Abstract
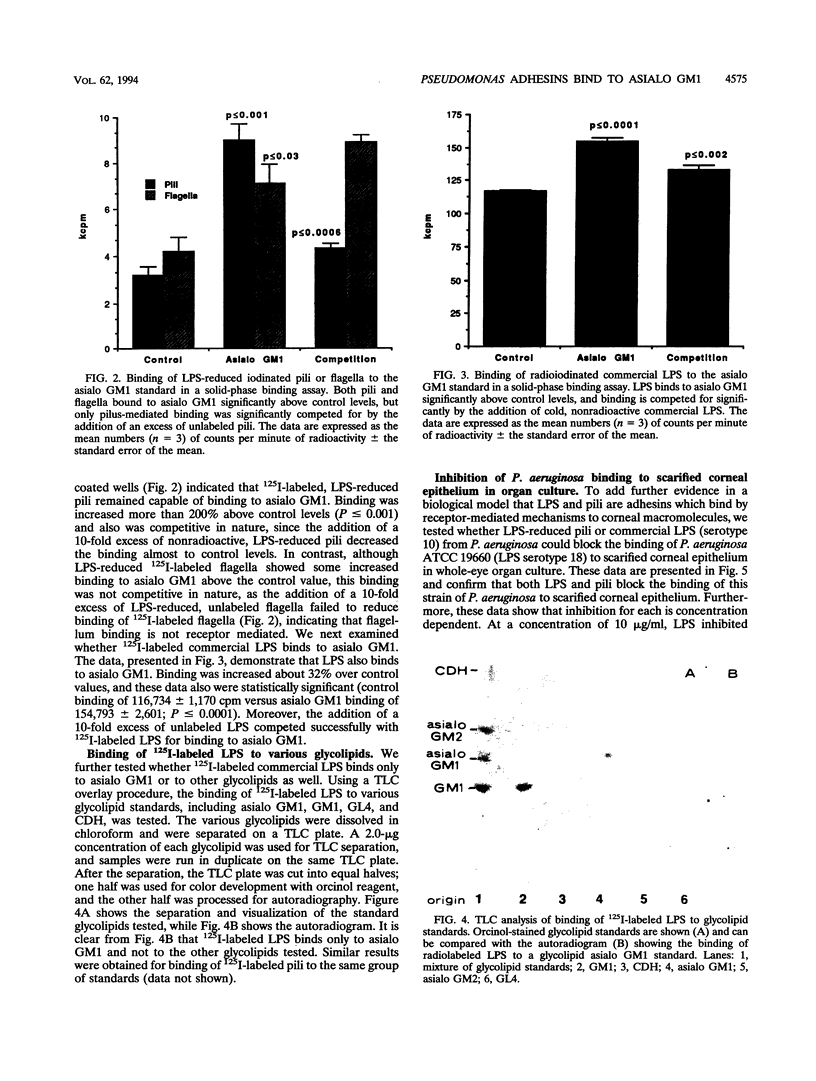
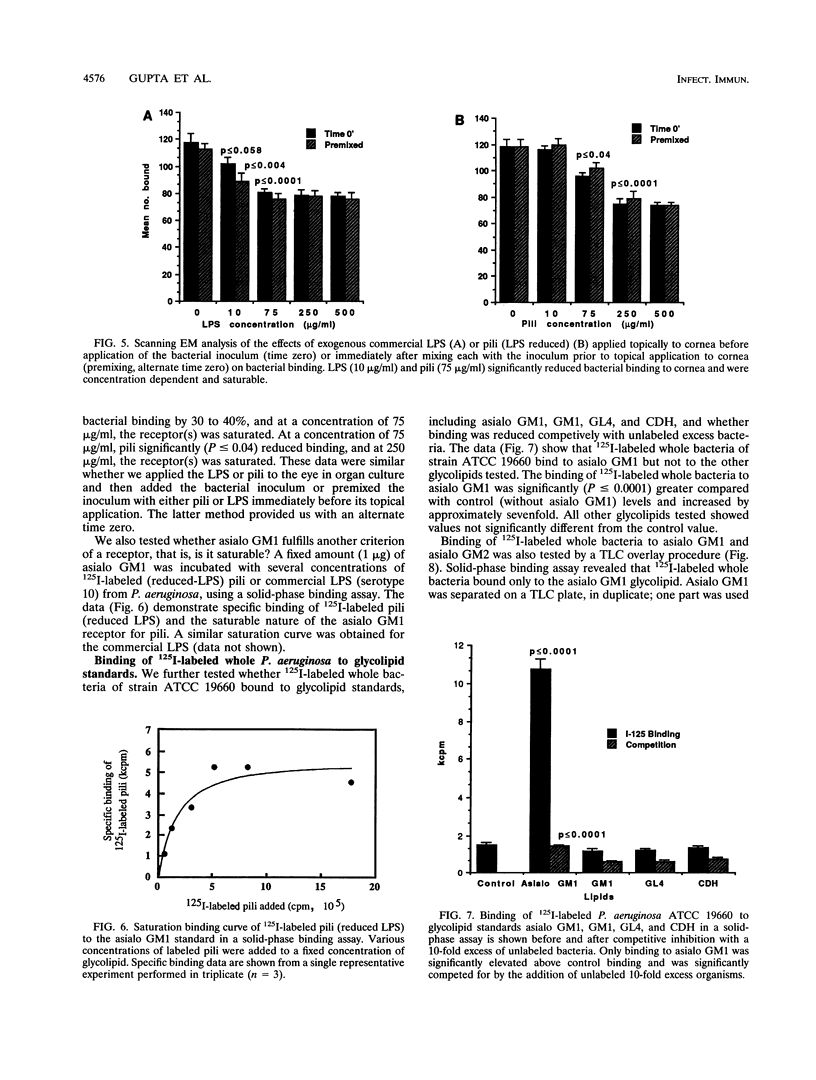
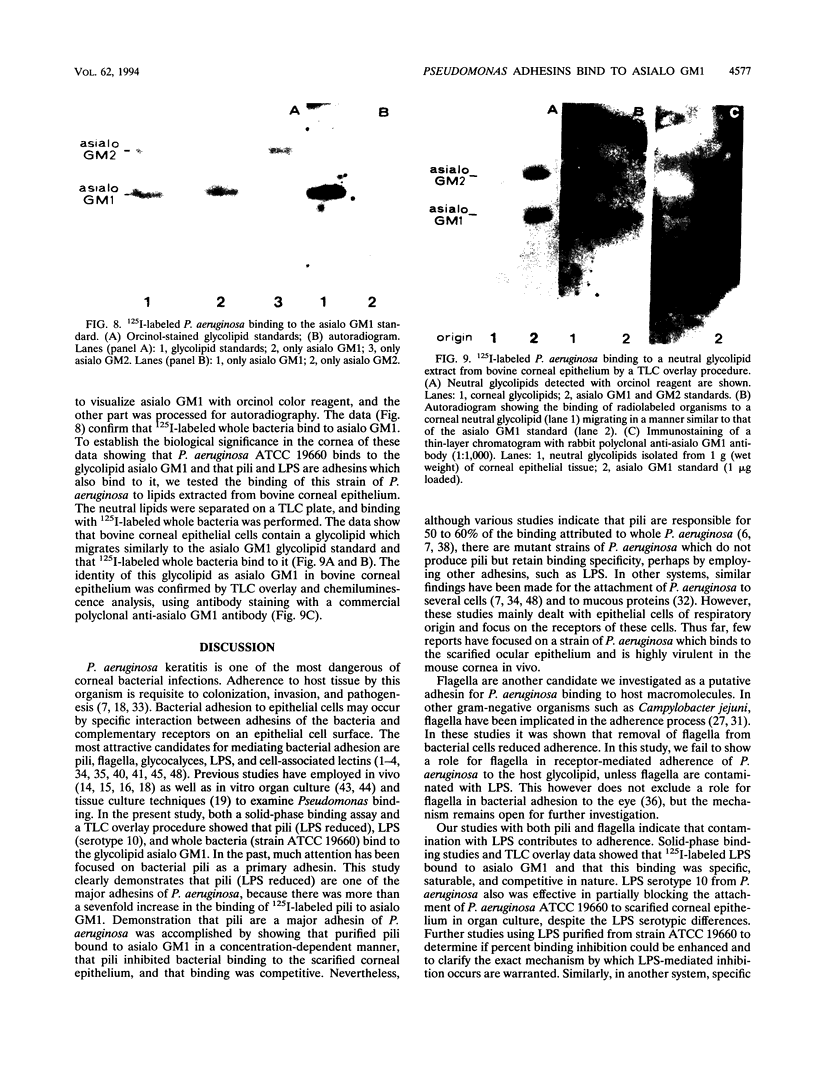
This study investigated which adhesins of Pseudomonas aeruginosa interact with the glycolipid asialo GM1, using solid-phase binding and thin-layer chromatography assays. Radioiodinated pili and flagella contaminated with lipopolysaccharide (LPS) bound to the glycolipid. When LPS was reduced to acceptable levels in pilus and flagellum samples, only pili specifically bound to the glycolipid. Commercial, radiolabeled LPS as well as whole bacteria of strain ATCC 19660 also bound to asialo GM1. Binding was specific, competitive, and saturable. Organ cultures of whole mouse eyes and scanning electron microscopy techniques were used also, and strain ATCC 19660 was inhibited from corneal binding by exogenous pili or commercial LPS and inhibition was concentration dependent for both. Binding of radiolabeled strain ATCC 19660 bacteria to neutral lipids extracted from bovine corneal epithelial tissue showed that the bacteria bound to a glycolipid which migrated at a position similar to that of an asialo GM1 standard and that the glycolipid stained positively with an antibody specific for asialo GM1. The data provide evidence that pili (reduced LPS) and LPS of P. aeruginosa bind to asialo GM1 glycolipid and that the glycolipid is not restricted to the mouse cornea.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attridge S. R., Rowley D. The role of the flagellum in the adherence of Vibrio cholerae. J Infect Dis. 1983 May;147(5):864–872. doi: 10.1093/infdis/147.5.864. [DOI] [PubMed] [Google Scholar]
- Baker N. R., Minor V., Deal C., Shahrabadi M. S., Simpson D. A., Woods D. E. Pseudomonas aeruginosa exoenzyme S is an adhesion. Infect Immun. 1991 Sep;59(9):2859–2863. doi: 10.1128/iai.59.9.2859-2863.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N., Hansson G. C., Leffler H., Riise G., Svanborg-Edén C. Glycosphingolipid receptors for Pseudomonas aeruginosa. Infect Immun. 1990 Jul;58(7):2361–2366. doi: 10.1128/iai.58.7.2361-2366.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Bélanger M., Dubreuil D., Harel J., Girard C., Jacques M. Role of lipopolysaccharides in adherence of Actinobacillus pleuropneumoniae to porcine tracheal rings. Infect Immun. 