Abstract
To explore whether nitrogen retention can differ on an isonitrogenous diet by changing when protein is consumed, we performed a short-term study in older individuals (64.5 ± 2.0 years) performing daily exercise while in energy balance. Participants consumed an isonitrogenous–isocaloric diet with the timing of a protein or carbohydrate beverage after exercise (protein after exercise [PRO], carbohydrate after exercise [CHO]) versus earlier in the day. Three-day mean energy balance (PRO: 202 ± 36 kcal and CHO: 191 ± 44 kcal; p = .68) did not differ between trials, but 3-day mean nitrogen balance was significantly more positive in the PRO (1.2 ± 0.32 g N) trial than the CHO trial (0.8 ± 0.45 g N; p < .05). Older individuals were better able to maintain nitrogen balance by simply changing when a portion of an identical amount of daily protein was consumed.
Keywords: Aerobic exercise, Protein intake, Frailty
THE importance of preventing frailty in the aging population has been discussed in detail (1). Physical activity and the anabolic effect of feeding serve to maintain body protein content. There is much discussion on how these anabolic stimuli, independently and in conjunction, change in older individuals (2) because whole body protein content and lean body mass decline with age (3). These declines in protein content and lean body mass can lead to changes in body composition that increase risk for chronic disease and disability (4). Thus, there is a practical need to devise easy-to-follow strategies to ensure that whole body protein content is maintained in older individuals.
The nitrogen balance (NBAL) method is classically not only used to determine adequate protein intakes for various populations but can also be used to measure whole body protein balance in response to exercise or nutritional interventions. The premise of this technique is that because protein is the body’s main nitrogen-containing compound, a fluctuation in nitrogen reflects a change in total body protein (5). Although our group and others primarily use stable isotopic methods for determining protein turnover, NBAL is still an appropriate method when assessing relatively short-term changes in protein balance. Studies using the NBAL method have primarily focused on the amount of dietary protein required to maintain NBAL in older individuals, arguing for (6,7) or against (8) increasing the protein recommendation above the recommended daily allowance. Largely overlooked in this discussion are the independent factors of energy balance and timing of protein intake, which if accounted for may explain the disparate results from other studies. If energy intake is inadequate, a negative NBAL and loss of lean body mass will be the result (9). Conversely, when energy balance becomes more positive, nitrogen losses are attenuated (10). During an energy deficit, the body oxidizes more amino acids to supplement ATP production. Furthermore, because building proteins is costly from an energetic standpoint (11), it follows that synthesis of nonvital proteins will be downregulated during an energy shortage. The decrease in energy intake that occurs progressively through each decade of life after the age of 20 years may contribute to the age-related decrement of body protein mass (12).
The anabolic effect of exercise and subsequent protein nutrition may play an important role in therapeutic treatments for older individuals to preserve muscle mass. When energy balance is maintained, moderate-intensity exercise can cause NBAL to become more positive by increasing nitrogen retention (13,14). In young men, the addition of 1 hour of exercise spares 2.5 mg N/kg body weight/day (14). There is evidence that whole body protein synthesis increases acutely following vigorous aerobic exercise (15) and that the consumption of a protein-containing beverage can increase this anabolic effect more than an isocaloric carbohydrate-containing beverage (C. Murphy and B. F. Miller, unpublished data). Finally, there is evidence that longer term aerobic training increases basal whole body protein turnover in young (16) and older individuals (17) and that postexercise protein supplementation augments muscular strength, mass, and bone gains in older women after a long-term weight-lifting program (18). The increase in synthesis of individual proteins, like those in muscle, may conserve nitrogen from protein breakdown and allow whole body protein balance to become more positive (19). We have described the net effect of exercise and nutrition as an optimization, where physical activity provides an anabolic stimulus and protein nutrition provides the building blocks to maximize the response (20).
Previous studies have mainly investigated resistance exercise as a strategy to maintain lean body mass, even though aerobic exercise effectively increases whole body protein turnover (as previously mentioned) and also increases muscle fiber size (21). Because walking is the most common form of exercise among the elderly individuals (22) and is commonly recommended to older individuals (23), low-intensity aerobic exercise could be considered a potential method to maintain lean body mass. To our knowledge, there is only a single NBAL study that compares the timing of a protein-containing mixed-meal beverage with a control beverage after aerobic-type exercise (24). In the study by Roy and colleagues, young female endurance athletes in negative energy balance were better able to maintain NBAL when protein instead of placebo was consumed immediately postexercise. The two conditions were isoenergetic and isonitrogenous but differed only in the timing of the macronutrient intake.
The current study investigated the concept that even if the exact same diet is consumed simply changing the timing of the protein can improve NBAL in older individuals. To do so, we studied a group of older participants under strictly controlled macronutrient intake and energy balance. An exercise stimulus of low- to moderate-intensity aerobic exercise was used because it is easily attainable in this population. We hypothesized that in older participants in energy balance, consumption of a protein-containing drink after exercise would increase 3-day NBAL compared with a condition in which exercise was also performed, but the protein drink was consumed at some other point in the day.
METHODS
Study Overview
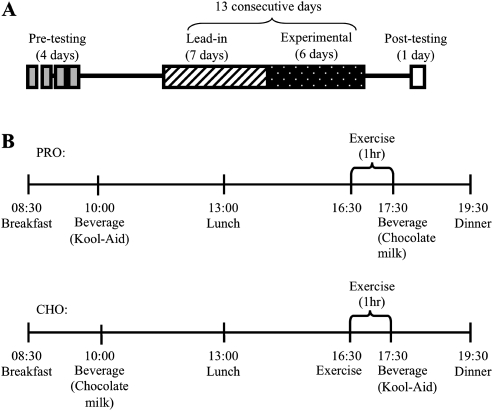
The study consisted of four distinct periods: preexperimental testing, a 7-day lead-in period, a 6-day inpatient/experimental period, and posttesting (Figure 1). The metabolic information obtained during pretesting was used to plan lead-in and experimental diets. The 7-day lead-in diet allowed a period for each participant to adapt to the level of protein provided during the inpatient/experimental period (5). The protocol was approved by the Colorado State University Institutional Review Board and the Colorado Multiple Institutional Review Board for human participants research.
