-3,4-dithiolate(2-); TfO− = triflate
(a) Pincer Ligand and Mononuclear Complexes. N,N′-Bis(2,6-dimethylphenyl)-2,6-pyridinedicarboxamide (H2pyN2Me2)
A solution of pyridine-2,6-dicarbonylchloride (1.02 g, 5.00 mmol) at 0° in THF (150 mL) was added slowly to a mixture of 2, 6-dimethylaniline (1.21 g, 10 mmol) and triethylamine (1.41 mL, 10 mmol). The reaction mixture was warmed to room temperature, stirred for 5 h, and filtered. The light yellow residue obtained by removal of solvent was treated with hexane (10 mL) and washed with a small volume of dichloromethane/ether (1:5 v/v) to give the pure product as a white powder (1.58 g, 85%). 1H NMR (CDCl3): δ 2.27 (s, 12), 7.13 (m, 6), 8.14 (t, 1), 8.51 (d, 2) 9.17 (s, 2).
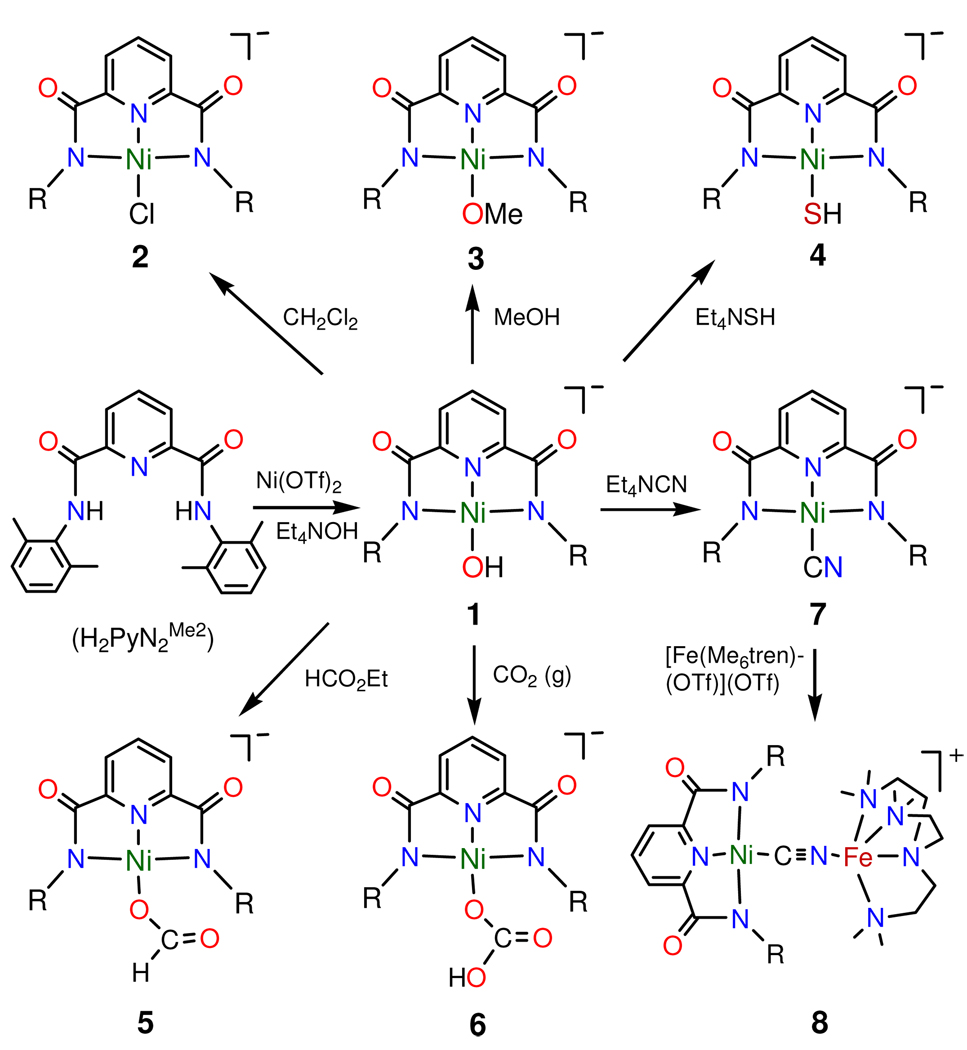
Synthetic reactions for complexes are summarized in Figure 2.
Figure 2.
Scheme showing the preparation of pincer complexes [Ni(pyN2Me2)L]1−, including hydroxo complex 1 and complexes 2–7 derived from it. Binuclear 8 is obtained from 7.
(Et4N)[Ni(pyN2Me2)(OH)]
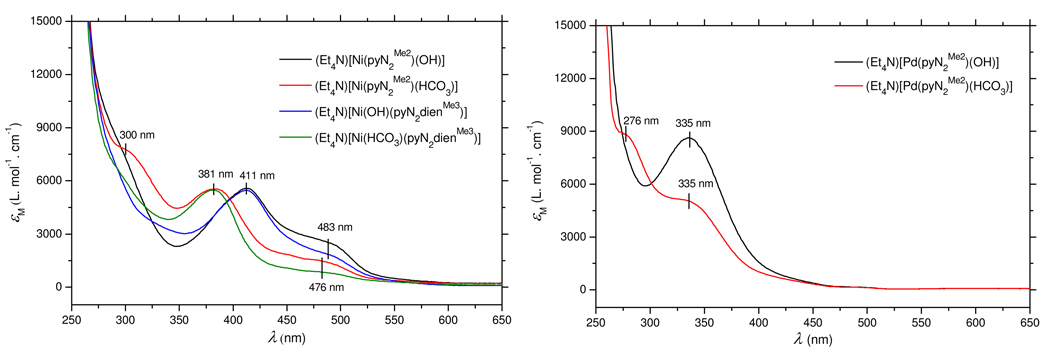
H2pyN2Me2 (149 mg, 0.40 mmol) and Ni(OTf)2 (143 mg, 0.40 mmol) were stirred in THF (5 mL) for 2 h. The light yellow suspension was treated with Et4NOH (25% in methanol, 353 mg, 0.60 mmol) and stirred for 2 h to give a red suspension. A second equal portion of Et4NOH was added and the mixture was stirred for 10 h to afford a deep red solution and some sticky precipitate, which was removed by filtration through celite. Hexane (10 mL) was added to the filtrate resulting in separation of a red oil. Addition of ether (5 mL) to the oil resulted in a solid, which was washed with THF and ether and dried to give the product as a red crystalline solid (138 mg, 60%). IR (KBr): νOH 3594 cm−1. Absorption spectrum (DMF): λmax (εM) 411 (5500), 483 (sh, 2600) nm. 1H NMR (CD2Cl2): δ −4.95 (s, 1), 2.48 (s, 12), 6.88 (m, 6), 7.51 (d, 2), 7.87 (t, 1). Anal. Calcd. for C31H42N4NiO3: C, 64.49; H, 7.33; N, 9.70. Found: C, 64.36; H, 7.40; N, 9.69.