1990 Nov;58(11):3523–3530. doi: 10.1128/iai.58.11.3523-3530.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doig P., Sastry P. A., Hodges R. S., Lee K. K., Paranchych W., Irvin R. T. Inhibition of pilus-mediated adhesion of Pseudomonas aeruginosa to human buccal epithelial cells by monoclonal antibodies directed against pili. Infect Immun. 1990 Jan;58(1):124–130. doi: 10.1128/iai.58.1.124-130.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doig P., Todd T., Sastry P. A., Lee K. K., Hodges R. S., Paranchych W., Irvin R. T. Role of pili in adhesion of Pseudomonas aeruginosa to human respiratory epithelial cells. Infect Immun. 1988 Jun;56(6):1641–1646. doi: 10.1128/iai.56.6.1641-1646.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnenfeld E. D., Cohen E. J., Arentsen J. J., Genvert G. I., Laibson P. R. Changing trends in contact lens associated corneal ulcers: an overview of 116 cases. CLAO J. 1986 Jul-Sep;12(3):145–149. [PubMed] [Google Scholar]
- Fletcher E. L., Fleiszig S. M., Brennan N. A. Lipopolysaccharide in adherence of Pseudomonas aeruginosa to the cornea and contact lenses. Invest Ophthalmol Vis Sci. 1993 May;34(6):1930–1936. [PubMed] [Google Scholar]
- Hansson G. C., Karlsson K. A., Larson G., Strömberg N., Thurin J. Carbohydrate-specific adhesion of bacteria to thin-layer chromatograms: a rationalized approach to the study of host cell glycolipid receptors. Anal Biochem. 1985 Apr;146(1):158–163. doi: 10.1016/0003-2697(85)90410-5. [DOI] [PubMed] [Google Scholar]
- Hazlett L. D., Masinick S., Barrett R., Rosol K. Evidence for asialo GM1 as a corneal glycolipid receptor for Pseudomonas aeruginosa adhesion. Infect Immun. 1993 Dec;61(12):5164–5173. doi: 10.1128/iai.61.12.5164-5173.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett L. D., Masinick S., Berk R. S., Zheng Z. Proteinase K decreases Pseudomonas aeruginosa adhesion to wounded cornea. Exp Eye Res. 1992 Oct;55(4):579–587. doi: 10.1016/s0014-4835(05)80171-x. [DOI] [PubMed] [Google Scholar]
- Hazlett L. D., Moon M. M., Strejc M., Berk R. S. Evidence for N-acetylmannosamine as an ocular receptor for P. aeruginosa adherence to scarified cornea. Invest Ophthalmol Vis Sci. 1987 Dec;28(12):1978–1985. [PubMed] [Google Scholar]
