Abstract
Certain micronutrients are protective against cognitive decline. We examined whether there is any uniform pattern of circulating micronutrients cross–culturally that are associated with successful cognitive aging. For the U.S. sample, we used the stored serum/plasma of 115 participants, collected in Oregon, USA. The Okinawa sample consisted of 49 participants selected using similar inclusion criteria as the Oregon sample, from the Keys to Optimal Cognitive Aging Project. All participants were aged 85 years and older without cognitive impairment. We found that the Okinawan elders used fewer vitamin supplements but had similar levels of vitamin B12 and α-tocopherol, lower folate and γ-tocopherol, compared with Oregonian elders. That is, we did not find a uniform pattern of circulating micronutrients, suggesting that micronutrients other than those examined here or other lifestyle factors than nutrition could play an important role in achieving successful cognitive aging.
Keywords: Oldest old, Circulating micronutrients, Healthy cognitive aging, Okinawa, Oregon
FACED with an aging population and a growing prevalence of dementia, it is critical to find an effective means to delay the onset of dementia and to find ways to slow the process of cognitive decline. Nutrition has been receiving much attention as a potential modifiable lifestyle factor leading to healthy cognitive aging. Although the oldest old (aged 85 years and older) are the fastest growing segment of the population both in the United States and in Japan (1), and although they face the highest risk of developing dementia due to the risk factor of age alone, the optimal intake or circulating levels of various micronutrients have not yet been established in this age group.
Two frequently studied dietary-related factors related to Alzheimer’s disease (AD), the most dominant type of dementia in the United States, are homocysteine and vitamin E. Elevated plasma total homocysteine concentrations have been found to be a strong risk factor for dementia, as well as AD, in epidemiological studies (2,3). Also, higher vitamin E intakes and serum tocopherol concentrations have been found to be protective against cognitive decline and dementia in epidemiological studies (4–9). However, with the exception of only a few trials (10), the majority of randomized clinical trials thus far have failed to support these observational study findings (11–16).
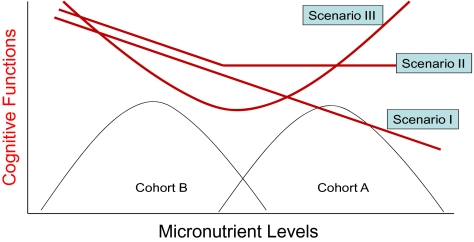
One possible reason for this discrepancy between epidemiological studies and clinical trials might be that healthy lifestyle comes with various components. As for nutrition, a balanced combination of several nutrients could be necessary to obtain positive health effects. Examining the serum/plasma micronutrient levels of multiple cohorts who achieved successful cognitive aging (ie, survival to oldest old with intact cognitive functions) could help clarify the possible combinations and optimal levels of micronutrients leading to healthy cognitive aging. Furthermore, if the association between cognitive function and micronutrients is not entirely linear over a wider spectrum of distribution of micronutrients, the study results based on a single cohort could mask important information. This issue is depicted by hypothetical associations between micronutrients and cognitive functions in Figure 1. For example, in this figure, cohort A has a higher level of a specific micronutrient compared with cohort B. If the association between a specific micronutrient level and cognitive function is linear (eg, as micronutrient levels increase, the cognitive function decreases), then we have a negative association between cognitive functions and the micronutrient in both cohort studies (depicted by the line indicated by scenario I). On the other hand, if the effect of a specific micronutrient on cognitive function levels off after a certain threshold as depicted by the line indicated by scenario II, then we might find a negative association between this micronutrient and cognitive function in cohort B but find no association in cohort A. Finally, if the association is inverse before and after a certain threshold level of micronutrient (scenario III), then we would find contradicting relationships between this micronutrient and cognitive functions between the studies conducted using cohorts A (negative association) and B (positive association). Therefore, comparisons of the absolute levels of circulating micronutrients obtained from groups of participants with very different dietary habits are a useful first step in the analysis of micronutrients and their effect on health.
Figure 1.
Hypothetical associations between cognitive functions and a specific micronutrient.
Here, we present the result of a small-scaled pilot study that compared circulating levels of micronutrients among cognitively healthy volunteers aged 85 years and older in Okinawa and Oregon. The variables compared include, total plasma homocysteine (serum B12 and folate as confounders), serum vitamin E (α- and γ-tocopherols), and serum sodium and potassium, factors linked to hypertension. Our hypothesis was that because both groups are survivors to the oldest-old age group with intact cognition (ie, achievers of successful cognitive aging), both groups would be similar in the levels of circulating micronutrients with regard to homocysteine, B12 and folate (nutrients necessary for homocysteine metabolism), and α- and γ-tocopherols (lipid-soluble antioxidants).
