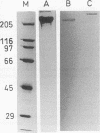
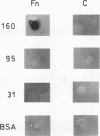
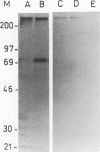
Abstract
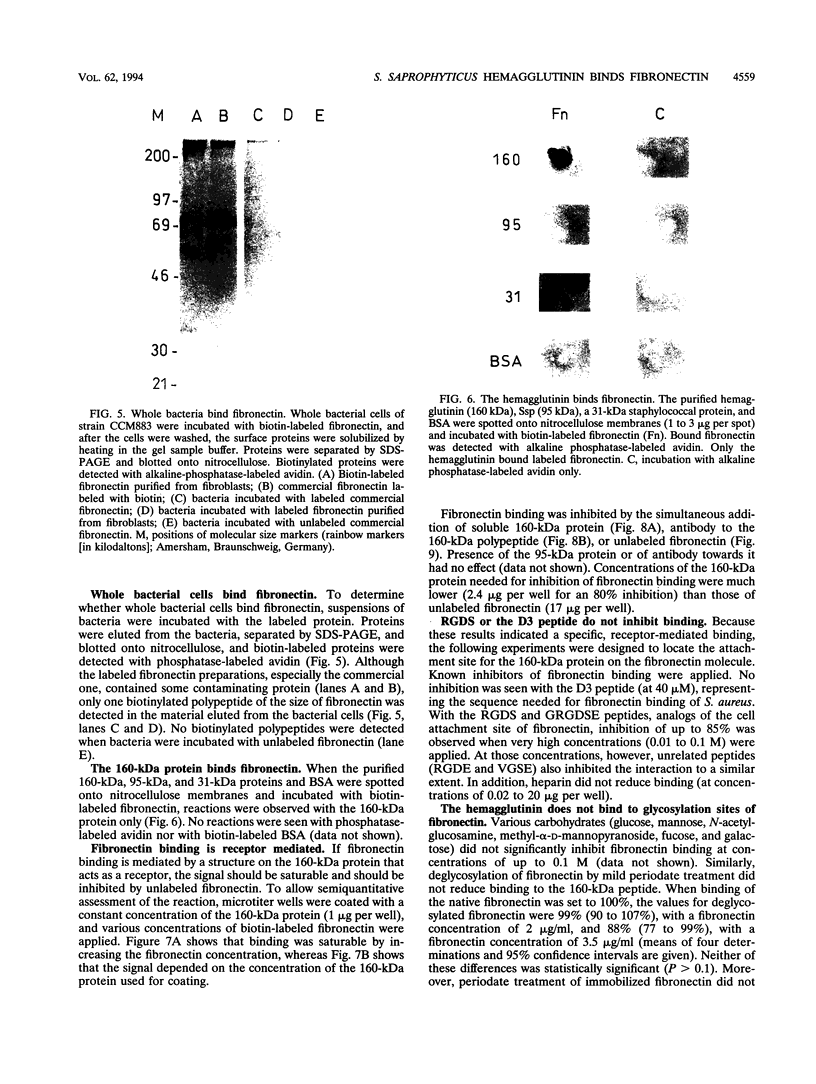
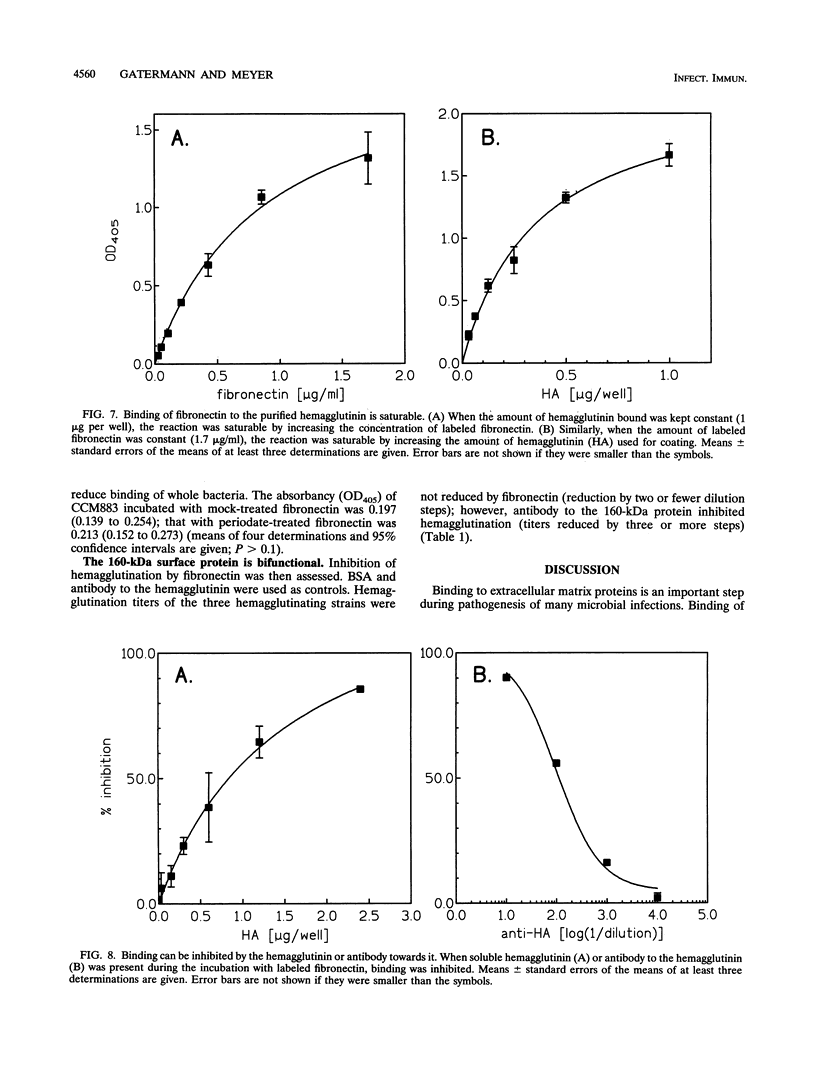
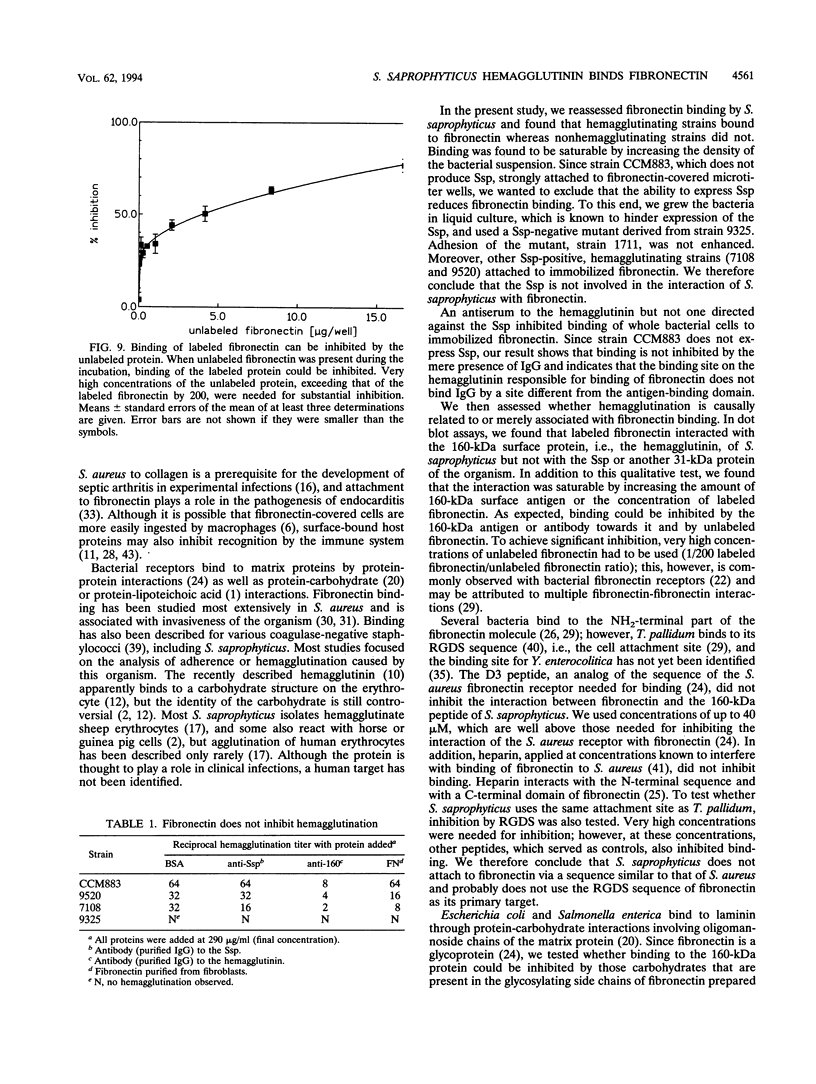
Attachment of microorganisms to host tissue is regarded as an important step in the pathogenesis of infections. Staphylococcus saprophyticus adheres to various epithelial cells and hemagglutinates sheep erythrocytes. The hemagglutinin has been identified, but a human target for this surface protein is still not known. In our report, we show that hemagglutinating strains of S. saprophyticus bind to immobilized fibronectin, whereas nonhemagglutinating strains do not. Bacterial binding was inhibited by antibody to the hemagglutinin but not by antibody to Ssp, another surface protein of S. saprophyticus. The purified hemagglutinin but not other surface proteins bound biotin-labeled fibronectin. Binding was saturable and could be inhibited by unbound hemagglutinin, unlabeled fibronectin, and by antibody to the hemagglutinin. We thus conclude that the hemagglutinin of S. saprophyticus may act as a fibronectin receptor in the human host. Heparin, the D3 peptide, or Arg-Gly-Asp-Ser (RGDS) containing peptides did not inhibit binding of fibronectin to the hemagglutinin, indicating that the binding site is different from that of Staphylococcus aureus or Treponema pallidum.
Full text
PDF

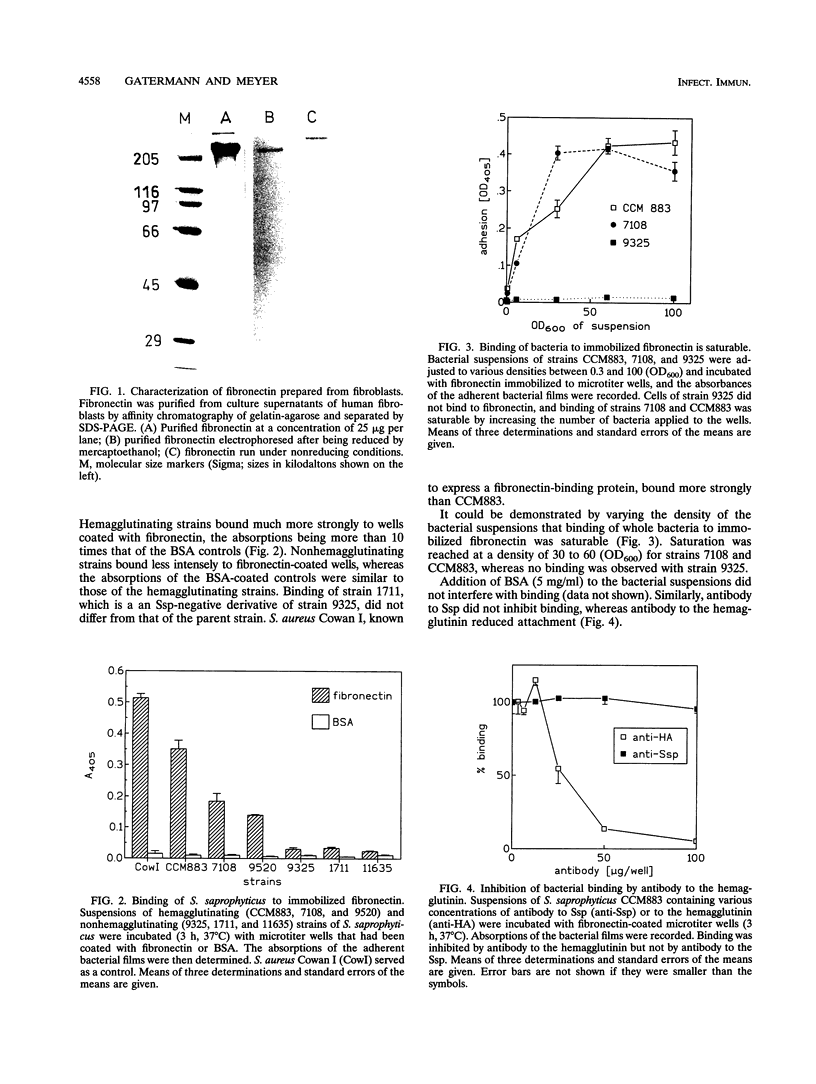


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachey E. H., Simpson W. A. The adherence of group A streptococci to oropharyngeal cells: the lipoteichoic acid adhesin and fibronectin receptor. Infection. 1982;10(2):107–111. doi: 10.1007/BF01816738. [DOI] [PubMed] [Google Scholar]
