Abstract
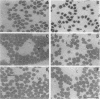
Genes for the MSP1a and MSP1b subunits of the Anaplasma marginale surface antigen complex MSP1 were previously cloned and expressed in Escherichia coli. We report here the localization of MSP1a and MSP1b polypeptides on the surface of recombinant E. coli by using a live cell indirect immunofluorescent antibody assay. Recombinant E. coli cells expressing the msp1 alpha gene or the msp1 beta gene encoding the MSP1a and MSP1b polypeptide subunits, respectively, were shown by a culture recovery adhesion assay and by direct microscopic examination to specifically adhere to bovine erythrocytes. This adhesion was more than additive when both genes were coexpressed in a single recombinant construct. Similarly, these recombinants hemagglutinated bovine erythrocytes in a microtiter hemagglutination assay. Inhibition of recombinant E. coli adhesion to bovine erythrocytes and hemagglutination inhibition were observed in the presence of homologous monospecific polyclonal antiserum raised against purified MSP1a or MSP1b polypeptide. These data suggest that the MSP1a and MSP1b polypeptides have functions as adhesins on A. marginale initial bodies, probably during erythrocyte invasion.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allred D. R., Hines S. A., Ahrens K. P. Isolate-specific parasite antigens of the Babesia bovis-infected erythrocyte surface. Mol Biochem Parasitol. 1993 Jul;60(1):121–132. doi: 10.1016/0166-6851(93)90035-v. [DOI] [PubMed] [Google Scholar]
- Allred D. R., McGuire T. C., Palmer G. H., Leib S. R., Harkins T. M., McElwain T. F., Barbet A. F. Molecular basis for surface antigen size polymorphisms and conservation of a neutralization-sensitive epitope in Anaplasma marginale. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3220–3224. doi: 10.1073/pnas.87.8.3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbet A. F., Allred D. R. The msp1 beta multigene family of Anaplasma marginale: nucleotide sequence analysis of an expressed copy. Infect Immun. 1991 Mar;59(3):971–976. doi: 10.1128/iai.59.3.971-976.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbet A. F., Palmer G. H., Myler P. J., McGuire T. C. Characterization of an immunoprotective protein complex of Anaplasma marginale by cloning and expression of the gene coding for polypeptide Am105L. Infect Immun. 1987 Oct;55(10):2428–2435. doi: 10.1128/iai.55.10.2428-2435.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barenkamp S. J., Leininger E. Cloning, expression, and DNA sequence analysis of genes encoding nontypeable Haemophilus influenzae high-molecular-weight surface-exposed proteins related to filamentous hemagglutinin of Bordetella pertussis. Infect Immun. 1992 Apr;60(4):1302–1313. doi: 10.1128/iai.60.4.1302-1313.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson L. A., Kar S., McLaughlin G., Ihler G. M. Entry of Bartonella bacilliformis into erythrocytes. Infect Immun. 1986 Nov;54(2):347–353. doi: 10.1128/iai.54.2.347-353.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsinghorst E. A., Baron L. S., Kopecko D. J. Penetration of human intestinal epithelial cells by Salmonella: molecular cloning and expression of Salmonella typhi invasion determinants in Escherichia coli. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5173–5177. doi: 10.1073/pnas.86.13.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriks I. S., Palmer G. H., McGuire T. C., Allred D. R., Barbet A. F. Detection and quantitation of Anaplasma marginale in carrier cattle by using a nucleic acid probe. J Clin Microbiol. 1989 Feb;27(2):279–284. doi: 10.1128/jcm.27.2.279-284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D. H., Kinden D. A., Buening G. M. Characterization of the inclusion limiting membrane of anaplasma marginale by immunoferritin labeling. Am J Vet Res. 1979 Jun;40(6):777–782. [PubMed] [Google Scholar]