- Hazlett L. D., Moon M., Berk R. S. In vivo identification of sialic acid as the ocular receptor for Pseudomonas aeruginosa. Infect Immun. 1986 Feb;51(2):687–689. doi: 10.1128/iai.51.2.687-689.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett L. D., Rosen D. D., Berk R. S. Age-related susceptibility to Pseudomonas aeruginosa ocular infections in mice. Infect Immun. 1978 Apr;20(1):25–29. doi: 10.1128/iai.20.1.25-29.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett L. D., Rosen D. D., Berk R. S. Pseudomonas eye infections in cyclophosphamide-treated mice. Invest Ophthalmol Vis Sci. 1977 Jul;16(7):649–652. [PubMed] [Google Scholar]
- Hazlett L. D., Wells P., Spann B., Berk R. S. Penetration of the unwounded immature mouse cornea and conjunctiva by Pseudomonas: SEM-TEM analysis. Invest Ophthalmol Vis Sci. 1980 Jun;19(6):694–697. [PubMed] [Google Scholar]
- Hazlett L., Barrett R., Klettner C., Berk R., Singh A. Pseudomonas aeruginosa: a probe to detail change with age in corneal receptor(s). Ophthalmic Res. 1991;23(3):141–146. doi: 10.1159/000267113. [DOI] [PubMed] [Google Scholar]
- Iida T., Katoh M., Matsuo Y. Adherence of Pseudomonas aeruginosa to a rabbit cornea cell line (SIRC) cells. Hiroshima J Med Sci. 1982 Dec;31(4):225–232. [PubMed] [Google Scholar]
- Irwin C. C., Irwin L. N. A simple rapid method for ganglioside isolation from small amounts of tissue. Anal Biochem. 1979 Apr 15;94(2):335–339. doi: 10.1016/0003-2697(79)90369-5. [DOI] [PubMed] [Google Scholar]
- Jones D. B. Early diagnosis and therapy of bacterial corneal ulcers. Int Ophthalmol Clin. 1973 Winter;13(4):1–29. [PubMed] [Google Scholar]
- Krivan H. C., Ginsburg V., Roberts D. D. Pseudomonas aeruginosa and Pseudomonas cepacia isolated from cystic fibrosis patients bind specifically to gangliotetraosylceramide (asialo GM1) and gangliotriaosylceramide (asialo GM2). Arch Biochem Biophys. 1988 Jan;260(1):493–496. doi: 10.1016/0003-9861(88)90473-0. [DOI] [PubMed] [Google Scholar]
- Krivan H. C., Roberts D. D., Ginsburg V. Many pulmonary pathogenic bacteria bind specifically to the carbohydrate sequence GalNAc beta 1-4Gal found in some glycolipids. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6157–6161. doi: 10.1073/pnas.85.16.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laibson P. R. Cornea and sclera. Arch Ophthalmol. 1972 Nov;88(5):553–574. doi: 10.1001/archopht.1972.01000030555018. [DOI] [PubMed] [Google Scholar]