- Beuth J., Ko H. L., Schumacher-Perdreau F., Peters G., Heczko P., Pulverer G. Hemagglutination by Staphylococcus saprophyticus and other coagulase-negative staphylococci. Microb Pathog. 1988 May;4(5):379–383. doi: 10.1016/0882-4010(88)90065-4. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Roth M., Hugo F. Biotinylation: a simple method for labelling complement component C8 with preservation of functional activity. J Immunol Methods. 1989 Jul 6;121(1):61–66. doi: 10.1016/0022-1759(89)90420-1. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Eriksen H. O., Espersen F., Clemmensen I. Opsonic activity of fibronectin in the phagocytosis of Staphylococcus aureus by polymorphonuclear leukocytes. Eur J Clin Microbiol. 1984 Apr;3(2):108–112. doi: 10.1007/BF02014326. [DOI] [PubMed] [Google Scholar]
- Furtado G. C., Cao Y., Joiner K. A. Laminin on Toxoplasma gondii mediates parasite binding to the beta 1 integrin receptor alpha 6 beta 1 on human foreskin fibroblasts and Chinese hamster ovary cells. Infect Immun. 1992 Nov;60(11):4925–4931. doi: 10.1128/iai.60.11.4925-4931.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatermann S., John J., Marre R. Staphylococcus saprophyticus urease: characterization and contribution to uropathogenicity in unobstructed urinary tract infection of rats. Infect Immun. 1989 Jan;57(1):110–116. doi: 10.1128/iai.57.1.110-116.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatermann S., Kreft B., Marre R., Wanner G. Identification and characterization of a surface-associated protein (Ssp) of Staphylococcus saprophyticus. Infect Immun. 1992 Mar;60(3):1055–1060. doi: 10.1128/iai.60.3.1055-1060.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatermann S., Marre R., Heesemann J., Henkel W. Hemagglutinating and adherence properties of Staphylococcus saprophyticus: epidemiology and virulence in experimental urinary tract infection of rats. FEMS Microbiol Immunol. 1988 Dec;1(3):179–185. doi: 10.1111/j.1574-6968.1988.tb02372.x. [DOI] [PubMed] [Google Scholar]