- Goodger W. J., Carpenter T., Riemann H. Estimation of economic loss associated with anaplasmosis in California beef cattle. J Am Vet Med Assoc. 1979 Jun 15;174(12):1333–1336. [PubMed] [Google Scholar]
- Hultgren S. J., Abraham S., Caparon M., Falk P., St Geme J. W., 3rd, Normark S. Pilus and nonpilus bacterial adhesins: assembly and function in cell recognition. Cell. 1993 Jun 4;73(5):887–901. doi: 10.1016/0092-8674(93)90269-v. [DOI] [PubMed] [Google Scholar]
- Isberg R. R., Falkow S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature. 1985 Sep 19;317(6034):262–264. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- Isberg R. R., Leong J. M. Cultured mammalian cells attach to the invasin protein of Yersinia pseudotuberculosis. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6682–6686. doi: 10.1073/pnas.85.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul R., Roy K. L., Wenman W. M. Cloning, expression, and primary structure of a Chlamydia trachomatis binding protein. J Bacteriol. 1987 Nov;169(11):5152–5156. doi: 10.1128/jb.169.11.5152-5156.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieser S. T., Eriks I. S., Palmer G. H. Cyclic rickettsemia during persistent Anaplasma marginale infection of cattle. Infect Immun. 1990 Apr;58(4):1117–1119. doi: 10.1128/iai.58.4.1117-1119.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocan K. M., Teel K. D., Hair J. A. Demonstration of Anaplasma marginale Theiler in ticks by tick transmission, animal inoculation, and fluorescent antibody studies. Am J Vet Res. 1980 Feb;41(2):183–186. [PubMed] [Google Scholar]
- Kogure K., Simidu U., Taga N. A tentative direct microscopic method for counting living marine bacteria. Can J Microbiol. 1979 Mar;25(3):415–420. doi: 10.1139/m79-063. [DOI] [PubMed] [Google Scholar]
- McGarey D. J., Allred D. R. Characterization of hemagglutinating components on the Anaplasma marginale initial body surface and identification of possible adhesins. Infect Immun. 1994 Oct;62(10):4587–4593. doi: 10.1128/iai.62.10.4587-4593.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire T. C., Palmer G. H., Goff W. L., Johnson M. I., Davis W. C. Common and isolate-restricted antigens of Anaplasma marginale detected with monoclonal antibodies. Infect Immun. 1984 Sep;45(3):697–700. doi: 10.1128/iai.45.3.697-700.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Falkow S. Evidence for two genetic loci in Yersinia enterocolitica that can promote invasion of epithelial cells. Infect Immun. 1988 May;56(5):1242–1248. doi: 10.1128/iai.56.5.1242-1248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenegro-James S., James M. A., Benitez M. T., Leon E., Baek B. K., Guillen A. T. Efficacy of purified Anaplasma marginale initial bodies as a vaccine against anaplasmosis. Parasitol Res. 1991;77(2):93–101. doi: 10.1007/BF00935421. [DOI] [PubMed] [Google Scholar]
- Oberle S. M., Palmer G. H., Barbet A. F. Expression and immune recognition of the conserved MSP4 outer membrane protein of Anaplasma marginale. Infect Immun. 1993 Dec;61(12):5245–5251. doi: 10.1128/iai.61.12.5245-5251.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberle S. M., Palmer G. H., Barbet A. F., McGuire T. C. Molecular size variations in an immunoprotective protein complex among isolates of Anaplasma marginale. Infect Immun. 1988 Jun;56(6):1567–1573. doi: 10.1128/iai.56.6.1567-1573.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G. H., Barbet A. F., Cantor G. H., McGuire T. C. Immunization of cattle with the MSP-1 surface protein complex induces protection against a structurally variant Anaplasma marginale isolate. Infect Immun. 1989 Nov;57(11):3666–3669. doi: 10.1128/iai.57.11.3666-3669.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G. H., Barbet A. F., Davis W. C., McGuire T. C. Immunization with an isolate-common surface protein protects cattle against anaplasmosis. Science. 1986 Mar 14;231(4743):1299–1302. doi: 10.1126/science.3945825. [DOI] [PubMed] [Google Scholar]