- Lipford G. B., Feng Q., Wright G. L., Jr A method for separating bound versus unbound label during radioiodination. Anal Biochem. 1990 May 15;187(1):133–135. doi: 10.1016/0003-2697(90)90430-h. [DOI] [PubMed] [Google Scholar]
- McSweegan E., Walker R. I. Identification and characterization of two Campylobacter jejuni adhesins for cellular and mucous substrates. Infect Immun. 1986 Jul;53(1):141–148. doi: 10.1128/iai.53.1.141-148.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Guay C., Pier G. B. Pseudomonas aeruginosa adhesins for tracheobronchial mucin. Infect Immun. 1987 Mar;55(3):600–603. doi: 10.1128/iai.55.3.600-603.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., McNiece M. T., Polack F. M. Adherence of Pseudomonas aeruginosa to the injured cornea: a step in the pathogenesis of corneal infections. Ann Ophthalmol. 1981 Apr;13(4):421–425. [PubMed] [Google Scholar]
- Ramphal R., Sadoff J. C., Pyle M., Silipigni J. D. Role of pili in the adherence of Pseudomonas aeruginosa to injured tracheal epithelium. Infect Immun. 1984 Apr;44(1):38–40. doi: 10.1128/iai.44.1.38-40.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert R. W., Das N. D., Zam Z. S. Adherence properties of Pseudomonas pili to epithelial cells of the human cornea. Curr Eye Res. 1982;2(5):289–293. doi: 10.3109/02713688209000772. [DOI] [PubMed] [Google Scholar]
- Rudner X. L., Hazlett L. D., Berk R. S. Systemic and topical protection studies using Pseudomonas aeruginosa flagella in an ocular model of infection. Curr Eye Res. 1992 Aug;11(8):727–738. doi: 10.3109/02713689209000747. [DOI] [PubMed] [Google Scholar]
- Rudner X. L., Zheng Z., Berk R. S., Irvin R. T., Hazlett L. D. Corneal epithelial glycoproteins exhibit Pseudomonas aeruginosa pilus binding activity. Invest Ophthalmol Vis Sci. 1992 Jun;33(7):2185–2193. [PubMed] [Google Scholar]
- Saiman L., Ishimoto K., Lory S., Prince A. The effect of piliation and exoproduct expression on the adherence of Pseudomonas aeruginosa to respiratory epithelial monolayers. J Infect Dis. 1990 Mar;161(3):541–548. doi: 10.1093/infdis/161.3.541. [DOI] [PubMed] [Google Scholar]
