Abstract
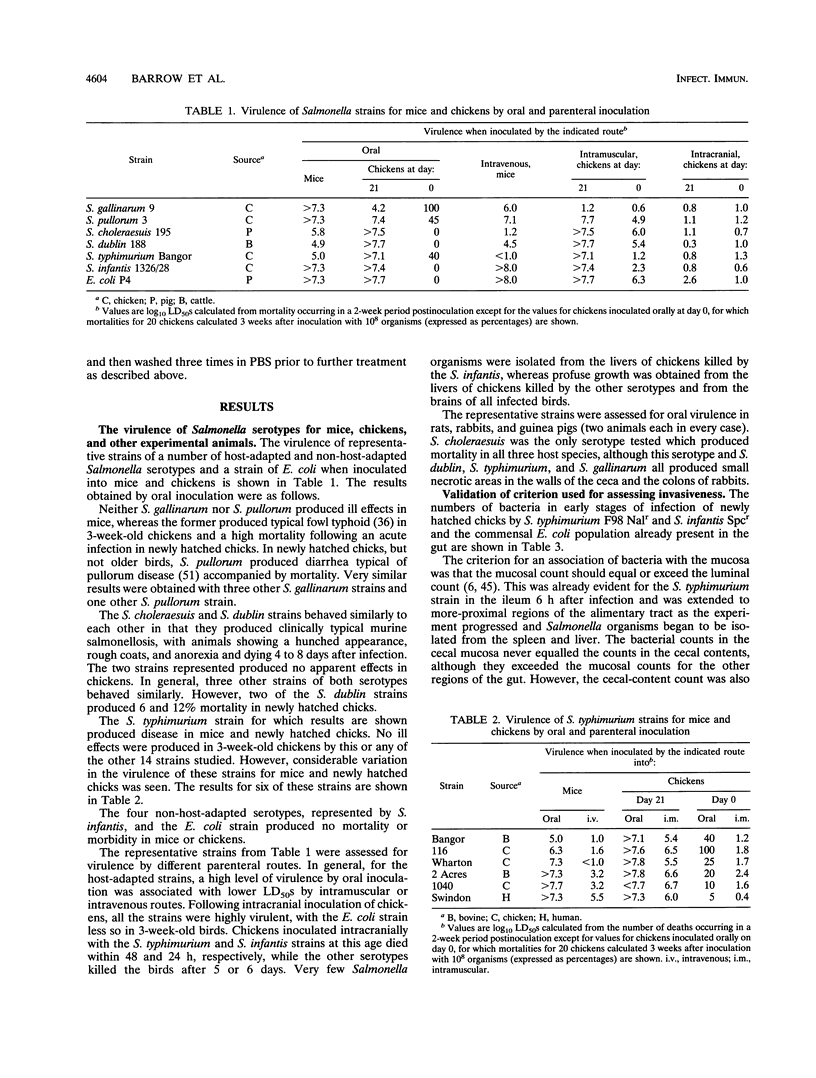
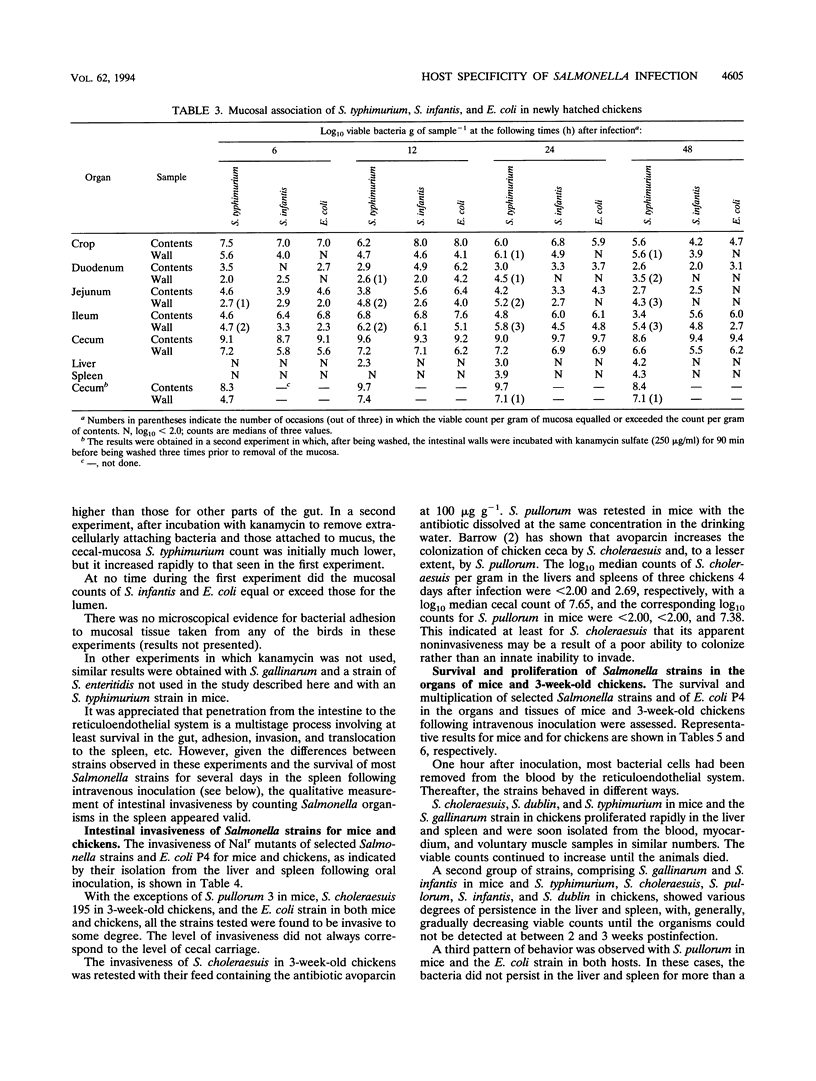
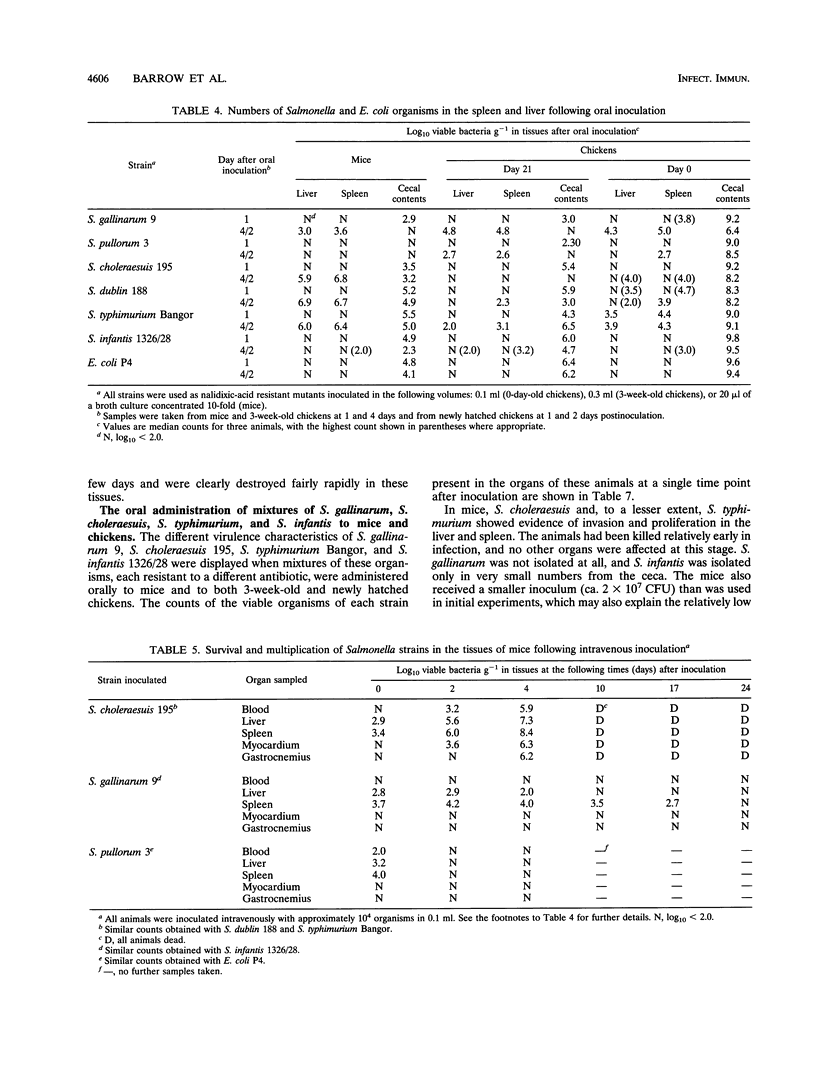
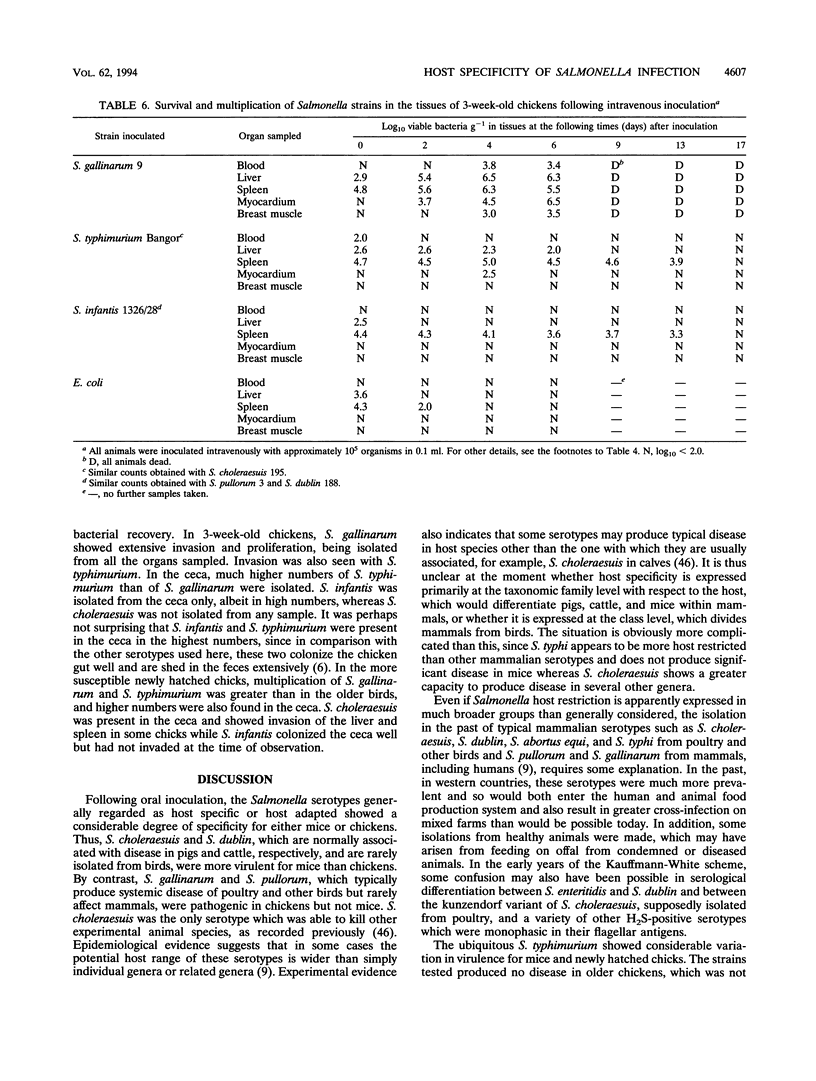
By experimental infection, host-specific Salmonella serotypes were shown to demonstrate specificities for chickens, mice, and other laboratory animals. Following oral inoculation, four strains of Salmonella gallinarum and two S. pullorum strains, isolated from diseased poultry, were more virulent for chickens than for mice. By contrast, four strains each of S. choleraesuis and S. dublin, isolated from diseased pigs and cattle, respectively, were more virulent for mice than for chickens. These results were also reflected in the degree of virulence expressed after parenteral inoculation. In addition, S. choleraesuis, but not other serotypes, killed rats, guinea pigs, and rabbits. S. typhimurium strains varied widely in their virulence, and some strains were virulent for both mice and chickens. Four other serotypes isolated from poultry or human food poisoning cases and a nonpathogenic Escherichia coli strain were much less virulent for both experimental host species. Most of the host-specific Salmonella serotypes studied were able to colonize the distal alimentary tract and invade the tissues in both mice and chickens to various degrees. There was, however, a greater difference in the ability to survive and multiply in the visceral organs, particularly the spleen and the liver, once invasion had occurred which correlated with the virulence for the host species involved.