Abstract
Pancreatic cancer is the fourth leading cause of cancer death in the United States. The prognosis of the disease is very negative since the cancer has usually metastasized by the time a patient manifests symptoms. Although combination therapy shows some promise, new drugs to treat the disease are needed. Given our interest in finding new therapies for pancreatic cancer, we sought to determine whether the known cytotoxic activity of the batzellines extended to pancreatic cancer cell lines. The batzellines are pyrroloiminoquinones alkaloids obtained from the deep water Caribbean sponge Batzella sp (family Esperiopsidae, order Poecilosclerida). We show here that batzellines exhibit selective cytotoxicity towards the pancreatic cancer cell lines AsPC-1, Panc-1, BxPC-3, and MIA PaCa2 compared to the normal African green monkey cell line Vero. The batzellines cause cytotoxicity by inducing cell cycle arrest that is mediated by their ability to intercalate into DNA and/or inhibit Topoisomerase II activity. The cytotoxic abilities of Isobatzellines A and C against pancreatic cancer cell lines, their low toxicity against normal cells and their reported ability to be synthesized makes them interesting compounds with potential chemotherapeutic effects that may merit further research.
Keywords: Natural Products, Pancreatic Cancer, Drug Discovery, Mechanism of Action
Introduction
The American Cancer Society estimates that 37,170 new cases of pancreatic cancer were detected and about 33,370 deaths due to pancreatic cancer occurred in 2007, making it the fourth leading cause of cancer death in the United States (1). The grim prognosis of pancreatic cancer results from the fact that by the time a patient displays symptoms, the cancer has already metastasized (1). Treatment of pancreatic cancer involves surgery, radiation therapy, chemotherapy or a combination of the three. The current chemotherapy for advanced pancreatic cancer is gemcitabine, a drug that inhibits DNA synthesis (1–3). Another lead compound in the treatment of pancreatic cancer is 5-fluorouracil. Combination therapy using gemcitabine and 5-fluorouracil is only slightly more effective than gemcitabine alone (2). Furthermore, there is no good therapy to treat pancreatic tumors that become refractory to gemcitabine (3). The prognosis of pancreatic cancer patients under current treatments is poor, and new drugs to treat the disease are needed.
The batzellines are pyrroloiminoquinones alkaloids obtained from the deep water Caribbean sponge Batzella sp (family Esperiopsidae, order Poecilosclerida) (4–6) that exhibit antifungal activity as well as cytotoxicity against murine leukemia cells (4). They are structurally related to other marine compounds such as the makaluvamines and the discorhabdins that exhibit strong cytotoxicity against many different cancer cell lines(7). Furthermore, the makaluvamines exhibit potent anti-tumor activity and are currently under intense research as potential therapeutics. Makaluvamine A and C effectively diminished tumor load of nude mice injected with human ovarian cancer tumors(8) and makaluvamine H reduced tumor load of nude mice injected with nasopharyngeal cancer cells(9).
A comprehensive study of marine pyrroloiminoquinones in the NCI 60 cell line panel showed that the cytotoxic activity of these compounds extends to many human cancers (10). However, of the batzellines used in the present study, only isobatzelline C and isobatzelline E were included in that study and their mechanism of action was not ascertained. Furthermore, pancreatic cancer cell lines were not included in that panel (10). Given our interest in finding new therapies for pancreatic cancer, we sought to determine whether the cytotoxic activity of the batzellines extended to pancreatic cancer cell lines, and to determine the mechanism by which this cytotoxicity was induced.
Materials and Methods
Reagents
Batzelline A, Batzelline B, Isobatzelline A, Isobatzelline C, Isobatzelline D, Isobatzelline E, Secobatzelline A, and Secobatzelline B are part of the Harbor Branch Oceanographic Institute library of compounds isolated from marine organisms. Methanol and isopropanol were purchased from Fisher Scientific, Fair Lawn, NJ. Tris, Sodium Chloride, 3-[4,5-Dimethyl-2-thiazolyl]-2,5-diphenyl-2H-tetrazolium bromide (MTT), Bromophenol Blue, and 5-fluorouracil were purchased from Sigma Chemical Co., St. Louis, MO.