(Et4N)[Ni(pyN2Me2)Cl]
A solution of (Et4N)[Ni(pyN2Me2)(OH)] (29 mg, 0.050 mmol) in dichloromethane (1 mL) was allowed to stand for 4 d. Ether was diffused into the solution causing deposition of the product as block-like brown-red crystals (24 mg, 81%). 1H NMR (CD2Cl2): δ 2.42 (s, 12), 6.86 (m, 6), 7.57 (d, 2), 7.97 (t, 1).
(Et4N)[Ni(pyN2Me2)(OMe)]
The preceding method with use of methanol (1 mL) afforded a red crystalline product (21 mg, 71%). 1H NMR (CD2Cl2): δ 2.50 (s, 12), 3.26 (s, 3), 6.89 (m, 6), 7.54 (d, 2), 7.89 (t, 1).
(Et4N)[Ni(pyN2Me2)(SH)]
To a solution of (Et4N)[Ni(pyN2Me2)(OH)] (23 mg, 0.040 mmol) in DMF (1 mL)was added a solution of Et4NSH (9.8 mg, 0.040 mmol) in an equal volume of DMF. The reaction mixture was stirred for 50 min and filtered. Diffusion of ether into the deep red filtrate gave the product as red crystals (22 mg, 93%). 1H NMR (DMF-d7): δ −3.51 (s, 1), 2.36 (s, 12), 6.81 (m, 6), 7.66 (d, 2), 8.19 (t, 1). Anal. Calcd. for C31H42N4NiO2S: C, 62.74; H, 7.13; N, 9.44. Found: C, 62.64; H, 7.17; N, 9.44.
(Et4N)[Ni(pyN2Me2)(HCO2)]
A solution of (Et4N)[Ni(pyN2Me2)(OH)] (12 mg, 0.020 mmol) in DMF (0.5 mL) was layered with ethyl formate (4 mL). After 2 d, the product was collected as orange-red crystals (7.0 mg, 58%). IR (KBr): 1615 (s), 1587 (w), 1470 (m), 1437 (vw), 1368 (m), 1300 (m) cm−1. 1H NMR (CD2Cl2): δ 2.49 (s, 12), 6.85 (m, 6), 7.54 (d, 2), 7.94 (t, 1). Anal. Calcd. for C32H42N4NiO4: C, 63.49; H, 6.99; N, 9.26. Found: C, 63.85; H, 7.10; N, 9.11.
(Et4N)[Ni(pyN2Me2)(HCO3)]
A solution of (Et4N)[Ni(pyN2Me2)(OH)] (23 mg, 0.040 mmol) in DMF (2 mL) was bubbled with carbon dioxide for 15 min. Diffusion of ether into the red solution gave the product as orange-red crystals (21 mg, 85%). IR (KBr): 1618 (vs), 1585 (w), 1470 (m), 1439 (w), 1360 (s), 1310 (w) cm−1. Absorption spectrum (DMF): λmax (εM) 300 (sh, 7800), 381 (5500), 476 (sh, 1500) nm. 1H NMR (CD3CN): δ 2.40 (s, 12), 6.90 (s, 6), 7.63 (s, 2), 8.15 (t, 1). Anal. Calcd. for C32H42N4NiO5: C, 61.85; H, 6.81; N, 9.02. Found: C, 61.44; H, 7.07; N, 8.99.
(Et4N)[Ni(pyN2Me2)(CN)]
To a solution of (Et4N)[Ni(pyN2Me2)(OH)] (23 mg, 0.040 mmol) in DMF (1 mL) was added a solution of Et4NCN (9.4 mg, 0.060 mmol) in DMF (0.5 mL). The reaction mixture was stirred for 1 h and filtered. Ether diffusion into the filtrate caused separation of the product as orange-red crystals (19 mg, 81%). IR (KBr): νCN 2127 cm−1. 1H NMR (CD2Cl2): δ 2.40 (s, 12), 6.90 (m, 6), 7.69 (d, 2), 8.07 (t, 1).
[Ni(pyN2Me2)(CN)Fe(Me6tren)](OTf)
To a solution of (Et4N)[Ni(pyN2Me2)(CN)] (19 mg, 0.032 mmol) in DMF (1 mL) was added a solution of [Fe(Me6tren)(OTf)](OTf)24 (19 mg, 0.032 mmol) in DMF (1 mL). The reaction mixture was stirred for 40 min and filtered. Diffusion of ether into the filtrate affords the product as thin platelike light red crystals (12 mg, 42%). IR (KBr): νCN 2135 cm−1.
(Et4N)[Pd(pyN2Me2)(OH)]
H2pyN3Me2 (75 mg, 0.20 mmol) and [Pd(MeCN)4](OTf)225 (114 mg, 0.2 mmol) were stirred in acetonitrile for 90 h. Solvent was reduced to ca. 3 ml and ether (13 ml) added to deposit a yellow-brown solid, which was washed with acetonitrile/THF (2 mL, 1:3 v/v) and dried. The solid was dissolved in DMF/THF (4 mL, 1:3 v/v), and the yellow solution was treated with Et4NOH (25% in methanol, 118 mg, 0.20 mmol) and stirred for 4 h. The mixture was filtered and ether diffused into the filtrate to afford the product as light yellow-brown crystalline solid, which was washed with THF and dried in vacuo (88 mg, 70%). Absorption spectrum (DMF): λmax (εM) 335 (8600) nm. 1H NMR (DMF-d7): δ −3.14 (s, 1), 2.35 (s, 12), 6.83 (t, 2), 6.92 (d, 4), 7.74 (d, 2), 8.20 (t, 1).
(Et4N)[Pd(pyN2Me2)(HCO3)]
A solution of (Et4N)[Pd(pyN2Me2)(OH)] (25 mg, 0.040 mmol) in DMF was bubbled with carbon dioxide for 30 min. Diffusion of ether into the solution resulted in separation of the product as block-like yellow crystals (24 mg, 90%). IR (KBr): 1615 (vs), 1584 (w), 1469 (m), 1445 (s), 1366 (s), 1304 (w) cm−1. Absorption spectrum (DMF): λmax (εM) 276 (sh, 8800), 335 (sh, 5000) nm. 1H NMR (DMF-d7): δ 2.36 (s, 12), 6.78 (t, 2), 6.85 (d, 4), 7.74 (d, 2), 8.28 (t, 1).