- Gatermann S., Meyer H. G., Wanner G. Staphylococcus saprophyticus hemagglutinin is a 160-kilodalton surface polypeptide. Infect Immun. 1992 Oct;60(10):4127–4132. doi: 10.1128/iai.60.10.4127-4132.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goward C. R., Scawen M. D., Murphy J. P., Atkinson T. Molecular evolution of bacterial cell-surface proteins. Trends Biochem Sci. 1993 Apr;18(4):136–140. doi: 10.1016/0968-0004(93)90021-e. [DOI] [PubMed] [Google Scholar]
- Gunnarsson A., Mårdh P. A., Lundblad A., Svensson S. Oligosaccharide structures mediating agglutination of sheep erythrocytes by Staphylococcus saprophyticus. Infect Immun. 1984 Jul;45(1):41–46. doi: 10.1128/iai.45.1.41-46.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski E., Caparon M. Protein F, a fibronectin-binding protein, is an adhesin of the group A streptococcus Streptococcus pyogenes. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6172–6176. doi: 10.1073/pnas.89.13.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedman P., Ringertz O., Lindström M., Olsson K. The origin of Staphylococcus saprophyticus from cattle and pigs. Scand J Infect Dis. 1993;25(1):57–60. [PubMed] [Google Scholar]
- Herrmann M., Vaudaux P. E., Pittet D., Auckenthaler R., Lew P. D., Schumacher-Perdreau F., Peters G., Waldvogel F. A. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J Infect Dis. 1988 Oct;158(4):693–701. doi: 10.1093/infdis/158.4.693. [DOI] [PubMed] [Google Scholar]
- Hovelius B., Mårdh P. A. Haemagglutination by Staphylococcus saprophyticus and other staphylococcal species. Acta Pathol Microbiol Scand B. 1979 Feb;87B(1):45–50. doi: 10.1111/j.1699-0463.1979.tb02401.x. [DOI] [PubMed] [Google Scholar]
- Jönsson K., Signäs C., Müller H. P., Lindberg M. Two different genes encode fibronectin binding proteins in Staphylococcus aureus. The complete nucleotide sequence and characterization of the second gene. Eur J Biochem. 1991 Dec 18;202(3):1041–1048. doi: 10.1111/j.1432-1033.1991.tb16468.x. [DOI] [PubMed] [Google Scholar]
- Kukkonen M., Raunio T., Virkola R., Lähteenmäki K., Mäkelä P. H., Klemm P., Clegg S., Korhonen T. K. Basement membrane carbohydrate as a target for bacterial adhesion: binding of type I fimbriae of Salmonella enterica and Escherichia coli to laminin. Mol Microbiol. 1993 Jan;7(2):229–237. doi: 10.1111/j.1365-2958.1993.tb01114.x. [DOI] [PubMed] [Google Scholar]