Figure 1.
Study timeline (A) and daily timing of meals, exercise, and consumption of beverages during PRO + CHO and CHO trials (B). The time line in panel B occurs within the period labeled “Experimental” in panel A. The order of the PRO and CHO trials were randomized.
Participants
Nine healthy sedentary male (n = 2) and female (n = 7) participants between the ages of 55 and 75 years were recruited to participate in this study (Table 1). During an initial screening appointment, participants completed an informed consent and a medical and exercise history questionnaire. Each participant completed an online food preference and allergy questionnaire from the Clinical and Translational Research Center (CTRC) so that the study diets would contain acceptable food choices. Participants were nonsmokers and were not taking any medications, except one participant who was on an antidepressant. Because milk was used as an experimental beverage, volunteers reporting lactose intolerance were excluded. Additional exclusionary criteria included obesity (body mass index >30), recent orthopedic injuries that would impede the ability to exercise, conditions affecting food absorption or digestion, undiagnosed hypo- or hyperparathyroidism (thyroid stimulating hormone [TSH] <0.05 uU/mL or TSH >5.0 uU/mL), or a current illness or infection.
Table 1.
Participant Characteristics*
| Participants |
|||
| Characteristic | Male | Female | All Participants |
| Age, y | 62 ± 7 | 66 ± 2 | 65 ± 2 |
| Height, cm | 182 ± 0.5 | 157 ± 1.0 | 162 ± 4.0 |
| Weight, kg | 88.8 ± 2.2 | 57.3 ± 2.8 | 64.3 ± 5.1 |
| Body mass index, kg/m | 26.8 ± 0.8 | 23.3 ± 1.1 | 24.1 ± 1.0 |
| Body fat, % | 22.2 ± 0.5 | 34.4 ± 2.4 | 31.7 ± 2.5 |
| VO2max, ml/kg/min | 30.2 ± 0.4 | 23.6 ± 1.5 | 25.0 ± 1.5 |
Values are expressed as means ± SEM, males (n = 2), females (n = 7), and all participants (n = 9).
Pretesting
Each participant underwent a series of preexperimental testing, both to ensure their eligibility and to determine various metabolic and physical characteristics. On the first day of testing, a graded exercise test (Balke protocol) was completed on a treadmill under the supervision of a cardiologist. Individuals were excluded if the test indicated a hypertensive or ischemic response to exercise as determined by the supervising cardiologist. Participants returned to the laboratory on a separate morning after abstaining from exercise for 24 hours and completing an overnight fast. Body composition was determined using dual-energy x-ray absorptiometry scan (QDR 4500W; Hologic, Inc., Bedford, MA). Resting metabolic rate (RMR) was then measured to estimate 24-hour resting caloric expenditure (Parvomedics TrueOne 2400, Sandy, UT). During this test, a hood was placed over the head of the participant, and expired gases were collected for 45 minutes. The first 15 minutes were treated as an adaptive period to allow the participant to become accustomed to the experimental setup and to determine the appropriate flow rate. The data from the final 30 minutes predicted RMR using the Weir equation (25). On another day during the same week, participants completed an incremental exercise test on a cycle ergometer (Monark Excalibur, Groningen, The Netherlands) to determine VO2max by indirect calorimetry (Parvomedics TrueOne 2400). The maximal cycling protocol began at 50 W for the first minute, increasing by 20 W for women and 30 Watts for men every 2 minutes thereafter. The test was terminated when participants reached volitional exhaustion. It was determined that a true maximal level was reached if allthe following criteria were achieved: Exercise heart rate came within 10 beats of predicted maximal heart rate, respiratory exchange ratio (RER) was greater than 1.1, and VO2 values appeared to reach a plateau.
On a separate day, a submaximal steady-state cycling test was completed at an intensity that approximates a brisk walking pace (55% VO2max). The purpose of this test was to estimate energy expenditure (EE) during a bout of exercise at the given intensity. The American College of Sports Medicine (ACSM) leg cycle ergometry equation (23) was used to estimate the cycle ergometer work rate that would produce a steady-state exercise level at 55% of maximal VO2:
| (1) |
Using indirect calorimetry, EE was measured each minute. These EE values were averaged over 30 minutes of steady-state exercise, allowing a prediction of exercise EE in kcal/hour during the study’s inpatient daily exercise protocol (1 hour of cycling at 55% of VO2max). During the pretesting period, participants also completed a diet log by recording all foods and beverages that were ingested during three consecutive days (2 weekdays and 2 weekend day). The record was collected to provide an indication of normal dietary habits of the participants. Participants provided food labels for any specialty items and gave investigators specific information about the foods that were eaten, portion sizes, and how foods were prepared. All foods were entered into Food Intake Analysis System software (FIAS 3.99, Houston, TX) and analyzed. The data from the 3 days were averaged, providing an indication of habitual energy, macronutrient, and fiber intake.