PARTICIPANTS AND METHODS
Oregon Participants
We used the stored serum/plasma of 115 participants, collected between the years 2000 and 2001, who participated in the Dementia Prevention Study, a clinical trial of Ginkgo biloba for dementia prevention. Detailed description of this study is found elsewhere (17). Briefly, the Oregon Center for Complementary and Alternative Medicine in Neurological Disorders and National Institute on Ageing—Layton Aging and Alzheimer Disease Center conducted a 42-month, randomized, double-blind, placebo-controlled pilot trial of standardized Ginkgo biloba extract to determine the effect of Ginkgo biloba extract on cognitive decline among oldest-old participants. Entry criteria were (1) age 85 years or older, (2) Clinical Dementia Rating (CDR) scale (18) = 0, (3) no subjective memory complaint compared with their age peers, (4) normal memory function defined by an education-adjusted score on the Logical Memory subscale of the Wechsler Memory Scale (19), (5) Mini-Mental State Examination score (20) more than 23, and (f) free from depressive symptoms defined by Center for Epidemiological Studies-Depression scale (21) less than 4. Participants were recruited primarily through mass mailings to age-eligible individuals in the greater Portland area in the state of Oregon. We used the samples collected at the baseline “before” the participants received Ginkgo biloba and vitamin E supplements. Out of 122 participants in the Ginkgo biloba extract trial, we used the data on 115 participants with adequate amounts of stored serum/plasma for examining micronutrient assessed in this study.
Okinawa Participants
Participants in this study were selected from the Keys to Optimal Cognitive Aging Project (KOCOA), a pilot project whose aim was to examine nutrition and other lifestyle factors leading to healthy cognitive aging. The recruitment (baseline of the study) was conducted from November 2007 to March 2008. The participants were recruited using participants’ registers of senior clubs located in Ginowan city in Okinawa (approximately 10 km North of Naha city, the capital of Okinawa prefecture). Senior clubs, funded by the municipal government, offer various activities to the local seniors, which include handicrafts, games, traditional dance, and occasional lectures on topics of interest to the seniors.
Researchers in the current study visited all 22 senior centers located in Ginowan city, explained the aims of the study, and asked for volunteers. A request to join the study was made at the conclusion of each presentation. Of the 196 volunteers, aged 80 years and older, who participated in the Keys to Optimal Cognitive Aging Project study, we selected 49 participants using the following criteria for the current comparative pilot analysis: (1) age 85 years or older, (2) CDR (18) = 0, (3) no subjective memory complaints compared with their age peers, (4) normal memory function defined by the ability to recall the event of a blood draw at Ryukyu University (described later in detail), (5) Mini-Mental State Examination greater than or equal to 19 (20,22), and (6) free from depressive symptoms defined by the Japanese Geriatric Depression Scale 15-item version (23) less than 6.
Our study protocol included drawing a fasting venous blood sample at University of the Ryukyus Hospital followed by a face-to-face interview within 2 weeks after the blood draw. We asked the participants at the face-to-face interview to recall whether they visited the University of the Ryukyus Hospital during the last 2 weeks and if so, what they did. If the participants voluntarily recalled the event of blood draw without any problems, we defined that the participant had met the criteria (4) above. This procedure was used because an education-adjusted normative score on the Logical Memory subscale of the Wechsler Memory Scale (19) (the criteria used for the Oregon cohort) has not been validated in Japan. A cut-point of Mini-Mental State Examination of 19 (instead of 24) was used because the mean years of education of this group, as with the majority of the current Okinawan elders, was very low with 6.8 years (SD = 2.2). The low educational levels among the oldest old in Okinawa are mainly attributed to the interruption of schooling due to World War II. Based on the Mangus correction (24), we used the age- and education-adjusted Mini-Mental State Examination score of 19 to reflect the characteristics of the Okinawa cohort. H.H.D., who was trained in the CDR assessment protocol at Oregon Health & Science University, trained and supervised the interviewers at the Okinawa site for the CDR assessment. The informants required for completing CDR assessments were either the senior club activity directors who knew the participant very well or the participant’s family members. The data collected included basic demographic information, plasma/serum samples, height, weight, blood pressures, and various lifestyle factors. The study protocol was approved by the Institutional Review Boards of Oregon Health & Science University, Oregon State University, and University of the Ryukyus, School of Medicine.