Cell Culture
The pancreatic carcinoma cell lines AsPC-1 (CRL-1682), Panc-1 (CRL-1469), BxPC-3 (CRL-1687), and MIA PaCa2 (CRL-1420), as well as the monkey cell line Vero(CRL-1587) were obtained from American Type Culture Collection (ATCC; Manassas, VA) grown, aliquotted and stored under liquid nitrogen. Aliquots of those cells were thawed and grown in RPMI-1640 with 10% fetal bovine serum, 0.11mg/ml sodium pyruvate, 4.5g/L D-glucose, 18mM HEPES buffer, 100U/ml penicillin G sodium, 100μg/ml streptomycin sulfate, 0.25μg/ml amphotericin B, 2mM L-glutamine and 50μg/ml gentamicin (Complete RPMI). Cells were maintained in a humidified incubator at 37°C and 5% CO2.
Cell Viability Assay (MTT)
6000 cells/well in 200μl of media were plated in a 96-well tissue culture plate. Cells were allowed to adhere for 24 hours. At the end of this incubation, 100 μl of medium were removed from each test well and 100 μl of medium containing treatment were added. Treatment consisted of serial dilution of the batzelline compounds, 5-fluorouracil, or media with methanol. The cells were then incubated for 72 hours at 37°C and 5% CO2. After this incubation, 75 μl of a 5mg/ml solution of Methylthiazoletetrazolium (MTT) were added to each well. The cells were then incubated for 3 hours at 37°C. At the end of this incubation, cells were centrifuged, the supernatant removed and 200 μl acidified isopropyl alcohol (1:500 solution of hydrochloric acid to isopropanol) were added to each well. The plates were then shaken for 15 minutes and the absorbencies of these solutions measured at 570 nm with a plate reader (NOVOstar, BMG Labtech Inc., Durham, NC).
Cell Cycle Analysis
AsPC-1 cells were trypsinized and split onto a 6-well plate where they were allowed to adhere for 24 hours. Cells were then treated for 48 hours with 5 or 25 μg/ml of the batzellines, the vehicle control, no treatment, or 100nM paclitaxel as a positive control. At the end of treatment, cells were trypsinized, pelleted, fixed with ice cold ethanol and incubated at −20°C for an hour. Cells were then washed and stained with propidium iodide in the presence of RNAse A for 30 minutes at 37°C. Cells were then transferred to glass tubes and analyzed in a flow cytometer.
Western Blotting
After treatment with the respective compounds at the appropriate concentration, cells were lysed using ice cold Radio Immunoprecipitation (RIPA) buffer containing phosphatase and protease inhibitors (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM PMSF, 1 mM EDTA, 5 μg/ml Aprotinin, 5 μg/ml Leupeptin, 1% Triton x-100, 1% Sodium deoxycholate, 0.1% SDS, 2mM Na3VO4, 1mM NaF). Their protein concentration was quantitated using the Lowry method (BCA assay, Pierce, Rockford IL). 35 μg of protein per sample were loaded and ran in a pre-cast denaturing SDS-PAGE gel (Biorad, Hercules CA), which was then transferred to a polyvinylidene difluoride (PVDF) membrane, and blocked with 5% non-fat milk in Tris-buffered saline containing Tween-20 (TBST) buffer for one hour at room temperature. After repeated washing, the membrane was incubated with primary antibody overnight at 4°C, followed by repeated washing and a one hour incubation with horseradish peroxidase conjugated secondary antibody at room temperature. Detection of proteins was done with chemiluminescence (Amersham Biosciences, Piscataway, NJ), followed by exposure to X-ray film. The X-ray film was subjected to a densitometry analysis. The density was normalized using the density of the actin for each sample. The antibodies used were caspase 3 (Cell Signaling Technologies, Beverly, MA) and anti-DFF45 (Sigma, St. Louis, MO). Secondary antibodies were obtained from Jackson Immunobiologicals (West Grove, PA)
Decatenation Reaction
The decatenation reaction was carried out using a kit purchased from TopoGEN Inc., Port Orange, FL (Catalog #1001-2) and following the manufacturer’s instructions. All reactions, with the exception of a positive control lacking topoisomerase II, contained 0.2μg kinetoplast DNA, 1U topoisomerase II, and buffer (4.5mM ATP, 0.45M Tris-HCl, 1.1M NaCl, 91mM MgCl2, and 4.5mM Dithiothreitol). In addition, some reactions contained either 5μg/ml or 25μg/ml of each of the batzelline compounds. Other reactions contained 100μM etoposide (TopoGEN, Pt Orange, FL), as a positive control. The vehicle control reaction for the batzellines contained 0.1 or 0.5μl of methanol (Fisher Scientific, Fair Lawn, NJ), while the one for the etoposide contained 1μl dimethylsulfoxide (DMSO, American Type Culture Collection, Rockville, MD). All reactions were brought to a final volume of 20μl with deionized water. The samples were then incubated at 37°C for 30 minutes in a water bath. The reactions were terminated with the addition of 4μl 5X stop buffer/loading dye (5% Sarkosyl, 0.125% bromophenol blue, 25% glycerol), and loaded into a 1% agarose gel containing 0.5μg/ml ethidium bromide. The samples were resolved through electrophoresis and viewed with an ultraviolet light box (Stratagene, La Jolla, CA). An image of the gel was obtained using Eagle Sight software (Stratagene, La Jolla, CA) and analyzed using Image J software (NIH, Bethesda, MD). Optical density measurements were plotted using Microsoft Excel.