(b) Macrocyclic Ligand and Complexes
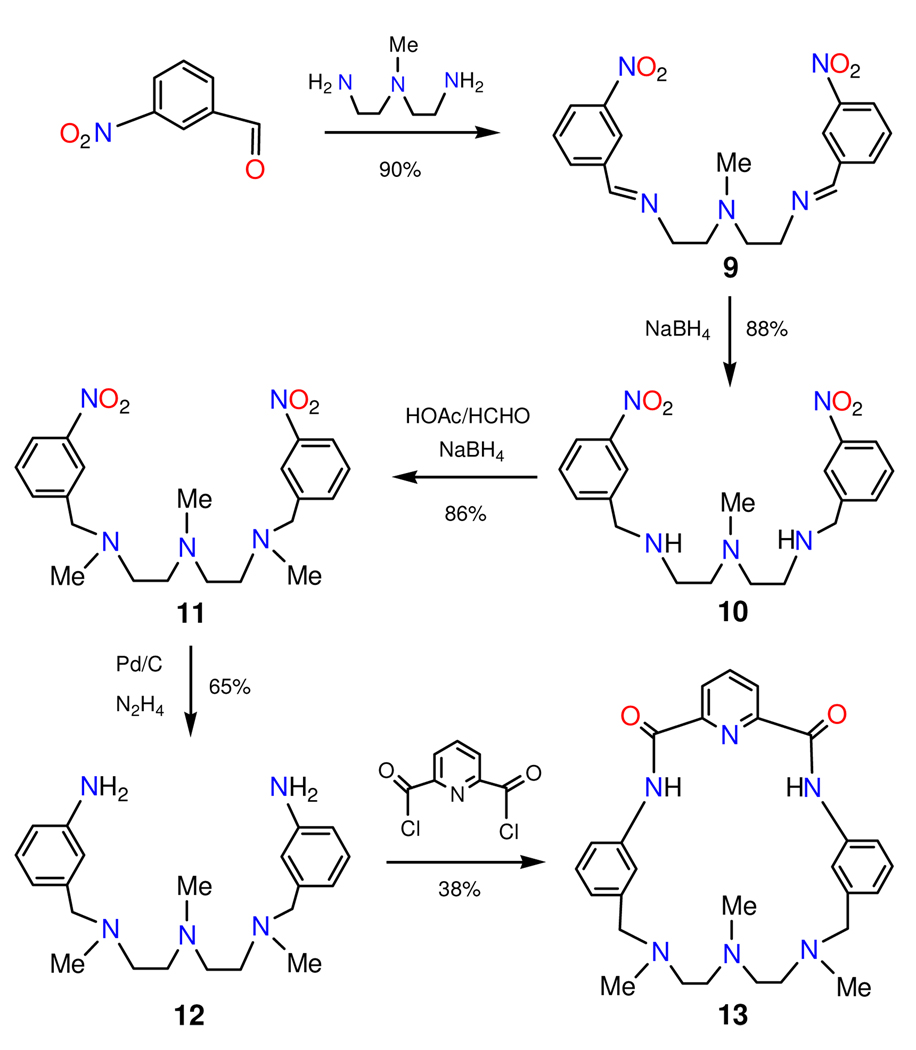
Reactions leading to the ligand and complexes are outlined in Figure 3 and Figure 4.
Figure 3.
Scheme for the synthesis of the binucleating pincer macrocycle ⊂-H2pyN2dienMe3 (13).
Figure 4.
Preparation of macrocyclic complexes [Ni(L)(⊂-pyN2dienMe3)]1− with L = OH− (14) and CN− (15) and bridged complexes [Ni(L)FeCl(⊂-pyN2dienMe3)] with L = OH− (17), HCO2− (18), and CN− (19).
Intermediates 9–12
3-Nitrobenzaldehyde (7.56 g, 50 mmol) and 3-N-methylaminopentane-1,5-diamine (2.93 g, 25 mmol) were reacted in ethanol (150 mL) for 12 h. The solid collected by filtration and a second batch obtained by volume reduction of the filtrate were combined and washed with ethanol to afford light yellow 9 (8.63 g, 90%; 1H NMR (CDCl3): δ 2.41 (s, 3), 2.83 (t, 4), 3.78 (t, 4), 7.51 (t, 2), 8.00 (d, 2), 8.21 (d, 2), 8.32 (s, 2), 8,48 (s, 2)). NaBH4 (3.40 g, 90 mmol) was added to a solution of 9 (8.63 g, 22.5 mmol) in methanol (150 mL) at 50° C. The solution was cooled to room temperature, solvent was removed, and the orange solid was extracted with dichloromethane/water (200 mL/100 mL). The organic layer was washed with water (2 ×100 mL), dried over MgSO4, and filtered. Removal of solvent gave 10 as a pale yellow oil (7.67 g, 88%; 1H NMR (CDCl3): δ 1.85 (s, br, 2), 2.20 (s, 3), 2.52 (t, 4), 2.70 (t, 4), 3.90 (s. 4), 7.45 (t, 2), 7.64 (d, 2), 8.07 (d, 2), 8.20 (s, 2)). To a solution of 10 (7.67 g, 20 mmol) in acetonitrile (540 mL) and acetic acid (60 mL) was added an aqueous solution of formaldehyde (28 mL, 37%). The mixture was stirred for 1 h, cooled to 0°C, and NaBH4 (3.74 g, 99 mmol) was slowly added. Stirring was continued for 2 h and for 36 h at room temperature. After filtration, solvent was removed from the filtrate to give a light yellow sticky solid, which was stirred in dichloromethane (200 mL). A solution of 2 M NaOH was added until pH was ca. 14. The aqueous layer was extracted with dichloromethane (2 × 50 mL) and the combined organic layers were washed with water (100 mL), dried over MgSO4 and filtered. Removal of solvent from the filtrate gave 11 as a light yellow oil (7.07 g, 86%; 1H NMR (CDCl3): δ 2.21 (s, 6), 2.22 (d, 3), 2.53 (m, 8), 3.59 (s, 4), 7.45 (t, 2), 7.64 (d, 2), 8.08 (d, 2), 8.19 (s, 2)). To a mixture of 11 (2.49 g, 6.0 mmol) and Pd/C (160 mg) in anhydrous ethanol was added a solution of N2H4·H2O (20 mL). The mixture was stirred for 30 min and for 12 h at 60°C and filtered. Volume reduction of the filtrate gave a light orange oil. The crude product was purified in the air on a silica column eluted with dichloromethane/methanol/NH4OH to give 12 as a light yellow oil (1.38 g, 65%; 1H NMR (CDCl3): δ 2.18 (s, 3), 2.19 (s, 6), 2.49 (m, 8), 3.39 (s, 2), 3.43 (s, 2), 3.68 (s, br, 4), 6.55 (d, 2), 6.66 (m, 4), 7.06 (t, 2)).