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmeyer R. M., McNern J. K., Bossio J. C., Rosenshine I., Finlay B. B., Galán J. E. Cloning and molecular characterization of a gene involved in Salmonella adherence and invasion of cultured epithelial cells. Mol Microbiol. 1993 Jan;7(1):89–98. doi: 10.1111/j.1365-2958.1993.tb01100.x. [DOI] [PubMed] [Google Scholar]
- Barrow P. A. Further observations on the effect of feeding diets containing avoparcin on the excretion of salmonellas by experimentally infected chickens. Epidemiol Infect. 1989 Apr;102(2):239–252. doi: 10.1017/s0950268800029915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow P. A., Huggins M. B., Lovell M. A., Simpson J. M. Observations on the pathogenesis of experimental Salmonella typhimurium infection in chickens. Res Vet Sci. 1987 Mar;42(2):194–199. [PubMed] [Google Scholar]
- Barrow P. A., Lovell M. A. Functional homology of virulence plasmids in Salmonella gallinarum, S. pullorum, and S. typhimurium. Infect Immun. 1989 Oct;57(10):3136–3141. doi: 10.1128/iai.57.10.3136-3141.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow P. A., Lovell M. A. The association between a large molecular mass plasmid and virulence in a strain of Salmonella pullorum. J Gen Microbiol. 1988 Aug;134(8):2307–2316. doi: 10.1099/00221287-134-8-2307. [DOI] [PubMed] [Google Scholar]