Lead-in Diet
Several (2–3) weeks after completing the pretesting, each participant completed a 7-day lead-in diet immediately followed by a 6-day inpatient stay at the University of Colorado-Denver CTRC (Figure 1). The lead-in diet was intended to provide an equal amount of protein to that of the inpatient diet, so that NBAL measurements made during the inpatient stay would not be a reflection of an acute adaptation to a new level of protein intake. During the 7-day lead-in period, participants reported to the Colorado State University (CSU) Nutrition Center each morning for weight measurement and breakfast. Participants were instructed to arrive in the same lightweight clothing each day and were weighed without shoes on the same scale (Detecto, Webb City, MO) each day. Breakfast was eaten at CSU under the supervision of study staff, and the remainder of each day’s food was prepared and placed in a soft cooler. Besides coming to the Nutrition Center each morning, participants were allowed to maintain their normal everyday activities. Thus, lunch, dinner, and snacks were consumed either at the participant’s home or at the place of work. All study diets (outpatient and inpatient) followed United States Department of Agriculture (USDA) nutritional guidelines and were created using Pronutra software (Viocare, Inc., Princeton, NJ). The macronutrient breakdown of the lead-in diet was as follows: 15% protein, 30% fat, and 55% carbohydrate taken as a percentage of total calories. Breakfast, lunch, and dinner comprised 30%, 30%, and 40% of calories, respectively. Participants were instructed to eat only the food that was provided to them by study staff and to eat everything in the cooler. If they were unable to eat any food items, they were asked to return the food the following day so that it could be weighed. Otherwise, it was assumed that the participants ate all foods provided to them and nothing else. Participants maintained energy balance (energy intake = EE) for the entire study period with caloric intake planned based on the participant’s estimated caloric expenditure. For the lead-in diet, each participant’s RMR was multiplied by an activity factor of 1.55–1.60, a range of activity factors that approximates those of free-living sedentary elderly individuals (26).
During the first 2 days of the lead-in period, participants wore an accelerometer (Actigraph GT1M, Pensacola, FL) to estimate calories expended during activities of daily living. Participants were instructed to wear the activity monitor on their waistband at all times except during sleeping or bathing. If activity counts were less than or equal to 1,952 per minute, then the Work-Energy Theorem was used to estimate EE:
| (2) |
The Freedson equation (27) was used to estimate EE when activity counts were greater than 1,952 per minute:
| (3) |
Total daily energy expenditure (TDEE) was estimated using the concept that the major components of EE are RMR, diet-induced thermogenesis (DIT), and activity (exercise and nonexercise) thermogenesis (28):
| (4) |
Because participants were sedentary, the EE predicted by the accelerometer was used for activity thermogenesis. The measured value for RMR was used, and DIT was estimated to be 10%–15% of TDEE (29). Free-living physical activity level (PAL) was calculated using the following equation:
| (5) |
Free-living energy balance was calculated using TDEE and energy intake as determined from the diet plans created by the study dietician:
| (6) |
Experimental Protocol (inpatient period)
The experimental protocol was implemented during a 6-day inpatient stay at the University of Colorado-Denver CTRC in which NBAL was continuously monitored. The study design was randomized crossover, so each participant completed two 3-day trials consecutively. Three-day diets were identical between the two conditions, so that energy, macronutrients, and foods consumed were exactly reproduced during each trial. Due to the well-known relationship between energy balance and NBAL, the purpose of the Cal room days was to directly measure EE and allow for calculation of energy balance.
On the evening before the 6-day inpatient study period, participants were admitted to the CTRC and provided dinner as the final meal of their lead-in diet. The study began the next morning with the start of the study diet and 24-hour urine collection. Day 1 and Day 6 were spent in a whole room calorimeter (Cal room), and the rest of the days were spent in a patient room on the CTRC. During the non–Cal room days, participants were allowed to leave the unit two times during the day for 30 minutes. Participants were instructed to consume only the diet provided to them by the CTRC staff and to exercise only during the 1-hour cycling bout prescribed as part of the study protocol. The study diets contained only noncaffeinated beverages, but participants were allowed to request noncaloric noncaffeinated beverages and to consume water ad libitum. Daily gowned-weight measurements were taken on the same digital scale every morning after voiding. Each day, the participants completed 1 hour of cycling exercise (Lode, Groningen, The Netherlands) at an intensity corresponding to 55% of VO2max as determined by the previously discussed steady-state exercise test. The exercise bout was intended to simulate a brisk walking pace and was tolerated well by all participants except for one who had some difficulty completing the full hour of exercise.
Two test beverages were consumed in both trials with the timing of one beverage in the morning and one beverage after exercise in the afternoon (Figure 1). In one trial (PRO), the morning drink was a 407-kcal Kool-Aid drink containing 100 g carbohydrate (57 g polycose, 50 g Kool-Aid, and 350 g water), whereas the postexercise beverage was a 248-kcal chocolate milk drink containing 15.3 g protein, 43.6 g carbohydrate, and 1.3 g fat (330 g skim milk, 4 g whey protein, and 42 g of chocolate syrup). In the second trial (CHO), the order of the drinks was reversed so that the protein-containing beverage was consumed in the morning, and the carbohydrate only drink was consumed after exercise. Besides the timing of the different beverage types in relation to the exercise bout, diets during the two trials were exactly the same. Calories for the beverages were deducted from total intake, and the remaining calories were divided as follows: 30% breakfast, 30% lunch, and 40% dinner. The macronutrient content of the inpatient diet was 13.5% protein, 21% fat, and 65.5% carbohydrate as a percentage of total calories. It was originally intended for the inpatient diet to provide the same macronutrient content as the lead-in diet (15% protein, 30% fat, and 55% carbohydrate). However, an error in the nutritional database for the sweetener used in the Kool-Aid beverage resulted in the provision of an additional 159 g of carbohydrate daily for each participant. The diets still aligned with the USDA’s acceptable macronutrient distribution ranges (45%–65% carbohydrate, 20%–35% fat, and 10%–35% protein) (30).