⊂-H2pyN2dienMe3
To a solution of Et3N (25 mL) in THF (350 mL) was added simultaneously solutions of 12 (1.38 g, 3.90 mmol) and pyridine-2,6-dicarbonylchloride (0.79 g, 3.90 mmol), each in THF (50 mL), by syringe pump over 24 h. The reaction mixture was stirred for 3 h, filtered, and the filtrate was evaporated to leave a yellow-orange solid. This residue was stirred with methanol (120 mL, 1 h) and the solution was filtered. The yellow filtrate was reduced to ca. 6 mL and heated to dissolve any solid. Cooling the solution caused separation of colorless crystals. The steps of filtrate volume reduction and cooling were repeated several more times to obtain additional amounts of white crystals. The crops were combined and dried to give the product as a white solid (0.72 g, 38%). 1H NMR (DMSO-d6): δ 2.13 (s, 6), 2.25 (s, 3), 2.53 (t, 4), 2.60 (t, 4), 3.51 (s, 4), 7.01 (d, 2), 7.35 (t, 2), 7.66 (s, 2), 8.30 (t, 1), 8.39 (m, 4), 11.22 (s, 2). Anal. Calcd. for C28H34N6O2: C, 69.11; H, 7.04; N, 17.27. Found: C, 68.01; H, 7.03; N, 17.01.
(Et4N)[Ni(OH)(⊂-pyN2dienMe3)]
The macrocycle (73 mg, 0.15 mmol) and Ni(OTf)2 (53 mg, 0.15 mmol) were mixed and stirred in DMF (2 mL) for 1 h to give a light yellow suspension. Et4NOH (25% in methanol, 118 mg, 0.20 mmol) was added and the mixture was stirred for 2 h forming a red suspension. A second portion of Et4NOH (147 mg, 0.25 mmol) was added and the mixture was stirred to 10 h to give a deep red solution with some sticky precipitate. The latter was removed by filtration through celite. Addition of ether (25 mL) to the filtrate and storage at −20°C for 2 d yielded the product as red crystals (53 mg, 51%). IR (KBr): νOH 3606 cm−1. Absorption spectrum (DMF): λmax (εM) 411 (5400), 483 (sh, 1900) nm. 1H NMR (DMF-d7): δ −4.40 (s, 1), 2.01 (s, 3), 2.20 (m, 4), 2.28 (m, 10), 3.35 (s, 4), 6.80 (d, 2), 7.05 (t, 2), 7.20 (s, 2), 7.28 (d, 2), 7.54 (d, 2), 8.06 (t, 1).
(Et4N)[Ni(HCO3)(⊂-pyN2dienMe3)]
A solution of (Et4N)[Ni(OH)(⊂-pyN2dienMe3)] (21 mg, 0.030 mmol) in DMF (1.5 mL) was bubbled with carbon dioxide for 15 min. Diffusion of ether in the red solution caused separation of the product as orange-red crystals (17 mg, 77%). IR (KBr): 1656 (s), 1619 (vs), 1580 (m), 1481 (w), 1456 (m), 1369 (m), 1328 (s) cm−1. Absorption spectrum (DMF): λmax (εM) 381 (5500), 476 (sh, 880) nm. 1H NMR (CD3CN): δ 1.88 (s, 3), 2.21 (br, s, 4), 2.32 (s, 6), 2.42 (br, s, 4), 3.43 (br, s, 4), 6.78 (d, 2), 6.90 (d, 2), 7.02 (s, 2), 7.09 (t, 2), 7.75 (d, 2), 8.01 (t, 1).
(Et4N)[Ni(CN)(⊂-pyN2dienMe3)]
To a solution of (Et4N)[Ni(OH)(⊂-pyN2dienMe3)] (34.5 mg, 0.050 mmol) in DMF (2 mL) was added a solution of Et4NCN (7.8 mg, 0.050 mmol) in DMF (1 mL). The reaction mixture was stirred for 1 h and filtered. Addition of ether (15 ml) to the red filtrate deposited the product as an orange crystalline solid (26 mg, 75%). IR (KBr): νCN 2129 cm−1. 1H NMR (CD2Cl2): δ 2.24 (s, 3), 2.32 (s, 6), 2.49 (m, 4), 2.60 (m, 4), 3.51 (s, 4), 6.86 (d, 2), 7.03 (d, 2), 7.08 (t, 2), 7.41 (s, 2), 7.66 (d, 2), 8.00 (t, 1).
[Ni(OH)FeCl(⊂-pyN2dienMe3)]
To a solution of (Et4N)[Ni(OH)(⊂-pyN3dienMe3)] (28 mg, 0.040 mmol) in DMF (2 mL) was added a solution of FeCl2 (5.1 mg, 0.040 mol) in DMF (1 mL). The reaction mixture was stirred for 1 h, filtered through celite, and ether was slowly diffused into the deep red filtrate over 3 d. The crystalline solid was washed with acetonitrile (5 mL) and dried to afford the product as red crystals (10 mg, 38%). IR (KBr): νOH 3597 cm−1. Anal. Calcd. for C28H33ClFeN6NiO3: C, 51.61; H, 5.11; N, 12.90; Fe, 8.57; Ni, 9.01. Found: C, 51.44; H, 5.39; Fe, 8.47; N, 12.79; Ni, 8.88.
[Ni(HCO2)FeCl(⊂-pyN2dienMe3)]
To a solution of (Et4N)[Ni(OH)(⊂-pyN2dienMe3)] (28 mg, 0.040 mmol) in DMF (1 mL) was added a solution of FeCl2 (5.1 mg, 0.040 mmol) in DMF (1 mL). The reaction mixture was stirred for 1 h and filtered through celite. The deep red filtrate was layered with ethyl formate (4 mL) for 4 d, filtered, and ether diffused into the filtrate to deposit the product as a red crystalline solid (8.0 mg, 30%). IR (KBr): 1631 (vs), 1599 (vs), 1485 (w), 1462 (m), 1441 (w), 1366 (s), 1311 (w) cm−1.
[Ni(CN)FeCl(⊂-pyN2dienMe3)]
To a solution of (Et4N)[Ni(CN)(⊂-pyN2dienMe3)] (26 mg, 0.037 mmol) in DMF (1 mL) was added a solution of FeCl2 (4.7 mg, 0.050 mmol) in DMF (0.5 mL). The reaction mixture was stirred for 1 h, filtered, and ether was added to the orange-red filtrate. The deposited solid was washed with acetonitrile (3 mL), stirred with methanol (1 mL) to form a suspension, which was filtered. The solid was dissolved in Me2SO/DMF/methanol (1:1:1 v/v/v, 1.5 mL) and ether added by diffusion to give the product as orange crystals (8.0 mg, 33%). IR (KBr): νCN 2154 cm−1.
Attempted Reactions with Carbon Monoxide
No reaction was detected when CO was bubbled through DMF solutions of NiII hydroxo complexes 1 and 14 for 15–20 min. The same treatment of 17 led to an off-white ill-defined solid after ether diffusion into the reaction mixture, and with PdII hydroxo complex 20 a black precipitate formed within ca. 2 min and no further reaction occurred after 20 min. Under similar conditions, all complexes but 17 readily formed soluble bicarbonate complexes with CO2.