- Saiman L., Prince A. Pseudomonas aeruginosa pili bind to asialoGM1 which is increased on the surface of cystic fibrosis epithelial cells. J Clin Invest. 1993 Oct;92(4):1875–1880. doi: 10.1172/JCI116779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Okinaga K. Role of pili in the adherence of Pseudomonas aeruginosa to mouse epidermal cells. Infect Immun. 1987 Aug;55(8):1774–1778. doi: 10.1128/iai.55.8.1774-1778.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Okinaga K., Saito H. Role of pili in the pathogenesis of Pseudomonas aeruginosa burn infection. Microbiol Immunol. 1988;32(2):131–139. doi: 10.1111/j.1348-0421.1988.tb01372.x. [DOI] [PubMed] [Google Scholar]
- Singh A., Hazlett L. D., Berk R. S. Characterization of Pseudomonas aeruginosa adherence to mouse corneas in organ culture. Infect Immun. 1990 May;58(5):1301–1307. doi: 10.1128/iai.58.5.1301-1307.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., Hazlett L., Berk R. S. Characterization of pseudomonal adherence to unwounded cornea. Invest Ophthalmol Vis Sci. 1991 Jun;32(7):2096–2104. [PubMed] [Google Scholar]
- Spurr-Michaud S. J., Barza M., Gipson I. K. An organ culture system for study of adherence of Pseudomonas aeruginosa to normal and wounded corneas. Invest Ophthalmol Vis Sci. 1988 Mar;29(3):379–386. [PubMed] [Google Scholar]
- Tjia K. F., van Putten J. P., Pels E., Zanen H. C. The interaction between Neisseria gonorrhoeae and the human cornea in organ culture. An electron microscopic study. Graefes Arch Clin Exp Ophthalmol. 1988;226(4):341–345. doi: 10.1007/BF02172964. [DOI] [PubMed] [Google Scholar]
- Tramont E. C. Adhesion of Neisseria gonorrhoeae and disease. Ciba Found Symp. 1981;80:188–201. doi: 10.1002/9780470720639.ch12. [DOI] [PubMed] [Google Scholar]
- Wollenweber H. W., Morrison D. C. Synthesis and biochemical characterization of a photoactivatable, iodinatable, cleavable bacterial lipopolysaccharide derivative. J Biol Chem. 1985 Dec 5;260(28):15068–15074. [PubMed] [Google Scholar]
- Woods D. E., Straus D. C., Johanson W. G., Jr, Berry V. K., Bass J. A. Role of pili in adherence of Pseudomonas aeruginosa to mammalian buccal epithelial cells. Infect Immun. 1980 Sep;29(3):1146–1151. doi: 10.1128/iai.29.3.1146-1151.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Levin S. M., Jong M. T., Chad Z., Kabbash L. G. CR3 (CD11b/CD18) expresses one binding site for Arg-Gly-Asp-containing peptides and a second site for bacterial lipopolysaccharide. J Exp Med. 1989 Jan 1;169(1):175–183. doi: 10.1084/jem.169.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]