TDEE for the inpatient stay was first approximated, and caloric intake was subsequently planned based on the estimate. The estimate for TDEE in the Cal room was lower than in the non–Cal room days because ambulatory activity levels are lower when restricted to the Cal room. To estimate TDEE, RMR was multiplied by an activity factor (AF; 1.35 for Cal room days and 1.45 for non–Cal room days [E. L. Melanson, unpublished observations]). Then, exercise EE was estimated from steady-state VO2 results, and an additional 20% of exercise EE calories were added to account for excessive postexercise oxygen consumption (31):
| (7) |
Participants entered the Cal room at 07:45 AM on Day 1 and Day 6 and exited at 07:15 AM the following morning. The 12′ × 12′ room contained a bed, toilet and sink, computer desk, television, and bicycle ergometer. Participants were instructed not to nap during the day as standard protocol for the calorimeter room. An infrared analyzer (ABB; Houston, TX) was used to measure CO2, whereas a paramagnetic analyzer was used to measure O2 (Siemens, Norcross, GA). Urine N was used to calculate 24-hour protein oxidation, with 1 g of urine N reflecting 6.25 g of oxidized protein. EE and substrate oxidation were calculated from oxygen consumption and the nonprotein respiratory quotient based on the equations of Jequier and colleagues (32). All measurements were taken as 1-minute averages and recorded to a data file.
Participants wore an accelerometer (Actigraph GT1M) during the entire inpatient stay, which was removed during cycling exercise, sleep, and showering. Data from the accelerometer allowed estimation of EE from activity. TDEE of noncalorimeter days was estimated using activity EE, previously measured RMR, and EE from both DIT and exercise as measured in the Cal room (values taken as an average of the 2 days spent in the room). Again, energy intake was determined from the diet plans created by the study dietician. If a participant left any study foods unconsumed, CTRC staff weighed the leftovers, and the value was reported to the dietician to allow accurate diet analyses. TDEE was measured on the Cal room days and estimated on the non–Cal room days as described previously.
Nitrogen Balance
To determine urinary nitrogen during the inpatient stay, 24-hour urine samples were collected beginning with the first void on Day 1, and two 10-mL aliquots from the total collection were frozen and stored for later analysis. The samples were analyzed for nitrogen by a chemiluminescent technique (33) using an Antek 7000 Elemental Nitrogen Analyzer (PAC, Houston, TX). NBAL was calculated as:
| (8) |
Because 6.25 g of protein contain an average of 1 g of nitrogen, N intake was calculated as (protein intake [grams]/6.25). Daily miscellaneous N losses and fecal N losses were estimated at 5 mg/kg body weight and 2 g, respectively (34). The 3-day averages of NBAL during PRO and CHO were compared to test the hypothesis that timing milk intake after aerobic exercise improves NBAL in older adults. Urinary creatinine was analyzed to measure completeness of urine collections. Collection was considered complete if daily creatinine was within 2 SDs of each participant’s mean value.
Posttesting
Within 1 week after the inpatient period, participants reported to CSU and underwent a dual-energy x-ray absorptiometry scan and a final weight measurement.
Statistical Analysis
NBAL is our primary outcome measure. In a previous study with 5 days of underfeeding (9), the authors reported a mean decrease of NBAL of 3.3 ± 1.0 g/24 hours (mean ± SD). From this result, we have conservatively estimated a difference of 1.5 g/24 hours for our participants and performed a two-tailed paired t test comparison of EXP with CON time periods with a significance (α) of .05 and a power (1 − β) of .95 (DSTPLAN, University of Texas M. D. Anderson Cancer Center, Department of Biomathematics). The resulting sample size is 8. Therefore, we assigned 10 participants per group to account for potential dropouts. NBAL data, Cal room oxidation data, and pre- and postbody weight/body composition measurements were analyzed using student’s paired t tests. Diet-related variables (free-living, lead-in, inpatient energy, and macronutrient intake) and longitudinal body weight were analyzed using one-way repeated measures analysis of variance. The Student Newman–Keuls post hoc test was used to determine where differences occurred. Variables were tested at a level of significance of p < .05. Data are presented as mean ± standard error of the mean unless indicated otherwise.
RESULTS
Energy Balance
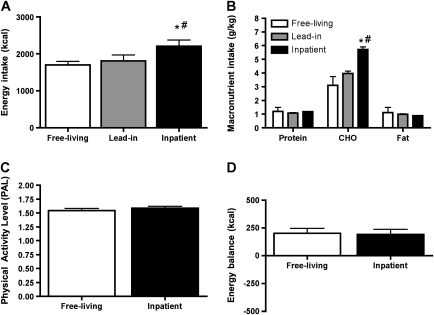
Total calories and macronutrient intake during free-living (from 3-day self-reported diet records), lead-in, and inpatient diets are shown in Figure 2A and B. A surplus of 160 kcal (in the form of CHO) was provided during the inpatient diets due to an error in the nutritional information for the Kool-Aid beverage. Free-living PAL and inpatient PAL were not significantly different (Figure 2C). During the inpatient stay, PAL was consistent across conditions (1.60 ± 0.04 in CHO and 1.61 ± 0.04 in PRO). During the lead-in diet, estimated mean daily energy balance was −103 ± 66.4 kcal. Participants were near energy balance during the inpatient stay, with a mean daily energy balance of 196 ± 21 kcal. Additionally, energy balance was consistent between the PRO and CHO trials (Figure 2D).
Figure 2.
Energy intake during free-living, lead-in, and inpatient diets (A); macronutrient intake during free-living, lead-in, and inpatient diets (B); activity level (PAL) during free-living and inpatient periods (C); and energy balance (energy in − energy out) during the inpatient period (D). Values are mean ± SEM, n = 9. *Different than free living (p < .001). #, Different than lead-in (p < .01).