- Kuusela P. Fibronectin binds to Staphylococcus aureus. Nature. 1978 Dec 14;276(5689):718–720. doi: 10.1038/276718a0. [DOI] [PubMed] [Google Scholar]
- Lindgren P. E., Speziale P., McGavin M., Monstein H. J., Hök M., Visai L., Kostiainen T., Bozzini S., Lindberg M. Cloning and expression of two different genes from Streptococcus dysgalactiae encoding fibronectin receptors. J Biol Chem. 1992 Jan 25;267(3):1924–1931. [PubMed] [Google Scholar]
- McGavin M. H., Krajewska-Pietrasik D., Rydén C., Hök M. Identification of a Staphylococcus aureus extracellular matrix-binding protein with broad specificity. Infect Immun. 1993 Jun;61(6):2479–2485. doi: 10.1128/iai.61.6.2479-2485.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavin M. J., Raucci G., Gurusiddappa S., Hök M. Fibronectin binding determinants of the Staphylococcus aureus fibronectin receptor. J Biol Chem. 1991 May 5;266(13):8343–8347. [PubMed] [Google Scholar]
- Mosher D. F. Physiology of fibronectin. Annu Rev Med. 1984;35:561–575. doi: 10.1146/annurev.me.35.020184.003021. [DOI] [PubMed] [Google Scholar]
- Paulsson M., Ljungh A., Wadström T. Rapid identification of fibronectin, vitronectin, laminin, and collagen cell surface binding proteins on coagulase-negative staphylococci by particle agglutination assays. J Clin Microbiol. 1992 Aug;30(8):2006–2012. doi: 10.1128/jcm.30.8.2006-2012.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier T. P., Kehoe M. A., Whitnack E., Dockter M. E., Beachey E. H. Fibrinogen binding and resistance to phagocytosis of Streptococcus sanguis expressing cloned M protein of Streptococcus pyogenes. Infect Immun. 1989 Jan;57(1):29–35. doi: 10.1128/iai.57.1.29-35.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor R. A., Christman G., Mosher D. F. Fibronectin-induced agglutination of Staphylococcus aureus correlates with invasiveness. J Lab Clin Med. 1984 Oct;104(4):455–469. [PubMed] [Google Scholar]