DNA Intercalation Assay
This assay was performed as described by Dijoux et al (10). Briefly, 0.5 μg of genomic salmon testes DNA (Calbiochem, La Jolla, CA) along with 1.27 μM ethidium bromide were plated on a black 96-well plate and allowed to equilibrate for half an hour at room temperature. Then, 5 or 25μg/ml of the batzellines, the vehicle control methanol, 100 μM etoposide (negative control) or 1 μM doxorubicin (positive control) were added and allowed to equilibrate for half an hour at room temperature. Fluorescence was read at an excitation of 340 and an emission at 590 with a plate reader (NOVOstar, BMG Labtech Inc., Durham, NC).
Statistics
Statistical analysis of the data sets to determine mean, standard deviation, variance, and standard error of the mean was performed using Microsoft Excel. To determine whether inhibition of target expression by our compounds was statistically different from the untreated samples and/or the vehicle control, these two sets of data were compared using the Student’s T Test. A p value ≤ 0.05 was considered significant. Outliers were detected through the Grubbs Test.
Results
The isolation and chemical characterization of the batzellines used in this study were the subject of prior publications (4–6). They all share planar structures that contain a variant of a tricyclic pyrrolo[4,3,2-de]quinoline core ring structure (Figure 1). The presence or absence of distinct side chains is what differentiates most members of this family.
Figure 1. Structures of the Batzellines.
The cytotoxic effects of the batzellines were determined in four different pancreatic cell lines—Panc-1, AsPC-1, BxPC-3, and MIA-PaCa2—as well as in the Vero cell line, an epithelial cell line from the kidney tissue of an African green monkey. These pancreatic cancer lines are thought to represent the spectrum of pancreatic cancer’s metastatic potential. The results (Table 1) show that Isobatzelline A, Isobatzelline C, Isobatzelline D and Secobatzelline A exhibited cytotoxicity in all pancreatic cell lines with a minimum concentration of compound required to produce 50% cytotoxicity (IC50) of less than 10μM. Furthermore, their cytotoxic effects on AsPC-1, BxPC3 and MIA PaCa2 were far superior to 5-fluorouracil, a current treatment for pancreatic cancer. Interestingly, the batzellines exhibited much less cytotoxicity in the Vero cells, suggesting a preferential cytotoxic ability towards tumor cell lines.
Table 1.