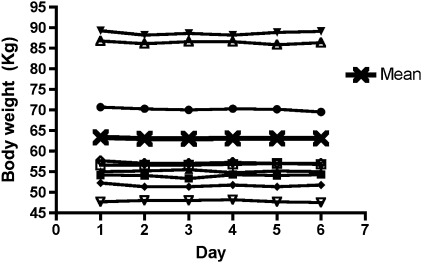
Body Weight
Body weight remained constant throughout the experimental period (Figure 3), indicating that participants remained in energy balance.
Figure 3.
Daily body weight during inpatient period. Legend indicates individual and group means (n = 9).
Exercise
Average workload of the exercise bouts was 44 ± 8.7 W. The average measured heart rate (Polar FS1, Lake Success, NY) during the cycling was 106 ± 8.5 beats per minute.
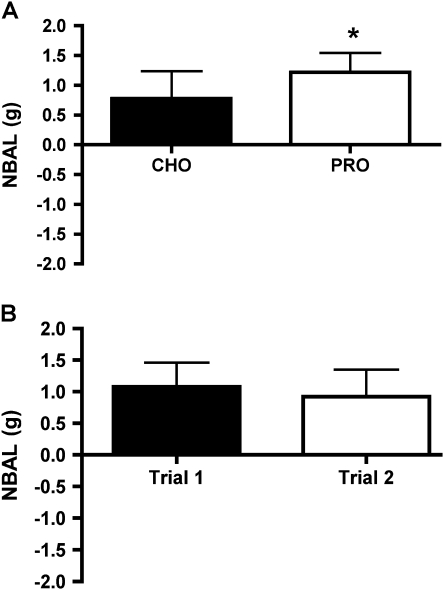
Nitrogen Balance
Three-day average NBAL was 57% higher during the PRO condition than the CHO condition (Figure 4A). In addition, there was no significant effect of Cal room stay versus non–Cal room stays (p = .18) or the order of the trials with the randomized first trial and second trials exhibiting similar NBAL (Figure 4B).
Figure 4.
Three-day individual and group mean nitrogen balance (NBAL) by treatment (A) and NBAL of Trial 1 (inpatient Days 1–3) versus Trial 2 (inpatient Days 4–6) independent of treatment (B). Values are means ± SEM, n = 9. *Different than CHO trial (p < .05).
DISCUSSION
In the current study, older participants completed two 3-day periods in which an isocaloric and isonitrogenous diet was consumed with only the timing of protein consumption in relation to an exercise bout differing between the 3-day periods. The primary finding of this study was that when protein was consumed immediately after easy to moderate-intensity exercise rather than earlier in the day, NBAL was more positive. These results indicate that the timing of protein nutrition is an important consideration in making protein intake recommendations in older individuals.
Nitrogen Balance
When chocolate milk (PRO) was consumed postexercise, NBAL was more positive than when the chocolate milk was taken in the morning and Kool-Aid (CHO) was consumed postexercise. Thus, there was greater potential for body protein accretion when PRO ingestion was timed immediately after exercise, which aligns with previous research on protein supplementation (18,35). However, body protein mass does not continue growing indefinitely, and NBAL adapts when a ceiling for body tissue mass is approached. For example, during conditions of strenuous exercise and a 30% energy surplus, NBAL becomes acutely positive and then diminishes over time (13). Still, based on the current study, there is greater potential for NBAL to remain positive when protein is consumed immediately after exercise. In support of this, when older women consumed a protein supplement after resistance exercise, about 0.9 kg fat free mass was gained over 24 weeks (18). Based on the results of the current study, consuming protein after aerobic-type exercise may help older individuals achieve a slightly positive nitrogen/protein balance. Whether this nutritional strategy results in long-term positive NBAL remains unclear.
Because no direct measurements of protein synthesis or breakdown were made during this study, it cannot be determined whether the positive protein balance resulted from increased synthesis, decreased breakdown, or a combination thereof. Also, conclusions cannot be drawn about where the protein accrual occurred. However, based on previous research, it is surmised that muscle protein synthesis (MPS) increased in response to the exercise stimulus (36), and the consumption of leucine-containing chocolate milk optimized this response by increasing MPS beyond the level reached with carbohydrate alone (37). It has previously been demonstrated with stable isotopic tracers that short-term positive muscle protein balance acutely stimulated by exercise and essential amino acid (EAA) ingestion reflects a 24-hour response (38).
Current Dietary Recommendations
The study diets followed USDA dietary recommendations for the percentage of total kilocalories that should come from each macronutrient: 45%–65% carbohydrate, 20%–35% fat, and 10%–35% protein (30). Inpatient protein intake was 1.2 g/kg, which is more than the 0.8 g/kg recommendation for older individuals but representative of a typical protein intake for this population (39). Indeed, mean self-reported habitual protein intake was 1.2 ± 0.1 g/kg and was maintained by the study diet. Thus, the lead-in period might have been unnecessary for some participants but allowed an adaptation period for those with higher or lower free-living protein intakes. Furthermore, our demonstration that the order of inpatient stay did not affect NBAL outcomes strengthens the argument that participants were habituated to the protein intake in our study.
Whether protein recommendations for older adults should be higher than 0.8 g/kg remains controversial (6–8). Regardless, MPS and accretion are blunted in older individuals when provided the same amount of EAAs as their younger counterparts (40,41). Adding a higher proportion of leucine allows MPS to be maximally stimulated in older individuals (42). Based on the EAA and leucine content of previous interventions that used milk (43) and whey protein (44), the chocolate milk beverage consumed by the participants in the current study contained ∼6.8 g EAAs with 1.7 g leucine. Although ∼7 g EAAs does not maximally stimulate synthesis in older individuals (41,45), the combination of a leucine-containing protein source with exercise was a sufficient stimulus to make protein balance more positive when diet provided adequate protein and energy. The inclusion of milk in dietary protein guidelines would be relatively simple because it is an easily attained protein source.