- Proctor R. A. Fibronectin: a brief overview of its structure, function, and physiology. Rev Infect Dis. 1987 Jul-Aug;9 (Suppl 4):S317–S321. doi: 10.1093/clinids/9.supplement_4.s317. [DOI] [PubMed] [Google Scholar]
- Proctor R. A. The staphylococcal fibronectin receptor: evidence for its importance in invasive infections. Rev Infect Dis. 1987 Jul-Aug;9 (Suppl 4):S335–S340. doi: 10.1093/clinids/9.supplement_4.s355. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Schennings T., Heimdahl A., Coster K., Flock J. I. Immunization with fibronectin binding protein from Staphylococcus aureus protects against experimental endocarditis in rats. Microb Pathog. 1993 Sep;15(3):227–236. doi: 10.1006/mpat.1993.1073. [DOI] [PubMed] [Google Scholar]
- Schmidt K. H., Mann K., Cooney J., Köhler W. Multiple binding of type 3 streptococcal M protein to human fibrinogen, albumin and fibronectin. FEMS Immunol Med Microbiol. 1993 Aug;7(2):135–143. doi: 10.1111/j.1574-695X.1993.tb00392.x. [DOI] [PubMed] [Google Scholar]
- Schulze-Koops H., Burkhardt H., Heesemann J., Kirsch T., Swoboda B., Bull C., Goodman S., Emmrich F. Outer membrane protein YadA of enteropathogenic yersiniae mediates specific binding to cellular but not plasma fibronectin. Infect Immun. 1993 Jun;61(6):2513–2519. doi: 10.1128/iai.61.6.2513-2519.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Koops H., Burkhardt H., Heesemann J., von der Mark K., Emmrich F. Plasmid-encoded outer membrane protein YadA mediates specific binding of enteropathogenic yersiniae to various types of collagen. Infect Immun. 1992 Jun;60(6):2153–2159. doi: 10.1128/iai.60.6.2153-2159.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer P., Gleyzal C., Guerret S., Etienne J., Grimaud J. A. Induction of a putative laminin-binding protein of Streptococcus gordonii in human infective endocarditis. Infect Immun. 1992 Feb;60(2):360–365. doi: 10.1128/iai.60.2.360-365.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottile J., Schwarzbauer J., Selegue J., Mosher D. F. Five type I modules of fibronectin form a functional unit that binds to fibroblasts and Staphylococcus aureus. J Biol Chem. 1991 Jul 15;266(20):12840–12843. [PubMed] [Google Scholar]