- Barrow P. A., Simpson J. M., Lovell M. A., Binns M. M. Contribution of Salmonella gallinarum large plasmid toward virulence in fowl typhoid. Infect Immun. 1987 Feb;55(2):388–392. doi: 10.1128/iai.55.2.388-392.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumstead N., Barrow P. A. Genetics of resistance to Salmonella typhimurium in newly hatched chicks. Br Poult Sci. 1988 Sep;29(3):521–529. doi: 10.1080/00071668808417078. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B., Blanden R. V. Infection-immunity in experimental salmonellosis. J Exp Med. 1966 Oct 1;124(4):601–619. doi: 10.1084/jem.124.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. Serum mediated killing of three group D salmonellas. J Gen Microbiol. 1967 Feb;46(2):247–253. doi: 10.1099/00221287-46-2-247. [DOI] [PubMed] [Google Scholar]
- Dunlap N. E., Benjamin W. H., Jr, Berry A. K., Eldridge J. H., Briles D. E. A 'safe-site' for Salmonella typhimurium is within splenic polymorphonuclear cells. Microb Pathog. 1992 Sep;13(3):181–190. doi: 10.1016/0882-4010(92)90019-k. [DOI] [PubMed] [Google Scholar]
- Elsinghorst E. A., Baron L. S., Kopecko D. J. Penetration of human intestinal epithelial cells by Salmonella: molecular cloning and expression of Salmonella typhi invasion determinants in Escherichia coli. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5173–5177. doi: 10.1073/pnas.86.13.