Plasma/Serum Micronutrients
For both the Oregon and the Okinawan groups, plasma and serum were promptly separated by centrifugation at 4°C for 15 minutes at 500g and stored at −80°C until analyzed. To increase the validity of the analysis of micronutrients potentially sensitive to laboratory variation, we used the same laboratory for both groups. Serum α- and γ-tocopherols were extracted and measured using high-pressure liquid chromatography as described by Podda and coworkers (25), by the Traber Lab at Linus Pauling Institute at Oregon State University. Plasma homocysteine, serum B12, and serum folate were measured with an automated chemiluminescence assay system (Immulite; Siemens, Deerfield, IL) by the Oregon Clinical and Translational Research Laboratory at Oregon Health & Science University. Total cholesterol, triglycerides, sodium, and potassium, were measured at SRL Inc. (Tokyo, Japan) for the Okinawan samples and at the Oregon Clinical and Translational Research Laboratory at Oregon Health & Science University for the Oregonian samples, using standard clinical assays.
Statistical Analysis
We compared the basic demographic characteristics and micronutrient levels between the two cohorts using chi-square statistics and logistic regression models (controlling for age and sex) for categorical variables, and t test, Wilcoxon rank sum test and linear regression models (controlling for age and sex) for continuous variables. For the α- and γ-tocopherols, we also compared those values adjusted by total cholesterol and triglycerides.
RESULTS
Tables 1–4 show the demographic characteristics of the two cohorts and micronutrient levels. For the nutrients whose abnormal values are already established, we also reported them as a categorical variable using the established cut-point in addition to means.
Table 1.
Basic Demographic Characteristics of Okinawa and Oregon Cohorts
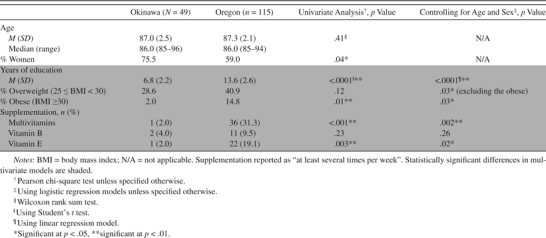 |
Table 2.
Comparisons of Proportions of Participants With Hypertension and Circulating Sodium and Potassium Between Okinawa and Oregon Cohorts
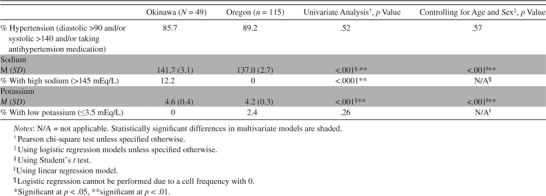 |
Table 3.
Comparisons of Circulating Homocysteine, Folate, and Vitamin B12 Between Okinawa and Oregon Cohorts
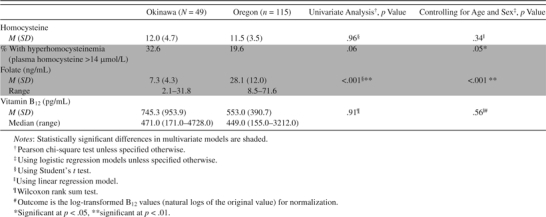 |
Table 4.
Comparisons of Serum α- and γ-Tocopherols Between Okinawa and Oregon Cohorts
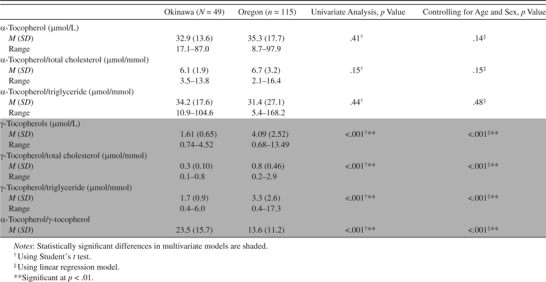 |
Contrary to our hypothesis, we found various differences between the two groups of the relatively healthy cognitively intact elderly participants aged 85 years and older. Fifteen percent of the Oregon group were obese, whereas only 2% of the Okinawan group were obese (p = .03, controlling for age and sex) using the World Health Organization standard classification for obesity of body mass index (BMI) ≥30 (26) (Table 1). Although the World Health Organization BMI cutoff points have been suggested to be used as an international standard for classification of obesity (27), World Health Organization has also acknowledged that Asians generally have higher body fat percentage compared with Caucasians and experience elevated risks at lower levels of BMI and have further recommended BMI cutoff points of 23 (moderate to high risk) and 27.5 (high to very high risk) for Asians instead of 25 and 30 for Caucasians, respectively. However, Asian countries (such as Japan) often adopt their own country-specific standards as well. If we use the very strict country-specific classification adopted by Japan (BMI ≥25) (28), then the percentage of participants from Okinawa with obesity would increase to 30.6%. The self-reported usage of vitamin supplements was much lower in Okinawa (Table 1). Both study groups had a high proportion of those with hypertension (Table 2). None of the Oregon elders had high sodium concentrations (>145 mEq/L), whereas 12% of the Okinawan elders had this profile (p < .001). On the other hand, none of the Okinawan elders had low potassium (≤3.5 mEq/L), whereas 2% of the Oregon elders had that profile with a statistically significant difference (p < .001).