X-ray Structure Determinations
The structures of the 15 compounds in Tables S1– S3 were determined; for brevity they are referred to by their anion or cation number. Diffraction-quality crystals were obtained from the following solvents: DMF/ether (1, 4, 6–8, 14, 17, 20, 21), dichloromethane/ether (2), methanol/ether (3, 13), DMF/ethyl formate (5, 18), Me2SO/DMF/methanol/ether (19). Diffraction data were collected on a Bruker CCD area detector diffractometer equipped with an Oxford 700 low temperature apparatus. Single crystals were coated with Paratone-N oil and mounted on a Nylon loop. Structures were solved by direct methods using the SHELX program package.26 Hydrogen atoms were not added to the cations or solvate molecules in 1–3, 6, 7, 14, 18, and 20 owing to disordered carbon atoms but are included in the compound formulas. In 13, hydrogen atoms of two HCl molecules were not located from Fourier maps but are included for charge balance. The hydrogen atoms of the -OH groups in 1, 14, 17, the -SH group in 4, and the -HCO3 group in 6 and 21 were located from difference Fourier maps and refined isotropically. Crystal data and refinement details are given in Tables S1–S3.27
Other Physical Measurements
Absorption spectra were determined with a Cary 50 Bio spectrophotometer. 1H NMR spectra were recorded on a Varian AM-400 instrument. Infrared spectra were obtained with a Nicolet 5PCFT-IR spectrometer.
Results and Discussion
Because the CO2 intermediate (b) and cyanide-inhibited C-clusters (Figure 1(c,d)) involve binding to nickel, we first focused attention on reactions at NiII in an NNN pincer ligand environment. Reaction of deprotonated N,N'-bis(phenyl)-2,6-pyridinedicarboxamide and NiCl2(dme) in a 2:1 mol ratio affords octahedral [Ni(pyN2)2]2−.28 When the reaction is conducted with a 1:1 ratio, we find the same complex formed (in reduced yield) owing to the favorable d-electron stabilization of octahedral d8. However, with 1:1 deprotonated N,N'-bis(2,6-diisopropylphenyl)-2,6-pyridinedicarboxamide and NiII, the planar mono-pincer complexes [Ni(pyN2 iPr2)L] (L = OH2, PMe3) result.29 Lesser steric hindrance that destabilizes a bis-pincer complex occurs with 2,6-dimethylphenyl N-substituents, as shown by formation of the [Ni(pyN2Me2)L]1− series of complexes 1–7 (Figure 2).
Mononuclear NiII Binding and Structures
The key species in the foregoing series is hydroxo complex 1, readily formed by deprotonation of the pincer ligand with Et4NOH in the presence of Ni(OTf)2 in THF and obtained in 60% yield. Compared to the double bridge NiII(OH)2NiII 30,31 and the supported bridge Ni-(OH)-Ni,32,33 the terminal NiII-OH group is uncommon,23,34–37 and is usually stabilized by hydrogen bonding or, as is the case here, by steric protection. The upfield –OH resonance (δ −4.95), similar in position to other Ni-OH groups,30,38 is indicative of nucleophilic reactivity, as displayed in reaction with dichloromethane (2, 81%) and reactions 1 with ethyl formate (5, 58%), and CO2 (6, 85%)). The complex deprotonates methanol (3, 71%), and undergoes substitution by L = HS− (4, 93%) or CN− (7, 81%) in reaction 2. Products were isolated as Et4N+ salts in the indicated yields. It was also demonstrated that 7 reacts with [Fe(Me6tren)(OTf)]1+ to form binuclear complex 8, obtained as the triflate salt (42%).
| (1) |
| (2) |
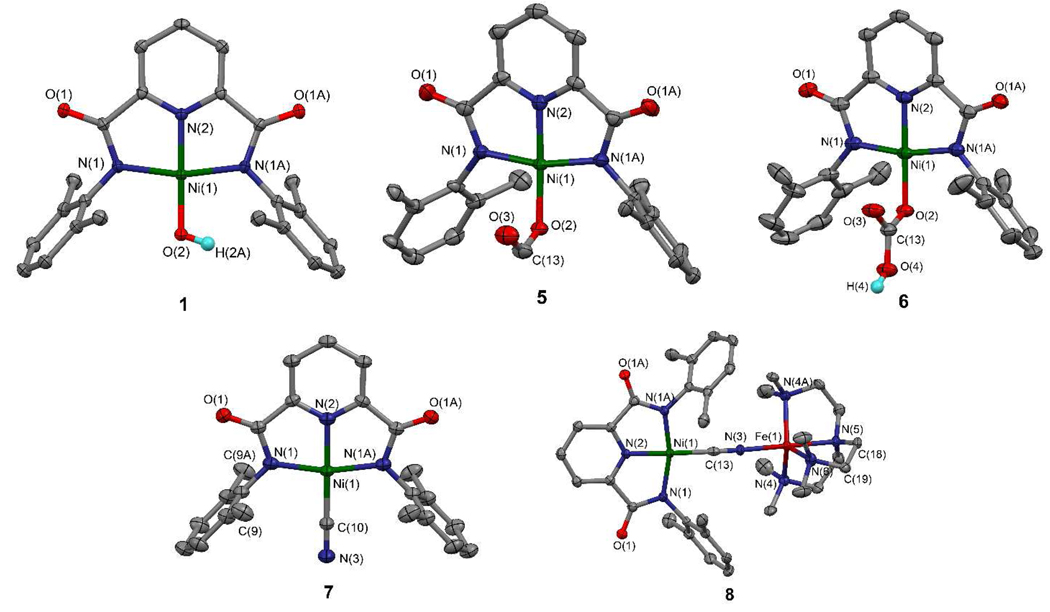
The complexes 1–7 are planar; structures of hydroxo, formate, bicarbonate, and cyanide complexes are set out in Figure 5. Because dimensions of the NiN3 portions of the coordination units are practically invariant, only the values for 1 are given. Cyanide, an inhibitor of CODH which binds at nickel (Figure 1), forms a linear Ni-C≡N unit in 7. Reaction with ethyl formate gives 5 with bent formate binding (Ni-O2-C13 = 114.2(5)°) and a dihedral angle of 90° between the formate and NiN3 planes. Bicarbonate complex 6 was prepared by passing CO2 through a DMF solution of 1, and can also be obtained by exposure of 1 to the air in an immediate reaction. As observed in Figure 6, UV-visible spectral changes associated with the reaction are very clear, resulting in a shift of the most intense band from 411 nm to 381 nm. Bicarbonate binding is very similar to formate, coordinating in the η1 mode with Ni-O2-C13 = 113.4(3)° and a CO3/NiN3 dihedral angle of 90°. These are the initial examples of the reaction of a terminal NiII-OH group with a formate ester or CO2 itself, leading to the first structure proofs of the binding modes NiII-(η1-OCHO) and NiII-(η1-OC(O)OH) (vide infra). In comparison to these reactions, 1 does not react with CO.