Cytotoxicity of Batzellines in Pancreatic Cancer Cell Lines
| Compound | MW | Panc-1 | AsPC-1 | IC50 (μM) BxPC-3 | MIA PaCa2 | Vero |
|---|---|---|---|---|---|---|
| Batzelline A | 282.75 | >17.683 | >17.683 | >17.683 | >17.683 | >17.683 |
| Batzelline B | 268.75 | >18.607 | >18.607 | >18.607 | >18.607 | >18.607 |
| Isobatzelline A | 281.75 | 9.37±0.536 | 1.736±0.415 | 2.392±0.218 | 4.342±0.22 | >17.746 |
| Isobatzelline C | 235 | 9.978±0.384 | 1.723±0.168 | 1.311±0.185 | 2.343±.0977 | >21.277 |
| Isobatzelline D | 279 | 5.723±0.253 | 1.477±0.187 | 1.48±0.18 | 2.672±0.295 | 15.701±1.17 |
| Isobatzelline E | 233 | >21.459 | >21.459 | >21.459 | >21.459 | >21.459 |
| Secobatzelline A | 255 | 10.389±1.158 | 3.623±0.801 | 4.095±0.146 | 5.626±0.739 | 14.03±1.53 |
| Secobatzelline B | 256 | 17.372±.281 | >19.531 | >19.531 | >19.531 | >19.531 |
| 5-Fluorouracil | 130.1 | 5.734±0.682 | >38.432 | 5.56±1.64 | 8.314±0.357 | 5.193±0.289 |
| Gemcitabine* | 299.66 | 7.2 | >250 | >250 | 14.5 | NA |
values obtained from Li et al (14) NA: Not available. n=4 ± SE
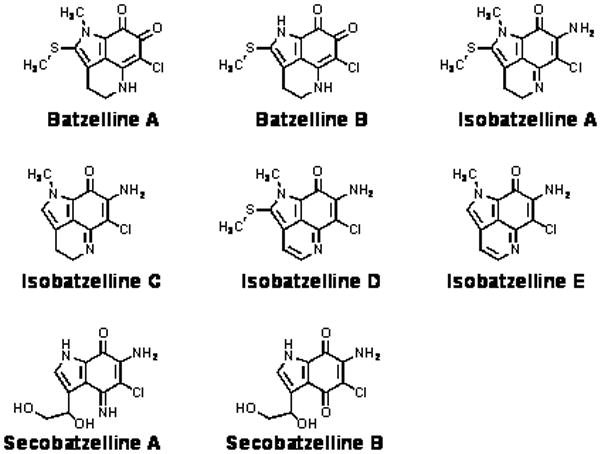
Next, we sought to determine the mechanism by which the batzellines were inducing cell death. Two arbitrary doses—5 and 25 μg/ml—were chosen for mechanism of action studies. These doses are higher than the IC50 representing ~20 and 100μM respectively of the batzellines (see Table 2 for exact conversions), but allowed us to recapitulate cell death in a shorter time. Since reports of structurally related marine pyrroloiminoquinones cite inhibition of topoisomerase II as their mechanism of action (8–10), we decided to test if the batzellines shared this property. Topoisomerase II is an enzyme that allows DNA strands to move through each other for synthesis of the lagging strand during DNA replication. Inhibiting this enzyme prevents synthesis of the lagging strand thus stopping DNA replication and inducing cell cycle arrest and apoptosis (11). Topoisomerase II inhibitors are widely used as chemotherapy in the treatment of cancer(12). Through an in vitro decatenation reaction of kinetoplast DNA we determined that Batzelline A, Batzelline B, Isobatzelline A, Isobatzelline E, and Secobatzelline A significantly inhibited topoisomerase II activity at a dose of 25μg/ml (Figure 2). No significant topoisomerase II inhibition was found at the lower dose of 5μg/ml (Data not shown). The best inhibitors of topoisomerase II activity were Isobatzelline E (95% inhibition) and Batzelline B (63% inhibition), which are not among the most cytotoxic of the batzellines at 72 hours of treatment. Isobatzelline A, C and D which exhibit the highest cytotoxicity against all cell lines tested did not inhibit topoisomerase II under the conditions tested, suggesting an alternative mechanism of action for those batzellines.
Table 2.
Summary of Activity of Batzellines on AsPC-1 cells at 25 μg/ml
| Compound | μM | % Inhibition topoisomeraseII | Cell cycle arrest | % DNA intercalation |
|---|---|---|---|---|
| Batzelline A | 88.42 | 58* | S phase | 18 |
| Batzelline B | 93.02 | 63* | S phase | 21 |
| Isobatzelline A | 88.73 | 36* | S phase | 54** |
| Isobatzelline C | 106.38 | 27 | S phase | 56** |
| Isobatzelline D | 89.61 | 26 | S phase | 47** |
| Isobatzelline E | 107.30 | 95* | G2 | 27 |
| Secobatzelline A | 98.04 | 61* | S phase | 34** |
| Secobatzelline B | 97.66 | 13 | S phase | 17 |
p≤0.05;
p≤0.005
Figure 2. Isobatzelline E, Batzelline B and Secobatzelline A Inhibit Topoisomerase II Activity.
a) Decatenation of kinetoplast DNA by topoisomerase II in the presence of the batzelline compounds, etoposide (positive control), methanol or DMSO (vehicle controls) was visualized through electrophoresis. b) Gels were subjected to densitometry analysis and the values of seven separate experiments were plotted using Microsoft Excel ± standard error of the mean and their statistical significance determined through the student T test.