Folate levels were much higher in the Oregon cohort than in the Okinawa cohort (p < .001), and the proportion of those with hyperhomocysteinemia was higher in the Okinawa cohort (p = .05) after controlling for age and sex (Table 3), although the latter finding would not be significant if correcting for multiple testing. Among E vitamins, α-tocopherol levels were similar between the two cohorts, with or without adjustments of total cholesterol or triglycerides (Table 4). However, the Oregon group had higher levels of γ-tocopherol (p < .001). After adjusting for total cholesterol and triglycerides, the difference in γ-tocopherol remained significant (p < .001 for both outcomes).
We conducted a post hoc analysis. We examined whether homocysteine was associated with folate and B12. The linear regression models were run with homocysteine levels being dependent variables and age, sex, folate, and serum B12 being covariates. Folate (coefficient = −.32, p = .04) and B12 (coefficient = −.001, p = .04) in the Okinawa cohort were negatively associated with homocysteine levels, but age and sex were not. In contrast, in the Oregon cohort, folate (coefficient = −.02, p = .39) and B12 (coefficient = −.001, p = .10) were not associated with homocysteine levels, but age was significantly associated with the level of homocysteine (coefficient = .33, p = .04).
DISCUSSION
To our knowledge, this is the first study that has compared the levels of micronutrients among the oldest old with intact cognitive function (CDR = 0) cross–culturally. Contrary to our hypothesis, major differences were found in their circulating micronutrient levels.
The Oregon elders had much higher folate levels. This is likely due to the fact that it is mandatory in the United States to fortify cereals and flour with folate. According to recent National Health and Nutrition Examination Survey results (29), red blood cell folate and serum folate levels among the U.S. population significantly increased after mandatory folate fortification in 1998. Serum folate levels increased with age among those aged 12 years and older according to National Health and Nutrition Examination Survey data (29). The serum folate level of the Oregon elders examined in the current study was higher than those aged 60 years and older reported by National Health and Nutrition Examination Survey, probably due to the age of our cohort and their relatively high educational levels. The latter could result in better dietary habits with adequate micronutrients and a higher rate of supplementation.
Is such a high blood folate level among the Oregon cohort a positive health factor? A recent interventional study found that high serum folate increased risk for cancer and all-cause mortality in ischemic cardiac disease patients (30). The increase in mortality was mainly due to cancer, but the putative mechanism(s) are not known. With regard to cognitive outcomes, the Cochrane Database of Systematic Reviews on the association between folate supplementation and cognitive functions are inconclusive (31). One study showed that those with low serum vitamin B12 status (<148 pmol/L) and high serum folate concentrations (>59 nmol/L) were at higher risk of cognitive impairment compared with those with low vitamin B12, but with normal serum folate concentrations (32). Also, a faster rate of cognitive decline was found among those with folate intakes exceeding 400 μg/day compared with those with lower intakes (33). The Oregon cohort had high serum B12 (ranging from 155 to 3212 pg/mL; ie, 114–2369 pmol/L). Only six participants (5.2%) had a value lower than 148 pmol/L, with none having lower than the critical deficiency threshold of 111 pmol/L. By these criteria, there does not appear to be an increased risk among the Oregon cohort in the current study. However, considering that the consumption of folate is high in the United States due to fortification and personal use of supplements, it may be prudent to establish guidelines for the oldest old regarding the average intake of vitamin B12 and folate because elderly persons tend to lose intrinsic factors in the digestive system and often lack the acidity to properly absorb B12 (34). The proportion of those with hyperhomocysteinemia was lower among the Oregon cohort compared with the Okinawa cohort, possibly due to high folate intake in the United States. If Okinawa elders could increase their folate intake, their homocysteine levels may decrease, as indicated by our post hoc analysis.