- Switalski L. M., Patti J. M., Butcher W., Gristina A. G., Speziale P., Hök M. A collagen receptor on Staphylococcus aureus strains isolated from patients with septic arthritis mediates adhesion to cartilage. Mol Microbiol. 1993 Jan;7(1):99–107. doi: 10.1111/j.1365-2958.1993.tb01101.x. [DOI] [PubMed] [Google Scholar]
- Switalski L. M., Rydén C., Rubin K., Ljungh A., Hök M., Wadström T. Binding of fibronectin to Staphylococcus strains. Infect Immun. 1983 Nov;42(2):628–633. doi: 10.1128/iai.42.2.628-633.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Baseman J. B., Alderete J. F. Fibronectin tetrapeptide is target for syphilis spirochete cytadherence. J Exp Med. 1985 Nov 1;162(5):1715–1719. doi: 10.1084/jem.162.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Water L., Destree A. T., Hynes R. O. Fibronectin binds to some bacteria but does not promote their uptake by phagocytic cells. Science. 1983 Apr 8;220(4593):201–204. doi: 10.1126/science.6338594. [DOI] [PubMed] [Google Scholar]
- Westerlund B., Korhonen T. K. Bacterial proteins binding to the mammalian extracellular matrix. Mol Microbiol. 1993 Aug;9(4):687–694. doi: 10.1111/j.1365-2958.1993.tb01729.x. [DOI] [PubMed] [Google Scholar]
- Whitnack E., Beachey E. H. Inhibition of complement-mediated opsonization and phagocytosis of Streptococcus pyogenes by D fragments of fibrinogen and fibrin bound to cell surface M protein. J Exp Med. 1985 Dec 1;162(6):1983–1997. doi: 10.1084/jem.162.6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward M. P., Young W. W., Jr, Bloodgood R. A. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J Immunol Methods. 1985 Apr 8;78(1):143–153. doi: 10.1016/0022-1759(85)90337-0. [DOI] [PubMed] [Google Scholar]