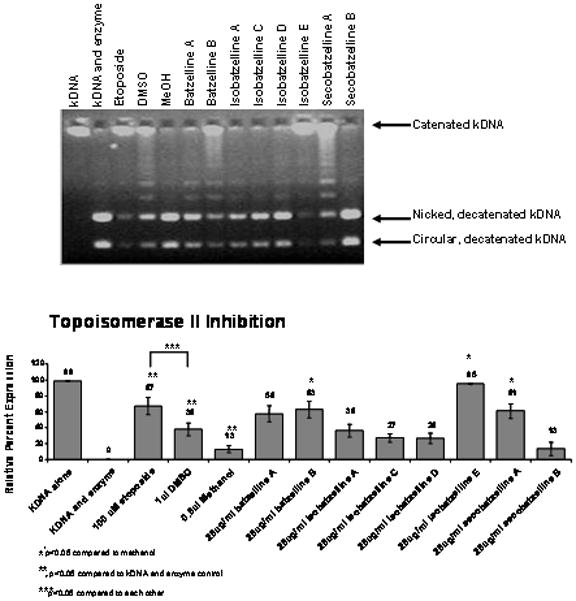
Since topoisomerase II inhibition leads to cell cycle arrest (13), cell cycle analysis was done on AsPC-1 cells treated with the batzellines to confirm our decatenation experiment. As shown on Figure 3, at the dose of 25 μg/ml all of the batzellines caused cell cycle arrest, but only Isobatzelline E gave us a very modest G2 arrest. The remainder of the batzellines tested caused cell cycle arrest at S phase suggesting that their mechanism of action is through interfering with DNA synthesis. Treatment with 5 μg/ml resulted in induction of S-phase cell cycle arrest by Batzelline A, Isobatzellines A, C, D and Secobatzelline A while Batzelline B, Isobatzelline E and Secobatzelline B did not have an effect at this dose (Data not shown).
Figure 3. Treatment of AsPC-1 Cells with the Batzellines induces Cell Cycle Arrest.
AsPC-1 cells were treated for 48 hours in the presence of methanol (vehicle control), media alone, 5 or 25 μg/ml of the batzellines, or 100 nM Paclitaxel (positive control) and subjected to cell cycle analysis by flow cytometry. Experiment was repeated 3 times and one representative experiment is shown.
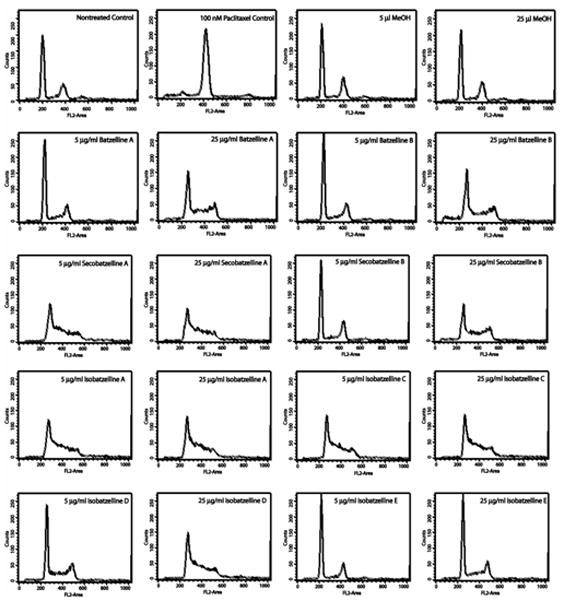
The cell cycle analysis and the assay for topoisomerase II inhibition suggested the existence of a different mechanism of action, while the structure of the batzellines suggested the strong possibility of their being DNA intercalators. Therefore, a fluorescence displacement assay was performed to test the ability of the batzellines to intercalate into DNA. Isobatzellines A, C, D and E and Secobatzelline A exhibited significant intercalation into DNA at both 5 and 25μg/ml (Figure 4). The best intercalators were isobatzelline A, C and D. Isobatzelline E and Secobatzelline A exhibited very modest intercalation.