Vitamin E also differed between Okinawans and Oregonians. Serum γ-tocopherol levels were significantly higher in Oregonians, even after adjustment for total cholesterol and/or triglycerides. Gamma-tocopherol is the major form of vitamin E in the U.S. diet and its antioxidative and anti-inflammatory properties beyond those found in α-tocopherol have been receiving attention recently (35). Ford and coworkers (36) studied the distribution of α- and γ-tocopherol levels among the U.S. population using data from the National Health and Nutrition Examination Survey. In Ford’s study, the mean α- and γ-tocopherol levels and those adjusted by total cholesterol among those aged 70 years and older, respectively, were 35.0 (α), 6.5 (α/total cholesterol), 4.2 (γ), and 0.8 (γ/total cholesterol). In our Oregon cohort, the comparable figures were 35.3, 6.7, 4.1, and 0.8, respectively, similar to the Ford study, but significantly higher than those found among the Okinawan group. Foods high in γ-tocopherol include hydrogenated corn and soybean oils; ingredients often included in processed foods, such as commercially baked goods (doughnut and other pastries, cookies, and crackers), deep-fried foods. snack foods such as potato chips, and some margarines. Morris and coworkers (37) found that higher consumption of γ-tocopherol was associated with unhealthy dietary behaviors, including higher intakes of saturated and trans fats. In their population-based Italian cohort study, Ravaglia and coworkers (38) showed that the beneficial effect of γ-tocopherol against dementia incidence was evident only in the middle tertile of γ-tocopherol. One of the potential explanations of this finding suggested by the authors is a poor diet possibly associated with high γ-tocopherol in the upper tertile. It is also possible that the high proportion of obesity observed in the Oregon cohort of oldest old could be partly due to a high consumption of processed foods that are convenient, highly available, and require little preparation.
Morris and coworkers (7) found that high intake of vitamin E from food, but not from supplements, was inversely associated with AD incidence. One explanation of this finding is that several tocopherol forms rather than α-tocopherol alone may be necessary for vitamin E to have a protective effect on cognitive health (37). The much lower γ-tocopherol levels found in the Okinawan cohort suggests that high serum levels of γ-tocopherol might not be necessary to achieve healthy cognitive aging. A controlled intervention study with γ-tocopherol would be required in order to more closely examine the effect of γ-tocopherol on cognitive health.
Finally, Oregon elders had a higher proportion of overweight and obese individuals based on the World Health Organization international standard classification. Obesity, especially midlife obesity, has been shown to have detrimental effects on cognitive health, along with higher risk of diabetes, a risk factor for not only vascular dementia but also for AD (39,40). On the other hand, distinguishing midlife and late-life obesity, one study (41) in the United States found that late-life underweight (BMI <20) increased risk of dementia over 5 years of follow-up, whereas being overweight (BMI 25.0–29.9) was not associated with an increased risk, and being obese (BMI ≥30) actually “reduced” the risk of dementia compared with having normal BMI in late life. Coinciding with this finding, a recent study based on Australian cohorts showed that for elders who had survived to the age of 70, mortality risk was lowest in those with a BMI classified as overweight over a 10-year period compared with those with normal weight or the obese participants (42). They suggested that current BMI requirements might be too strict for seniors. It would be interesting to explore whether those overweight in the cohorts examined here actually survive longer with intact cognition compared with those of normal weight. Further follow-up would be required to examine this issue.
Our study strengths include the fact that we compared the micronutrient levels of rare survivors among the elders (oldest old with CDR = 0), a rarely studied group with regard to micronutrient analysis. Additionally, within-cultural comparisons (eg, examining whether or not a specific micronutrient is associated with cognitive well-being “within” one culture or cohort) could suffer from the potential ceiling effects of a specific micronutrient. That is, if the majority of the cohort members have high or low levels of specific micronutrients, we might not be able to find a significant association between that nutrient and health due to the lack of variability in a within-cohort study. Cross-cultural comparisons of the absolute levels of micronutrients are useful in that they have the potential to shed light upon areas that within-cultural studies may not. Our study limitations include small sample size, especially for the Okinawan cohort. Even though we used similar inclusion criteria between the two cohorts, and both groups are healthy volunteers, the study findings may not be completely free from sample selection bias. Vitamin supplementations were based on self-report in Okinawa, which could suffer from reporting errors. The potential effect of medications on micronutrients were reported, including a long-term use of proton pump inhibitors being associated with B12 deficiency and elevated homocysteine (43,44) and drug-induced hypokalemmia (45). We believe that the effect of medications among relatively healthy volunteers examined in this study is not substantial, but further studies are required to confirm this notion. Oregon micronutrient analyses were conducted using aliquots stored for more than 8 years. Previous studies have confirmed the stability of serum and plasma α-tocopherol stored up to 15 years (46,47). Other studies have confirmed that vitamin B12 and homocysteine are stable for more than 29 years of storage (48). Folate has been shown to degrade after 17 years of storage and appears stable for up to 6 years (48). We may have underestimated the true difference in serum folate between our two cohorts because we cannot confirm that some folate degradation may have occurred in the Oregon samples as a consequence of storage duration in that cohort. Finally, this is a cross-sectional pilot study; although we excluded those with CDR ≥0.5, dementia-related pathology is known to start a decade or more before neurological symptoms become apparent. Therefore, reverse causation is also a possibility.