Importance of Timing Protein Intake After Exercise
Although exercise and protein nutrition have independent effects on muscle protein kinetics and NBAL, this study examined the combination of the two, with particular attention paid to the timing of the protein intake. The daily exercise bout completed by participants was 60 minutes of moderate-intensity (55% VO2max) cycling, fulfilling ACSM recommendations for cardiorespiratory exercise (23). The exercise was potent enough to provide an anabolic stimulus but attainable in terms of time, intensity, and expenditure. Free-living and inpatient PAL were similar and characteristic of previously studied older populations (46). Exercise at 55% of VO2max simulates a brisk walking pace and provides an effective anabolic stimulus, as aerobic exercise at only 40% of VO2max has been demonstrated to increase MPS (36). Over the long term, progressive endurance training programs stimulate some muscle hypertrophy (21), aligning with NBAL studies that indicate a nitrogen sparing and, thus, protein accreting effect with aerobic exercise while in energy balance (13,14). Also, because older individuals most commonly choose walking as a form of exercise (22), the proposed exercise intervention is a practical strategy for this population.
The influence of timed postexercise nutrition on NBAL was elucidated in a study of young female endurance athletes (24). Participants underwent two 7-day trials in which they increased their training volume and either consumed a mixed-meal beverage (POST) or a noncaloric placebo beverage (CON) after exercise. As in our study, diet was replicated during the two trials, so the timing of the beverage was the only difference. In Roy and colleagues, intake was not adjusted for the EE of exercise, so participants lost weight over both study periods. Even with the caloric deficit, there was a strong trend (p = .06) for NBAL (measured only on Day 5 and Day 6 of each trial) to be more positive in POST than in CON. Also, body mass declined by only 0.7 ± 0.2 kg in POST compared with 1.4 ± 0.4 in CON. This study shows that increased exercise levels combined with postexercise mixed-meal nutrition can influence NBAL to become more positive and preserve body mass, even in a small time frame (24).
Although carbohydrate alone after exercise increases MPS (47), protein and carbohydrate together augment the stimulus (48). The stimulatory effect of carbohydrate alone depends on the amount of carbohydrate provided (49). These data suggest that the unintended additional carbohydrate provided by the Kool-Aid beverage might have produced a more positive N balance than would have occurred if the Kool-Aid beverage were isocaloric with the chocolate milk. Despite the additional energy immediately postexercise during CHO, NBAL was still significantly more positive in PRO.
Experimental Considerations
For the purpose of this study, NBAL was the appropriate method because the goal was to integrate responses over a 3-day period rather than acute snapshots afforded by stable isotope studies, and it allowed for relatively free-living, yet controlled, conditions. However, it is acknowledged that measurement issues can sometimes lead to artificially positive NBAL values (50). For total accuracy, it is necessary to collect all forms of N excretion, including urine, feces, skin, sweat, and hair. Due to the impractical nature of such collections, fecal N and other miscellaneous N losses are often approximated (34), and previous studies have indicated that these miscellaneous losses do not change in studies similar to ours (51). On the N intake side, aliquots of food from the study diets can be analyzed for nitrogen content, which was not done in this study. However, the exact same food content was consumed in both conditions, and any measurement errors would be present during both conditions to the same degree. Therefore, even if the magnitude of positive NBAL was overestimated, the difference between the two conditions would remain constant.
Conclusion and Recommendation
Because the development of sarcopenia and loss of body protein happens gradually, an intervention with the potential to increase long-term nitrogen/protein balance was examined. We propose a rethinking of protein recommendations for older adults that goes beyond absolute quantity. Due to the potential for long-term positive protein balance and maintenance of muscle mass, the timing of a high-quality protein source following exercise should be included as part of current protein guidelines for the elderly. The accessible strategy of milk consumption after low- to moderate-intensity exercise provides a possible means for increasing nitrogen and protein balance in older individuals, a population that is at risk for frailty.
FUNDING
This work was funded by the Colorado Agriculture Experimental Station grant #COL00604, a career development support from National Institute on Aging K01AG031829-01 (B.F.M.), and the University of Colorado Denver Clinical and Translational Science Award (1UL1 RR025780).
Acknowledgments
The study participants are thanked for their exceptional dedication. Furthermore, we thank Archana Mande for designing the study diets and the University of Colorado-Denver CTRC staff for their help.