- Palmer G. H., Barbet A. F., Kuttler K. L., McGuire T. C. Detection of an Anaplasma marginale common surface protein present in all stages of infection. J Clin Microbiol. 1986 Jun;23(6):1078–1083. doi: 10.1128/jcm.23.6.1078-1083.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G. H., Barbet A. F., Musoke A. J., Katende J. M., Rurangirwa F., Shkap V., Pipano E., Davis W. C., McGuire T. C. Recognition of conserved surface protein epitopes on Anaplasma centrale and Anaplasma marginale isolates from Israel, Kenya and the United States. Int J Parasitol. 1988 Feb;18(1):33–38. doi: 10.1016/0020-7519(88)90033-1. [DOI] [PubMed] [Google Scholar]
- Palmer G. H., Kocan K. M., Barron S. J., Hair J. A., Barbet A. F., Davis W. C., McGuire T. C. Presence of common antigens, including major surface protein epitopes, between the cattle (intraerythrocytic) and tick stages of Anaplasma marginale. Infect Immun. 1985 Dec;50(3):881–886. doi: 10.1128/iai.50.3.881-886.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G. H., McGuire T. C. Immune serum against Anaplasma marginale initial bodies neutralizes infectivity for cattle. J Immunol. 1984 Aug;133(2):1010–1015. [PubMed] [Google Scholar]
- Palmer G. H., Oberle S. M., Barbet A. F., Goff W. L., Davis W. C., McGuire T. C. Immunization of cattle with a 36-kilodalton surface protein induces protection against homologous and heterologous Anaplasma marginale challenge. Infect Immun. 1988 Jun;56(6):1526–1531. doi: 10.1128/iai.56.6.1526-1531.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G. H., Waghela S. D., Barbet A. F., Davis W. C., McGuire T. C. Characterization of a neutralization-sensitive epitope on the Am 105 surface protein of Anaplasma marginale. Int J Parasitol. 1987 Oct;17(7):1279–1285. doi: 10.1016/0020-7519(87)90093-2. [DOI] [PubMed] [Google Scholar]
- RISTIC M., WATRACH A. M. Anaplasmosis. VI. Studies and a hypothesis concerning the cycle of development of the causative agent. Am J Vet Res. 1963 Mar;24:267–277. [PubMed] [Google Scholar]
- Schmiel D. H., Knight S. T., Raulston J. E., Choong J., Davis C. H., Wyrick P. B. Recombinant Escherichia coli clones expressing Chlamydia trachomatis gene products attach to human endometrial epithelial cells. Infect Immun. 1991 Nov;59(11):4001–4012. doi: 10.1128/iai.59.11.4001-4012.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Tebele N., McGuire T. C., Palmer G. H. Induction of protective immunity by using Anaplasma marginale initial body membranes. Infect Immun. 1991 Sep;59(9):3199–3204. doi: 10.1128/iai.59.9.3199-3204.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega C. A., Buening G. M., Green T. J., Carson C. A. In vitro cultivation of Babesia bigemina. Am J Vet Res. 1985 Feb;46(2):416–420. [PubMed] [Google Scholar]
- Williams E. I., Jones W. E. Blood transfusions during patent bovine anaplasmosis. Am J Vet Res. 1968 Mar;29(3):703–710. [PubMed] [Google Scholar]
- Winkler H. H. Rickettsial hemolysis: adsorption, desorption, readsorption, and hemagglutination. Infect Immun. 1977 Sep;17(3):607–612. doi: 10.1128/iai.17.3.607-612.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaugg J. L., Stiller D., Coan M. E., Lincoln S. D. Transmission of Anaplasma marginale Theiler by males of Dermacentor andersoni Stiles fed on an Idaho field-infected, chronic carrier cow. Am J Vet Res. 1986 Oct;47(10):2269–2271. [PubMed] [Google Scholar]