In sum, contrary to our hypothesis, there was no discernable protective micronutrient pattern. The Okinawan elders had lower folate and γ-tocopherol and a borderline higher proportion with hyperhomocysteinemia. The optimal lifestyle leading to healthy cognitive aging could consist of various components such as healthy diet, high physical activity, and social engagement, among other factors. Each component’s effect on overall cognitive health could vary and there could also be interaction effects among the components as shown in the study by Scarmeas and coworkers (49) and/or or gene–environment interactions. The oldest old examined here likely achieved healthy cognitive aging due to various factors and the interactions of these factors over the life course. Some factors may include shared genetic traits among families (50), whereas others may have unique social, historical, and epidemiological contexts, such as the population-wide experience of long-term caloric restriction among the Okinawans (51) and the potential associated physiological benefits (52). Some detrimental effects could be compensated for by protective factors. This means that there may potentially be many patterns that allow elders to survive with intact cognition, and we need to study the lifestyle “package” leading to healthy cognitive aging. Alternatively, we could explore the most effective means to sustain cognitive health within each component (nutrition, social engagement, physical activities, etc.) and recommend the best approach hoping that the interaction would yield combined and better outcomes. The results presented here are based on a pilot study. Follow-up of the current cohorts examined here and further cross-national comparisons of circulating micronutrients and other lifestyle factors could help clarify the lifestyle package leading to healthy cognitive aging.
FUNDING
This study was supported by the National Institute on Aging (K01AG023014 to H.H.D.), (P30 AG08017 to J.A.K.), the National Center for Complementary and Alternative Medicine (P50 AT00066 to J.A.K.), Linus Pauling Institute Research Grant (H.H.D.), Oregon Tax Check-off Grant (H.H.D.), the Center for Healthy Aging Research Pilot Grant at Oregon State University (H.H.D.), and Oregon Clinical and Translational Research Institution (UL1 RR024140).
Acknowledgments
We would like to express our sincere appreciation to Ms. Masayo Iha and Mr. Daisuke Higa, who acted as study coordinators for the Keys to Optimal Cognitive Aging Project and Ms. Wasserman, the study coordinator for the Dementia Prevention Study project. Faculty and staff from the University of the Ryukyus Hospital were also instrumental in the successful completion of the project. We also thank Dr. Satoshi Sasaki for his helpful advice. Finally, this study would not have been possible without the cooperation and support of the municipalities, public officials, families, and most importantly, the participants in our studies.
References
- 1.NIH. Why Population Aging Matters: A Global Perspective (Publication Number 07–6134) Washington, DC: National Institute on Health; 2007. [Google Scholar]
- 2.Seshadri S, Beiser A, Selhub J, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N Engl J Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 3.Ravaglia G, Forti P, Maioli F, et al. Homocysteine and folate as risk factors for dementia and Alzheimer disease. Am J Clin Nutr. 2005;82:636–643. doi: 10.1093/ajcn.82.3.636. [DOI] [PubMed] [Google Scholar]
- 4.Zandi PP, Anthony JC, Khachaturian AS, et al. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: the Cache County Study. Arch Neurol. 2004;61:82–88. doi: 10.1001/archneur.61.1.82. [DOI] [PubMed] [Google Scholar]
- 5.Morris MC, Beckett LA, Scherr PA, et al. Vitamin E and vitamin C supplement use and risk of incident Alzheimer disease. Alzheimer Dis Assoc Disord. 1998;12:121–126. doi: 10.1097/00002093-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Morris MC, Evans DA, Bienias JL, et al. Dietary intake of antioxidant nutrients and the risk of incident Alzheimer disease in a biracial community study. JAMA. 2002;287:3230–3237. doi: 10.1001/jama.287.24.3230. [DOI] [PubMed] [Google Scholar]
- 7.Morris MC, Evans DA, Bienias JL, Tangney CC, Wilson RS. Vitamin E and cognitive decline in older persons. Arch Neurol. 2002;59:1125–1132. doi: 10.1001/archneur.59.7.1125. [DOI] [PubMed] [Google Scholar]
- 8.Engelhart MJ, Geerlings MI, Ruitenberg A, et al. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA. 2002;287:3223–3229. doi: 10.1001/jama.287.24.3223. [DOI] [PubMed] [Google Scholar]