Figure 4. Isobatzelline A, C, D, E and Secobatzelline A Have the Ability to Intercalate into DNA.
Salmon Testes DNA was allowed to interact with ethidium bromide for 30 minutes. At the end of this incubation, 5 or 25 μg/ml of the batzellines, methanol (vehicle control), 1 μM Doxorubicin (positive control) or 100 μM Etoposide were added to each reaction and allowed to interact for 30 minutes. The intercalation of ethidium bromide into DNA provides a fluorescent signal. Displacement of ethidium bromide by a DNA intercalator was detected by a decrease in the fluorescent intensity. The fluorescent signal was read in a plate reader under excitation 340nm and emission 590nm and the average of 3 experiments ± standard deviation is shown. Statistical significance was determined through the student T test.
Discussion
Similar to other marine pyrroloiminoquinones, the batzellines appear to act through more than one mechanism of action. The main mechanisms appear to be the inhibition of topoisomerase II and their ability to interfere with DNA synthesis by intercalating into DNA (Table 2). Interestingly, the most potent of the batzellines against pancreatic cancer cell lines appear to be those that act mainly through DNA intercalation. Thus, Isobatzellines A, C, and D along with Secobatzelline A show strong cytotoxicity against pancreatic cell lines with poor cytotoxicity against the Vero cell line. They all exhibit lower IC50s than published values for gemcitabine for all cell lines except Panc-1 (14). All of the batzellines have IC50s below that observed for 5-fluorouracil, a chemotherapeutic drug currently used in the treatment of pancreatic cancer, in at least 3 of the cell lines used in this study, and are about 3-fold less potent than 5-fluorouracil in killing normal cells. This preferential cytotoxicity towards pancreatic cancer cells makes them interesting compounds. Both Batzelline B and Isobatzelline E appear to act mainly through their ability to inhibit topoisomerase II, although both also have a very modest ability to intercalate into DNA. However, many known topoisomerase II inhibitors are also DNA intercalators(15). While they exhibit low cytotoxicity compared to the other batzellines, when given extra time to act (e.g. more than the 72 hours at which our IC50s are obtained), they can effectively kill approximately 70% AsPC-1 at a dose of 25μg/ml (Data not shown). Batzelline A and Secobatzelline B are the least active members of the family in the assays used in this study. They induced little cytotoxicity and showed no DNA intercalation or topoisomerase II inhibition, although they were able to induce cell cycle arrest at S phase. Their mechanism for causing cell cycle arrest remains to be ascertained.
The structure of the batzellines predicted their ability to intercalate into DNA. However, there appears to be no single functional group within the batzellines that is responsible for topoisomerase II inhibitory activity. The isobatzellines differ primarily in the side chain substituents, yet while Isobatzelline E is a very good topoisomerase II inhibitor, only Isobatzelline A shows modest inhibitory activity while Isobatzellines C and D show none. The second best inhibitor of topoisomerase II activity is secobatzelline A, while structurally related secobatzelline B shows no activity. Furthermore, no strong similarities exist between isobatzelline E and secobatzelline A. When compared to other marine pyrroloiminoquinones, both Isobatzelline A and Isobatzelline C are structurally similar to makaluvamine A, an excellent inhibitor of topoisomerase II activity (8), yet only isobatzelline A inhibits topoisomerase II. Isobatzelline E, our best inhibitor, is structurally similar to makaluvamine B, a poor topoisomerase II inhibitor (8).
The cytotoxic abilities of Isobatzellines A, C, D and Secobatzelline A against pancreatic cancer cell lines along with their low toxicity against normal cells makes them interesting compounds with potential chemotherapeutic effects that merit further research. In addition, although the limited availability of marine compounds is a major obstacle to their use as cancer therapeutics, syntheses of isobatzelline A and C have been reported (7), making their widespread use as chemotherapeutics a possibility.
Acknowledgments
Funding for this project was provided by NIH/NCI 1R01CA093455-01A1, the State of Florida Center of Excellence in Biomedical & Marine Biotechnology (COE-HRE07), the Gertrude E. Skelly Charitable Foundation and the Health Resources & Services Administration Center for Sustainable Use of Marine Resources (4C76HF00231-01-04).