Figure 5.
Structures of five pincer complexes [Ni(pyN2Me2)L]1− with L = OH− (1), HCO2 − (5), HCO3− (6), CN− (7), and (CN)Fe(tren)1+ (8). Atom numbering schemes and 50% probability ellipsoids are shown. Selected bond distances (Å) and angles (deg): 1 – Ni-N1 1.911(2), Ni-N2 1.826(3), Ni-O2 1.825(3), N1-Ni-N2 82.73(7), N1-Ni-O2 97.29(6), N1-Ni-N1A 165.4(1), N2-Ni-O2 176.9(1); 5 – Ni-O2 1.857(5), C13-O2 1.27(1), C13-O3 1.21(1), Ni-O2-C13 114.2(5), O2-C13-O3 127.4(8); 6 – Ni-O2 1.817(4), C13-O2 1.253(7), C13-O3 1.286(7), C13-O4 1.293(6), Ni-O2-C13 113.4(3), O2-C13-O3 121.5(5), O2-C13-O4 118.5(5), O3-C13-O4 120.0(5); 7 – Ni-C10 1.849(7), C10-N3 1.15(1), Ni-C10-N3 180.0; 8 – Ni-C13 1.853(7), C13-N3 1.129(8), Fe-N3 2.085(5), av Fe-N(4–6) 2.146[8], N3-Fe-N5 179.9(2). Atoms N(1) and N(1A) and O(1) and O(1A) are related by a mirror plane. Disorder of the carbon atoms of a phenyl ring (5) and of methylene carbon atoms (8) over two equally populated positions is omitted; one position in shown for each molecule.
Figure 6.
Absorption spectra for the conversion of NiII and PdII hydroxo to bicarbonate complexes in DMF upon reaction with CO2. Upper: 1 → 5 and 14 → 16. Lower: 20 → 21.
Macrocyclic Mononuclear NiII and Binuclear NiIIFeII Systems
Having examined the reactivity of the NNN pincer NiII site in mononuclear complexes, a binuclear system containing this site and a binding site for FeII was sought. We utilized the binucleating macrocycle concept39,40 with a ligand design related to NCN pincer-crown ethers41,42 and binding sites differentiated for selective metal incorporation. The target macrocycle ⊂-H2pyN2dienMe3 (13) was synthesized via intermediates 9–12 by the reaction sequence in Figure 3, which results in an overall 17% yield from 3-nitrobenzaldehyde. Macrocycle formation was verified by the X-ray structure in Figure 7. The molecule is approximately planar except for the dien-type fragment whose N2C4 least-squares plane forms a dihedral angle of 82.3° with the least-squares plane of the remainder of the molecule (atom deviations of ≤ 0.20 Å).
Figure 7.
Structures of free macrocycle ⊂-H2pyN2dienMe3 (13), [Ni(OH)(⊂-pyN2dienMe3)]1− (14), and [Ni(L)FeCl(⊂-pyN2dienMe3)] with L = OH− (17), HCO2− (18), CN− (19). Atom numbering schemes and 50% probability ellipsoids are shown. Selected bond distances (Å) and angles (deg): 14 – Ni-N1 1.927(4), Ni-N2 1.821(6), Ni-O2 1.802(4), N1-Ni-N2 82.7(1), N1-Ni-O2 97.3(1); 17 – Ni-O3 1.927(4), Fe-O3 2.059(3), Fe-Cl 2.334(2), Ni-O3-Fe 140.0(2), Ni⋯Fe 3.747(2); 18 – Ni-O3 1.842(3), Fe-O4 2.032(3), Fe-Cl 2.252(2) C29-O3 1.216(6), C29-O4 1.236(5), Ni-O3-C29 135.9(3), O3-C29-O4 125.2(4), Fe-O4-C29 120.2(3), Ni⋯Fe 4.792(1); 19 – Ni-C29 1.827(3), C29-N7 1.145(4), Fe-N7 2.022(2), Ni-C29-N7 170.3(3), Fe-N7-C29 141.8(2), Ni⋯Fe 4.631(1). Complex 18 occurs as two independent molecules of very similar structure; the data refer to one structure. Disorder in the positions of certain nitrogen and carbon atoms in a 1:1 population in 14 is omitted; only one position is shown.
Preparations of mononuclear and binuclear macrocyclic complexes are summarized in Figure 4; structures are reported in Figure 7. Using the same procedure as for 1, NiII was taken up in the pincer site to yield hydroxo complex 14 (51%), which was readily converted to cyanide complex 15 by ligand substitution. Binding of NiII in the macrocycle results in a conformational change in the dien portion and reorientation of the phenyl rings from a dihedral angle of 77.6° with the NiN3 plane in 1 to 60.4° in 14. Both complexes have mirror symmetry, and metric features of their NiN3O coordination units are insignificantly different. Bicarbonate complex 16 was prepared from 14 and CO2. An X-ray structure was not determined, but the spectral comparison with 5 (Figure 6) demonstrates formation of the complex.
Three NiIIFeII complexes, with hydroxo (17), formate (18), and cyanide bridges (19), were prepared. Complexes 17 and 19 were obtained by insertion of FeII in reactions 3 with 14 (L = OH−, 38%) and 15 (L = CN−, 75%), whereas 18 was prepared from 17 generated in situ and ethyl formate in reaction 4. In these species, NiII is planar while the FeIIN3OCl units are intermediate between
| (3) |
| (4) |
trigonal pyramidal and square pyramidal (τ = 0.51 (17), 0.43 (19)) or approach trigonal bipyramidal (τ = 0.17, 0.19 (18)). The shape parameter τ = 0 for square pyramidal and 1 for trigonal bipyramidal structures.43 Ring conformations in the dien region are similar and the NiN3/phenyl dihedral angles (53.5–56.4°) indicate that inclusion of the bridge units has a minor effect on phenyl group orientations in the three complexes. Attempts to prepare complexes with hydroxycarbonyl and bridges were unsuccessful. In particular, 14, as 1, is unreactive toward CO and 17 did not yield a tractable product.
There are no prior reports of NiII-L-FeII bridges with L = OH− and HCO2−, and only one other case of a single intramolecular NiII-CN-FeII bridge.44 The most closely related bridges with these three linking groups are of the unsupported CuII-L-FeIII type45–48 and require one trivalent metal center for stability. Complexes 17–19 provide the first insights into structure modes of the three bridge groups between these elements. The bridges are supported but the macrocycle is sufficiently flexible so as to sustain bond sequences of different lengths, varying from 3.7 to 4.8 Å. Metric data for bridge units are summarized in Table 1. The hydroxo bridge in 17 features an angle of 140° and bond lengths that separate the metal centers by 3.75 Å. Relative to 14, the Ni-O interaction has increased by ca. 0.12 Å upon bridge formation. In 18, formate functions as an µ2:η2 bridge with the metal centers in a syn orientation, M-O-C bond angles of 136° (M = Ni) and120° (M = Fe), and a Ni⋯Fe distance of 4.79 Å. In 19, the Ni-CN and C≡N bonds are not significantly altered by bridge formation compared to 7. The complete Ni-C≡N-Fe bridge unit in 8 is linear, whereas in 19 the Ni-C≡N portion is linear but the Fe-N≡C sequence is bent (142°) to a larger extent than in [Ni(pdtc)CNFe(Me6tren)]1+ (166°),44 the only other molecular example of a single NiII-CN-FeII bridge. The bent configuration is probably due to macrocycle constraints which with a cyanide bridge impose a 4.63 Å Ni⋯Fe separation.