References
- 1.Fiatarone MA, O’Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe RR. Optimal nutrition, exercise, and hormonal therapy promote muscle anabolism in the elderly. J Am Coll Surg. 2006;202:176–180. doi: 10.1016/j.jamcollsurg.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Cohn SH, Vartsky D, Yasumura S, et al. Compartmental body composition based on total-body nitrogen, potassium, and calcium. Am J Physiol. 1980;239:E524–E530. doi: 10.1152/ajpendo.1980.239.6.E524. [DOI] [PubMed] [Google Scholar]
- 4.Evans WJ, Campbell WW. Sarcopenia and age-related changes in body composition and functional capacity. J Nutr. 1993;123:465–468. doi: 10.1093/jn/123.suppl_2.465. [DOI] [PubMed] [Google Scholar]
- 5.WHO. Protein and Amino Acid Requirements in Human Nutrition. Vol. 935. Geneva, Switzerland: WHO Press; 2007. pp. 1–235. [PubMed] [Google Scholar]
- 6.Campbell WW, Trappe TA, Wolfe RR, Evans WJ. The recommended dietary allowance for protein may not be adequate for older people to maintain skeletal muscle. J Gerontol A Med Sci. 2001;56:M373–M380. doi: 10.1093/gerona/56.6.m373. [DOI] [PubMed] [Google Scholar]
- 7.Gersovitz M, Motil K, Munro HN, Scrimshaw NS, Young VR. Human protein requirements: assessment of the adequacy of the current recommended dietary allowance for dietary protein in elderly men and women. Am J Clin Nutr. 1982;35:6–14. doi: 10.1093/ajcn/35.1.6. [DOI] [PubMed] [Google Scholar]
- 8.Campbell WW, Johnson CA, McCabe GP, Carnell NS. Dietary protein requirements of younger and older adults. Am J Clin Nutr. 2008;88:1322–1329. doi: 10.3945/ajcn.2008.26072. [DOI] [PubMed] [Google Scholar]
- 9.Friedlander AL, Braun B, Pollack M, et al. Three weeks of caloric restriction alters protein metabolism in normal-weight, young men. Am J Physiol Endocrinol Metab. 2005;289:E446–E455. doi: 10.1152/ajpendo.00001.2005. [DOI] [PubMed] [Google Scholar]
- 10.Calloway DH, Spector H. Nitrogen balance as related to caloric and protein intake in active young men. Am J Clin Nutr. 1954;2:405–412. doi: 10.1093/ajcn/2.6.405. [DOI] [PubMed] [Google Scholar]
- 11.Buttgereit F, Brand MD. A hierarchy of ATP-consuming processes in mammalian cells. Biochem J. 1995;312(Pt 1):163–167. doi: 10.1042/bj3120163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wakimoto P, Block G. Dietary intake, dietary patterns, and changes with age: an epidemiological perspective. J Gerontol A Biol Sci Med Sci. 2001;56, Spec No. 2:65–80. doi: 10.1093/gerona/56.suppl_2.65. [DOI] [PubMed] [Google Scholar]
- 13.Butterfield GE, Calloway DH. Physical activity improves protein utilization in young men. Br J Nutr. 1984;51:171–184. doi: 10.1079/bjn19840021. [DOI] [PubMed] [Google Scholar]
- 14.Todd KS, Butterfield GE, Calloway DH. Nitrogen balance in men with adequate and deficient energy intake at three levels of work. J Nutr. 1984;114:2107–2118. doi: 10.1093/jn/114.11.2107. [DOI] [PubMed] [Google Scholar]
- 15.Devlin JT, Brodsky I, Scrimgeour A, Fuller S, Bier DM. Amino acid metabolism after intense exercise. Am J Physiol. 1990;258:E249–E255. doi: 10.1152/ajpendo.1990.258.2.E249. [DOI] [PubMed] [Google Scholar]
- 16.Lamont LS, Patel DG, Kalhan SC. Leucine kinetics in endurance-trained humans. J Appl Physiol. 1990;69:1–6. doi: 10.1152/jappl.1990.69.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol. 2004;286:E92–E101. doi: 10.1152/ajpendo.00366.2003. [DOI] [PubMed] [Google Scholar]
- 18.Holm L, Olesen JL, Matsumoto K, et al. Protein-containing nutrient supplementation following strength training enhances the effect on muscle mass, strength, and bone formation in postmenopausal women. J Appl Physiol. 2008;105:274–281. doi: 10.1152/japplphysiol.00935.2007. [DOI] [PubMed] [Google Scholar]
- 19.Carraro F, Hartl WH, Stuart CA, Layman DK, Jahoor F, Wolfe RR. Whole body and plasma protein synthesis in exercise and recovery in human subjects. Am J Physiol. 1990;258:E821–E831. doi: 10.1152/ajpendo.1990.258.5.E821. [DOI] [PubMed] [Google Scholar]
- 20.Miller BF. Human muscle protein synthesis after physical activity and feeding. Exerc Sport Sci Rev. 2007;35:50–55. doi: 10.1097/jes.0b013e31803eac78. [DOI] [PubMed] [Google Scholar]
- 21.Harber MP, Konopka AR, Douglass MD, et al. Aerobic exercise training improves whole muscle and single myofiber size and function in older women. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1452–R1459. doi: 10.1152/ajpregu.00354.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McPhillips JB, Pellettera KM, Barrett-Connor E, Wingard DL, Criqui MH. Exercise patterns in a population of older adults. Am J Prev Med. 1989;5:65–72. [PubMed] [Google Scholar]
- 23.Whaley MH, Brubaker PH, Otto RM, Armstrong LE. ACSM’s Guidelines for Exercise Testing and Prescription. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 24.Roy BD, Luttmer K, Bosman MJ, Tarnopolsky MA. The influence of post-exercise macronutrient intake on energy balance and protein metabolism in active females participating in endurance training. Int J Sport Nutr Exerc Metab. 2002;12:172–188. doi: 10.1123/ijsnem.12.2.172. [DOI] [PubMed] [Google Scholar]
- 25.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pannemans DL, Westerterp KR. Energy expenditure, physical activity and basal metabolic rate of elderly subjects. Br J Nutr. 1995;73:571–581. doi: 10.1079/bjn19950059. [DOI] [PubMed] [Google Scholar]