- 9.Wengreen HJ, Munger RG, Corcoran CD, et al. Antioxidant intake and cognitive function of elderly men and women: the Cache County Study. J Nutr Health Aging. 2007;11:230–237. [PubMed] [Google Scholar]
- 10.Wouters-Wesseling W, Wagenaar LW, Rozendaal M, et al. Effect of an enriched drink on cognitive function in frail elderly persons. J Gerontol A Biol Sci Med Sci. 2005;60:265–270. doi: 10.1093/gerona/60.2.265. [DOI] [PubMed] [Google Scholar]
- 11.Sano M, Ernesto C, Thomas RG, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer's disease. The Alzheimer's Disease Cooperative Study. N Engl J Med. 1997;336:1216–1222. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- 12.Lewerin C, Matousek M, Steen G, Johansson B, Steen B, Nilsson-Ehle H. Significant correlations of plasma homocysteine and serum methylmalonic acid with movement and cognitive performance in elderly subjects but no improvement from short-term vitamin therapy: a placebo-controlled randomized study. Am J Clin Nutr. 2005;81:1155–1162. doi: 10.1093/ajcn/81.5.1155. [DOI] [PubMed] [Google Scholar]
- 13.Sun Y, Lu CJ, Chien KL, Chen ST, Chen RC. Efficacy of multivitamin supplementation containing vitamins B6 and B12 and folic acid as adjunctive treatment with a cholinesterase inhibitor in Alzheimer's disease: a 26-week, randomized, double-blind, placebo-controlled study in Taiwanese patients. Clin Ther. 2007;29:2204–2214. doi: 10.1016/j.clinthera.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 14.McMahon JA, Green TJ, Skeaff CM, Knight RG, Mann JI, Williams SM. A controlled trial of homocysteine lowering and cognitive performance. N Engl J Med. 2006;354:2764–2772. doi: 10.1056/NEJMoa054025. [DOI] [PubMed] [Google Scholar]
- 15.Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 16.Aisen PS, Schneider LS, Sano M, et al. High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trial. JAMA. 2008;300:1774–1783. doi: 10.1001/jama.300.15.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dodge HH, Zitzelberger T, Oken BS, Howieson D, Kaye J. A randomized placebo-controlled trial of Ginkgo biloba for the prevention of cognitive decline. Neurology. 2008;70:1809–1817. doi: 10.1212/01.wnl.0000303814.13509.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 19.Wechsler D. Wechsler Memory Scale (WMS-III) San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 22.Otsuka T, Homma A. Assessment Manual of Intellectual Function for the Demented Elderly (in Japanese) Tokyo, Japan: World Planning; 1991. [Google Scholar]
- 23.Niino N. [Prevalence of depressive symptoms among the elderly]. (in Japanese) Nippon Ronen Igakkai Zasshi. 1988;25:403–407. doi: 10.3143/geriatrics.25.403. [DOI] [PubMed] [Google Scholar]
- 24.Mungas D, Marshall SC, Weldon M, Haan M, Reed BR. Age and education correction of Mini-Mental State Examination for English and Spanish-speaking elderly. Neurology. 1996;46:700–706. doi: 10.1212/wnl.46.3.700. [DOI] [PubMed] [Google Scholar]
- 25.Podda M, Weber C, Traber MG, Packer L. Simultaneous determination of tissue tocopherols, tocotrienols, ubiquinols, and ubiquinones. J Lipid Res. 1996;37:893–901. [PubMed] [Google Scholar]
- 26.WHO. Report on a WHO Consultation on Obesity, Geneva, 3–5 June, 1997. WHO/NUT/NCD/98.1; Technical Report Series Number 894. Genava, Switzerland: World Health Organization; 2000. Obesity: preventing and managing the global epidemic. [PubMed] [Google Scholar]
- 27.WHO expert consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 28.Examination Committee of Criteria for ‘Obesity Disease’ in Japan. Japan Society for the Study of Obesity. New criteria for ‘obesity disease’ in Japan. Circ J. 2002;66:987–992. doi: 10.1253/circj.66.987. [DOI] [PubMed] [Google Scholar]
- 29.NCHS Data Brief. 2008. http://www.cdc.gov/nchs/data/databriefs/db06.htm, as of April 2010. [Google Scholar]
- 30.Ebbing M, Bonaa KH, Nygard O, et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA. 2009;302:2119–2126. doi: 10.1001/jama.2009.1622. [DOI] [PubMed] [Google Scholar]
- 31.Malouf R, Grimley Evans J. Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD004514.pub2. CD004514. [DOI] [PubMed] [Google Scholar]
- 32.Morris MS, Jacques PF, Rosenberg IH, Selhub J. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am J Clin Nutr. 2007;85:193–200. doi: 10.1093/ajcn/85.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris MC, Evans DA, Bienias JL, et al. Dietary folate and vitamin B12 intake and cognitive decline among community-dwelling older persons. Arch Neurol. 2005;62:641–645. doi: 10.1001/archneur.62.4.641. [DOI] [PubMed] [Google Scholar]