Table 1.
Bridge Dimensions (Å, deg) in Macrocyclic Complexes
| 17 | 18 | 19 | |
|---|---|---|---|
| Ni-O | 1.927(4) | 1.842(3) | --- |
| Fe-O | 2.059(3) | 2.032(3) | --- |
| Ni-C | --- | --- | 1.827(3) |
| Fe-N | --- | --- | 2.022(2) |
| Ni-O-Fe | 140.0(2) | --- | --- |
| Ni-O-C | --- | 135.9(3) | --- |
| Ni-C-N | --- | --- | 170.3(3) |
| Fe-O-C | --- | 120.2(3) | --- |
| Fe-N-C | --- | --- | 141.8(2) |
| O-C-O | --- | 125.2(4) | --- |
| Ni⋯Fe | 3.747(2) | 4.792(1) | 4.631(1) |
NiII and PdII Reactivity with CO and CO2
Prior reactions of NiII with CO2 have involved only binuclear hydroxo-bridged complexes, which lead to binuclear products containing µ2:η3-CO3 bridges with one or two chelate rings.30,31,49 In another case, reaction in methanol affords a µ2:η2 methylcarbonate bridge.33 These result underscore the unique NiII η1-bicarbonate binding mode in 6, whose ligand structure prevents bridge formation.
Carbonylation reactions of pincer complexes have recently been summarized.50 We briefly note several observations pertinent to this work with PCP pincer species. Reaction 5 (M = PdII) affords the η1 C-bound hydroxycarbonyl complex; with CO2 the product is the η1-bicarbonate complex.23 Reaction 6 (M = NiII, PdII) with a less hindered ligand forms a binuclear η2(C,O):µ2-CO2 product which with additional CO can be cleaved to the mononuclear PdII hydroxycarbonyl.22 These results suggested examination of reactions with the PdII analogue of the NiII-hydroxo complex 1.
| (5) |
| (6) |
Reaction of [Pd(MeCN)4]2+ and H2pyN2Me2 followed by addition of Et4NOH gave (Et4N)[20] (70%). The complex is isostructural with 1, having Pd-N1 = 2.023(4) Å, Pd-N2 = 1.906(7) Å, and Pd-O = 1.959(6) Å. Reaction with CO2 affords (Et4N)[21] (90%) with η1-bicarbonate ligation and Pd-O = 2.041(3) Å.27 Spectral changes parallel those with NiII but at higher energies (Figure 6). However, exposure to CO resulted in the formation of an insoluble black precipitate; no tractable product was obtained. It is now apparent that the NNN pincer environment, unlike PCP ligands, with NiII-OH or PdII-OH functional groups does not support either CO binding or formation of a hydroxycarbonyl complex.51
Summary
With use of a binucleating macrocycle whose NNN pincer and triamine portions provide selective metal binding sites, the first examples of NiII-L-FeII bridged molecules have been synthesized and structural modes of three bridging groups (µ2-OH−, µ2:η2-HCO2− and -CN−) established. Results include necessary supporting information on reactivity and structure at the NiII site in mononuclear NNN pincer complexes. This investigation is motivated by the biomimetic Ni-CODH C-cluster problem whose difficulties have been noted at the outset. The NiIIFeII molecules are not site analogues because of substantial structural departures from the native site (Figure 1), but their accessibility and demonstrated bridging capability offer the first indication that molecular NiII…FeII sites and bridges can be constructed. Any further elaboration of these binuclear systems should include reduced intermetal separation to ≲ 3.0 Å as in the C-cluster (Figure 1), which might prove advantageous for bridged hydroxycarbonyl formation from a CO reaction and unidentate linear or bent cyanide ligation. Ultimately, the bridging unit should be integrated into a Fe…NiFe3S4 bridged assembly, a future goal.
Supplementary Material
Acknowledgement
This research was supported by NIH Grant GM 28856. We thank Dr. S.-L. Zheng from assistance with crystallography.
Footnotes
Supporting Information Available. Crystallographic data for the 15 compounds in Tables S1–S3 and structures of compounds containing 2, 3, 4, 20 and 21 in Figure S1. This material is available free of charge via the Internet at http://pubs.acs.org.
Reference
- 1.Groysman S, Holm RH. Biochemistry. 2009;48:2310–2320. doi: 10.1021/bi900044e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbreviations are given in Chart I.