- 27.Freedson PS, Melanson E, Sirard J. Calibration of the computer science and applications, inc. accelerometer. Med Sci Sports Exerc. 1998;30:777–781. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 28.Donahoo WT, Levine JA, Melanson EL. Variability in energy expenditure and its components. Current Opin Clin Nutr Metab Care. 2004;7:599–605. doi: 10.1097/00075197-200411000-00003. [DOI] [PubMed] [Google Scholar]
- 29.D’Alessio DA, Kavle EC, Mozzoli MA, et al. Thermic effect of food in lean and obese men. J Clin Invest. 1988;81:1781–1789. doi: 10.1172/JCI113520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.United States Department of Health and Human Services and United States Department of Agriculture. Dietary Guidelines Advisory Committee: Dietary Guidelines for Americans, 2005. 6th ed. Washington, DC: G.P.O.; 2005. [Google Scholar]
- 31.Melanson EL, Gozansky WS, Barry DW, Maclean PS, Grunwald GK, Hill JO. When energy balance is maintained, exercise does not induce negative fat balance in lean sedentary, obese sedentary, or lean endurance-trained individuals. J Appl Physiol. 2009;107:1847–1856. doi: 10.1152/japplphysiol.00958.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jequier E, Acheson K, Schutz Y. Assessment of energy expenditure and fuel utilization in man. Annu Rev Nutr. 1987;7:187–208. doi: 10.1146/annurev.nu.07.070187.001155. [DOI] [PubMed] [Google Scholar]
- 33.Skogerboe KJ, Labbe RF, Rettmer RL, Sundquist JP, Gargett AM. Chemiluminescent measurement of total urinary nitrogen for accurate calculation of nitrogen balance. Clin Chem. 1990;36:752–755. [PubMed] [Google Scholar]
- 34.Calloway DH, Odell AC, Margen S. Sweat and miscellaneous nitrogen losses in human balance studies. J Nutr. 1971;101:775–786. doi: 10.1093/jn/101.6.775. [DOI] [PubMed] [Google Scholar]
- 35.Esmarck B, Andersen JL, Olsen S, Richter EA, Mizuno M, Kjaer M. Timing of postexercise protein intake is important for muscle hypertrophy with resistance training in elderly humans. J Physiol. 2001;535:301–311. doi: 10.1111/j.1469-7793.2001.00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheffield-Moore M, Yeckel CW, Volpi E, et al. Postexercise protein metabolism in older and younger men following moderate-intensity aerobic exercise. Am J Physiol. 2004;287:E513–E522. doi: 10.1152/ajpendo.00334.2003. [DOI] [PubMed] [Google Scholar]
- 37.Koopman R, Wagenmakers AJ, Manders RJ, et al. Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects. Am Physiol. 2005;288:E645–E653. doi: 10.1152/ajpendo.00413.2004. [DOI] [PubMed] [Google Scholar]
- 38.Tipton KD, Borsheim E, Wolf SE, Sanford AP, Wolfe RR. Acute response of net muscle protein balance reflects 24-h balance after exercise and amino acid ingestion. Am J Physiol. 2003;284:E76–E89. doi: 10.1152/ajpendo.00234.2002. [DOI] [PubMed] [Google Scholar]
- 39.Millward DJ, Roberts SB. Protein requirements of older individuals. Nutr Res Rev. 1996;9:67–87. doi: 10.1079/NRR19960006. [DOI] [PubMed] [Google Scholar]
- 40.Cuthbertson D, Smith K, Babraj J, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. Faseb J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 41.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr. 2005;82:1065–1073. doi: 10.1093/ajcn/82.5.1065. [DOI] [PubMed] [Google Scholar]
- 42.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol. 2006;291:E381–E387. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- 43.Wilkinson SB, Tarnopolsky MA, Macdonald MJ, Macdonald JR, Armstrong D, Phillips SM. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am J Clin Nutr. 2007;85:1031–1040. doi: 10.1093/ajcn/85.4.1031. [DOI] [PubMed] [Google Scholar]
- 44.Paddon-Jones D, Sheffield-Moore M, Katsanos CS, Zhang XJ, Wolfe RR. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Exp Gerontol. 2006;41:215–219. doi: 10.1016/j.exger.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 45.Paddon-Jones D, Sheffield-Moore M, Zhang XJ, et al. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol. 2004;286:E321–E328. doi: 10.1152/ajpendo.00368.2003. [DOI] [PubMed] [Google Scholar]
- 46.Pannemans DL, Halliday D, Westerterp KR. Whole-body protein turnover in elderly men and women: responses to two protein intakes. Am J Clin Nutr. 1995;61:33–38. doi: 10.1093/ajcn/61.1.33. [DOI] [PubMed] [Google Scholar]
- 47.Borsheim E, Cree MG, Tipton KD, Elliott TA, Aarsland A, Wolfe RR. Effect of carbohydrate intake on net muscle protein synthesis during recovery from resistance exercise. J Appl Physiol. 2004;96:674–678. doi: 10.1152/japplphysiol.00333.2003. [DOI] [PubMed] [Google Scholar]
- 48.Howarth KR, Moreau NA, Phillips SM, Gibala MJ. Coingestion of protein with carbohydrate during recovery from endurance exercise stimulates skeletal muscle protein synthesis in humans. J Appl Physiol. 2009;106:1394–1402. doi: 10.1152/japplphysiol.90333.2008. [DOI] [PubMed] [Google Scholar]
- 49.Miller SL, Tipton KD, Chinkes DL, Wolf SE, Wolfe RR. Independent and combined effects of amino acids and glucose after resistance exercise. Med Sci Sports Exerc. 2003;35:449–455. doi: 10.1249/01.MSS.0000053910.63105.45. [DOI] [PubMed] [Google Scholar]
- 50.Hegsted DM. Balance studies. J Nutr. 1976;106:307–311. [Google Scholar]
- 51.Phillips SM, Atkinson SA, Tarnopolsky MA, MacDougall JD. Gender differences in leucine kinetics and nitrogen balance in endurance athletes. J Appl Physiol. 1993;75:2134–2141. doi: 10.1152/jappl.1993.75.5.2134. [DOI] [PubMed] [Google Scholar]