References
- 1.All About Pancreatic Cancer. [electronic] 2008 Available from: http://www.cancer.org/docroot/CRI/CRI_2x.asp?sitearea=&dt=34.
- 2.Cardenes HR, Chiorean EG, Dewitt J, Schmidt M, Loehrer P. Locally advanced pancreatic cancer: current therapeutic approach. Oncologist. 2006 Jun;11(6):612–23. doi: 10.1634/theoncologist.11-6-612. [DOI] [PubMed] [Google Scholar]
- 3.Bergenfeldt M, Albertsson M. Current state of adjuvant therapy in resected pancreatic adenocarcinoma. Acta Oncol. 2006;45(2):124–35. doi: 10.1080/02841860600554238. [DOI] [PubMed] [Google Scholar]
- 4.Sun HH, Sakemi S, Burres N, McCarthy PJ. Isobatzellines A, B, C and D. Cytotoxic and Antifungal Pyrroloquinoline Alkaloids from the Marine Sponge Batzella sp. Journal of Organic Chemistry. 1990;55:4964–6. [Google Scholar]
- 5.Gunasekera SP, McCarthy PJ, Longley RE, Pomponi SA, Wright AE. Secobatzellines A and B, two new enzyme inhibitors from a deep-water Caribbean sponge of the genus Batzella. J Nat Prod. 1999 Aug;62(8):1208–11. doi: 10.1021/np990177a. [DOI] [PubMed] [Google Scholar]
- 6.Chang LC, Otero-Quintero S, Hooper JN, Bewley CA. Batzelline D and Isobatzelline E from the Indopacific Sponge Zyzzya fuliginosa. J Nat Prod. 2002;65:776–8. doi: 10.1021/np010581l. [DOI] [PubMed] [Google Scholar]
- 7.Antunes EM, Copp BR, Davies-Coleman MT, Samaai T. Pyrroloiminoquinone and related metabolites from marine sponges. Nat Prod Rep. 2005 Feb;22(1):62–72. doi: 10.1039/b407299p. [DOI] [PubMed] [Google Scholar]
- 8.Barrows LR, Radisky DC, Copp BR, et al. Makaluvamines, marine natural products, are active anti-cancer agents and DNA topo II inhibitors. Anticancer Drug Des. 1993 Oct;8(5):333–47. [PubMed] [Google Scholar]
- 9.Matsumoto SS, Haughey HM, Schmehl DM, et al. Makaluvamines vary in ability to induce dose-dependent DNA cleavage via topoisomerase II interaction. Anticancer Drugs. 1999 Jan;10(1):39–45. doi: 10.1097/00001813-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Dijoux MG, Schnabel PC, Hallock YF, et al. Antitumor activity and distribution of pyrroloiminoquinones in the sponge genus Zyzzya. Bioorg Med Chem. 2005 Nov 1;13(21):6035–44. doi: 10.1016/j.bmc.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 11.Kuo PL, Hsu YL, Kuo YC, Chang CH, Lin CC. The anti-proliferative inhibition of ellipticine in human breast mda-mb-231 cancer cells is through cell cycle arrest and apoptosis induction. Anticancer Drugs. 2005 Aug;16(7):789–95. doi: 10.1097/01.cad.0000171768.36317.93. [DOI] [PubMed] [Google Scholar]
- 12.Giles GI, Sharma RP. Topoisomerase enzymes as therapeutic targets for cancer chemotherapy. Med Chem. 2005 Jul;1(4):383–94. doi: 10.2174/1573406054368738. [DOI] [PubMed] [Google Scholar]
- 13.Larsen AK, Escargueil AE, Skladanowski A. From DNA damage to G2 arrest: the many roles of topoisomerase II. Prog Cell Cycle Res. 2003;5:295–300. [PubMed] [Google Scholar]
- 14.Li J, Zhu J, Melvin WS, Bekaii-Saab TS, Chen CS, Muscarella P. A structurally optimized celecoxib derivative inhibits human pancreatic cancer cell growth. J Gastrointest Surg. 2006 Feb;10(2):207–14. doi: 10.1016/j.gassur.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 15.McClendon AK, Osheroff N. DNA topoisomerase II, genotoxicity, and cancer. Mutat Res. 2007 Oct 1;623(1–2):83–97. doi: 10.1016/j.mrfmmm.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