- 3.Ragsdale SW, Kumar M. Chem. Rev. 1996;96:2515–2539. doi: 10.1021/cr950058+. [DOI] [PubMed] [Google Scholar]
- 4.Ragsdale SW. Crit. Rev. Biochem. Mol. Biol. 2004;39:165–195. doi: 10.1080/10409230490496577. [DOI] [PubMed] [Google Scholar]
- 5.Lindahl PA. Biochemistry. 2002;41:2097–2105. doi: 10.1021/bi015932+. [DOI] [PubMed] [Google Scholar]
- 6.Lindahl PA, Graham DE. Metal Ions Life Sci. 2007;2:357–415. [Google Scholar]
- 7.Drennan CL, Doukov TI, Ragsdale SW. J. Biol. Inorg. Chem. 2004;9:511–515. doi: 10.1007/s00775-004-0563-y. [DOI] [PubMed] [Google Scholar]
- 8.Dobbek H, Svetlitchnyi V, Liss J, Meyer O. J. Am. Chem. Soc. 2004;126:5382–5387. doi: 10.1021/ja037776v. [DOI] [PubMed] [Google Scholar]
- 9.Volbeda A, Fontecilla-Camps JC. J. Chem. Soc., Dalton Trans. 2005:3443–3450. doi: 10.1039/b508403b. [DOI] [PubMed] [Google Scholar]
- 10.Jeoung J-H, Dobbek H. Science. 2007;318:1461–1464. doi: 10.1126/science.1148481. [DOI] [PubMed] [Google Scholar]
- 11.Gong W, Hao B, Wei Z, Ferguson DJ, Tallant T, Krzycki JA, Chan MK. Proc. Natl. Acad. Sci. USA. 2008;105:9558–9563. doi: 10.1073/pnas.0800415105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kung Y, Doukov TI, Seravalli J, Ragsdale SW, Drennan CL. Biochemistry. 2009;48:7432–7440. doi: 10.1021/bi900574h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciurli S, Ross PK, Scott MJ, Yu S-B, Holm RH. J. Am. Chem. Soc. 1992;114:5415–5423. [Google Scholar]
- 14.Zhou J, Scott MJ, Hu Z, Peng G, Münck E, Holm RH. J. Am. Chem. Soc. 1992;114:10843–10854. [Google Scholar]
- 15.Zhou J, Raebiger JW, Crawford CA, Holm RH. J. Am. Chem. Soc. 1997;119:6242–6250. [Google Scholar]
- 16.Panda R, Berlinguette CP, Zhang Y, Holm RH. J. Am. Chem. Soc. 2005;127:11092–11101. doi: 10.1021/ja052381s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun J, Tessier C, Holm RH. Inorg. Chem. 2007;46:2691–2699. doi: 10.1021/ic062362z. [DOI] [PubMed] [Google Scholar]
- 18.Hikichi S, Ogihara T, Fujisawa K, Kitajima N, Akita M, Moro-oka Y. Inorg. Chem. 1997;36:4539–4547. doi: 10.1021/ic960903m. [DOI] [PubMed] [Google Scholar]
- 19.MacBeth CE;, Hammes BS, Young VG, Jr, Borovik AS. Inorg. Chem. 2001;40:4733–4741. [PubMed] [Google Scholar]
- 20.Bénisvy L, Halut S, Donnadieu B, Tuchagues J-P, Chottard J-C, Li Y. Inorg. Chem. 2006;45:2403–2405. doi: 10.1021/ic060100r. [DOI] [PubMed] [Google Scholar]
- 21.Bennett MA, Jin H, Willis AC. J. Organometal. Chem. 1993;451:249–256. [Google Scholar]
- 22.Cámpora J, Palma P, del Rio D, Álvarez E. Organometallics. 2004;23:1652–1655. [Google Scholar]
- 23.Johansson R, Wendt OF. Organometallics. 2007;26:2426–2430. [Google Scholar]
- 24.Britovsek GJP, England J, White AJP. Inorg. Chem. 2005;44:8125–8134. doi: 10.1021/ic0509229. [DOI] [PubMed] [Google Scholar]
- 25.Wendt OF, Kaiser N-FK, Elding LI. J. Chem. Soc., Dalton Trans. 1997:4733–4737. [Google Scholar]
- 26.Sheldrick GM. Acta Crystallogr. 2009;A64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 27.See paragraph at the end of this article for Supporting Information available.
- 28.Patra AK, Mukherjee R. Inorg. Chem. 1999;38:1388–1393. [Google Scholar]
- 29.Wasilke J-C, Wu G, Bu X, Kehr G, Erker G. Organometallics. 2005;24:4289–4297. [Google Scholar]
- 30.Carmona E, Marín JM, Palma P, Paneque M, Poveda ML. Inorg. Chem. 1989;28:1895–1900. [Google Scholar]
- 31.Kitajima N, Hikuchi S, Tanaka M, Moro-oka Y. J. Am. Chem. Soc. 1993;115:5496–5508. [Google Scholar]
- 32.Barrios AM, Lippard SJ. J. Am. Chem. Soc. 2000;122:9172–9177. [Google Scholar]
- 33.Kersting B. Angew. Chem. Int. Ed. 2001;40:3988–3990. [Google Scholar]
- 34.Orlandini A, Sacconi L. Inorg. Chem. 1976;15:78–85. [Google Scholar]
- 35.Meyer F, Kaifer E, Kircher P, Heinze K, Pritzkow H. Chem. Eur. J. 1999;5:1617–1630. [Google Scholar]
- 36.Cámpora J, Matas I, Palma P, Graiff C, Tiripicchio A. Organometallics. 2005;24:2827–2830. [Google Scholar]
- 37.Kieber-Emmons MT, Schenker R, Yap GPA, Brunold TC, Riordan CG. Angew. Chem. Int. Ed. 2004;43:6716–6718. doi: 10.1002/anie.200460747. [DOI] [PubMed] [Google Scholar]
- 38.López G, Garcia G, Sánchez G, Garcia J, Vicente C. J. Chem. Soc., Chem. Commun. 1989:1045–1046. [Google Scholar]
- 39.Gavrilova AL, Bosnich B. Chem. Rev. 2004;104:349–383. doi: 10.1021/cr020604g. [DOI] [PubMed] [Google Scholar]
- 40.Vigato PA, Tamburini S, Bertolo L. Coord. Chem. Rev. 2007;251:1311–1492. [Google Scholar]
- 41.Mahoney JM, Nawaratna GU, Beatty AM, Duggan PJ, Smith BD. Inorg. Chem. 2004;43:5902–5907. doi: 10.1021/ic0494859. [DOI] [PubMed] [Google Scholar]
- 42.Mahoney JM, Stucker KA, Jiang H, Carmichael I, Brinkmann NR, Beatty AM, Noll BC, Smith BD. J. Am. Chem. Soc. 2005;127:2922–2928. doi: 10.1021/ja0440295. [DOI] [PubMed] [Google Scholar]
- 43.Addison AW, Nageswara Rao T, Reedijk J, van Rijn J, Verschoor GC. J. Chem. Soc., Dalton Trans. 1984:1349–1356. [Google Scholar]
- 44.Huang D, Deng L, Sun J, Holm RH. Inorg. Chem. 2009;48:6159–6166. doi: 10.1021/ic900494u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scott MJ, Zhang HH, Lee SC, Hedman B, Hodgson KO, Holm RH. J. Am. Chem. Soc. 1995;117:568–569. [Google Scholar]
- 46.Scott MJ, Goddard CA, Holm RH. Inorg. Chem. 1996;35:2558–2567. doi: 10.1021/ic9512035. [DOI] [PubMed] [Google Scholar]
- 47.Scott MJ, Holm RH. J. Am. Chem. Soc. 1994;116:11357–11367. [Google Scholar]
- 48.Lim BS, Holm RH. Inorg. Chem. 1998;37:4898–4908. doi: 10.1021/ic9801793. [DOI] [PubMed] [Google Scholar]
- 49.Lozano AA, Sáez M, Pérez J, García L, Lezama L, Rojo T, López G, García G, Santana MD. Dalton Trans. 2006:3906–3911. doi: 10.1039/b604541c. [DOI] [PubMed] [Google Scholar]
- 50.Morales-Morales D. In: Modern Carbonylation Methods. Kollár L, editor. Wiley-VCH: Weinheim; 2008. pp. 27–64. [Google Scholar]
- 51.While reaction of CO with reduced Ni complexes remains a possibility, preliminary investigation of 1 (DMF) and 7 (acetonitrile) by cyclic voltammetry only quasi-reversible reductions at −1.40 V and −1.31 V, respectively, vs. SCE. Both complexes showed reversible oxidations (1, +0.67 V; 7, +1.04 V).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.