- 34.Evatt ML, Terry PD, Ziegler TR, Oakley GP. Association between vitamin B12-containing supplement consumption and prevalence of biochemically defined B12 deficiency in adults in NHANES III (Third National Health and Nutrition Examination Survey) Public Health Nutr. 2010;13:25–31. doi: 10.1017/S1368980009990279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang Q, Christen S, Shigenaga MK, Ames BN. gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr. 2001;74:714–722. doi: 10.1093/ajcn/74.6.714. [DOI] [PubMed] [Google Scholar]
- 36.Ford ES, Schleicher RL, Mokdad AH, Ajani UA, Liu S. Distribution of serum concentrations of alpha-tocopherol and gamma-tocopherol in the US population. Am J Clin Nutr. 2006;84:375–383. doi: 10.1093/ajcn/84.1.375. [DOI] [PubMed] [Google Scholar]
- 37.Morris MC, Evans DA, Tangney CC, et al. Relation of the tocopherol forms to incident Alzheimer disease and to cognitive change. Am J Clin Nutr. 2005;81:508–514. doi: 10.1093/ajcn.81.2.508. [DOI] [PubMed] [Google Scholar]
- 38.Ravaglia G, Forti P, Lucicesare A, et al. Plasma tocopherols and risk of cognitive impairment in an elderly Italian cohort. Am J Clin Nutr. 2008;87:1306–1313. doi: 10.1093/ajcn/87.5.1306. [DOI] [PubMed] [Google Scholar]
- 39.Haan MN. Therapy insight: type 2 diabetes mellitus and the risk of late-onset Alzheimer's disease. Nat Clin Pract Neurol. 2006;2:159–166. doi: 10.1038/ncpneuro0124. [DOI] [PubMed] [Google Scholar]
- 40.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Jr, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 2005;330:1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fitzpatrick AL, Kuller LH, Lopez OL, et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66:336–342. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flicker L, McCaul KA, Hankey GJ, et al. Body mass index and survival in older men and women aged 70 to 75 years. J Am Geriatr Soc. 2010;58:234–241. doi: 10.1111/j.1532-5415.2009.02677.x. [DOI] [PubMed] [Google Scholar]
- 43.Raghunath AS, O’Morain C, McLoughlin RC. Review article: the long-term use of proton-pump inhibitors. Aliment Pharmacol Ther. 2005;22(suppl 1):55–63. doi: 10.1111/j.1365-2036.2005.02611.x. [DOI] [PubMed] [Google Scholar]
- 44.Valuck RJ, Ruscin JM. A case-control study on adverse effects: H2 blocker or proton pump inhibitor use and risk of vitamin B12 deficiency in older adults. J Clin Epidemiol. 2004;57:422–428. doi: 10.1016/j.jclinepi.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 45.Gennari FJ. Disorders of potassium homeostasis. Hypokalemia and hyperkalemia. Crit Care Clin. 2002;18:273–288. doi: 10.1016/s0749-0704(01)00009-4. [DOI] [PubMed] [Google Scholar]
- 46.Comstock GW, Alberg AJ, Helzlsouer KJ. Reported effects of long-term freezer storage on concentrations of retinol, beta-carotene, and alpha-tocopherol in serum or plasma summarized. Clin Chem. 1993;39:1075–1078. [PubMed] [Google Scholar]
- 47.Brown Thomas J, Duewer DL, Kline MC, Sharpless KE. The stability of retinol, alpha-tocopherol, trans-lycopene, and trans-beta-carotene in liquid-frozen and lyophilized serum. Clin Chim Acta. 1998;276:75–87. doi: 10.1016/s0009-8981(98)00103-x. [DOI] [PubMed] [Google Scholar]
- 48.Hannisdal R, Gislefoss RE, Grimsrud TK, Hustad S, Morkrid L, Ueland PM. Analytical recovery of folate and its degradation products in human serum stored at -25 degrees C for up to 29 years. J Nutr. 2010;140:522–526. doi: 10.3945/jn.109.116418. [DOI] [PubMed] [Google Scholar]
- 49.Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer disease. JAMA. 2009;302:627–637. doi: 10.1001/jama.2009.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Willcox BJ, Willcox DC, He Q, Curb JD, Suzuki M. Siblings of Okinawan centenarians share lifelong mortality advantages. J Gerontol A Biol Sci Med Sci. 2006;61:345–354. doi: 10.1093/gerona/61.4.345. [DOI] [PubMed] [Google Scholar]
- 51.Willcox BJ, Willcox DC, Todoriki H, et al. Caloric restriction, the traditional Okinawan diet, and healthy aging: the diet of the world's longest-lived people and its potential impact on morbidity and life span. Ann N Y Acad Sci. 2007;1114:434–455. doi: 10.1196/annals.1396.037. [DOI] [PubMed] [Google Scholar]
- 52.Barzilai N, Bartke A. Biological approaches to mechanistically understand the healthy life span extension achieved by calorie restriction and modulation of hormones. J Gerontol A Biol Sci Med Sci. 2009;64:187–191. doi: 10.1093/gerona/gln061. [DOI] [PMC free article] [PubMed] [Google Scholar]