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Gumbiner B., Falkow S. Penetration of Salmonella through a polarized Madin-Darby canine kidney epithelial cell monolayer. J Cell Biol. 1988 Jul;107(1):221–230. doi: 10.1083/jcb.107.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON E. A. Salmonellosis in calves. Vet Rec. 1961 Dec 2;73:1284–1296. [PubMed] [Google Scholar]
- Galán J. E., Curtiss R., 3rd Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6383–6387. doi: 10.1073/pnas.86.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán J. E., Curtiss R., 3rd Distribution of the invA, -B, -C, and -D genes of Salmonella typhimurium among other Salmonella serovars: invA mutants of Salmonella typhi are deficient for entry into mammalian cells. Infect Immun. 1991 Sep;59(9):2901–2908. doi: 10.1128/iai.59.9.2901-2908.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán J. E., Ginocchio C., Costeas P. Molecular and functional characterization of the Salmonella invasion gene invA: homology of InvA to members of a new protein family. J Bacteriol. 1992 Jul;174(13):4338–4349. doi: 10.1128/jb.174.13.4338-4349.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., Curtiss R., 3rd Plasmid-associated virulence of Salmonella typhimurium. Infect Immun. 1987 Dec;55(12):2891–2901. doi: 10.1128/iai.55.12.2891-2901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., Danbara H., Guiney D. G., Lax A. J., Norel F., Rhen M. Molecular analysis of spv virulence genes of the Salmonella virulence plasmids. Mol Microbiol. 1993 Mar;7(6):825–830. doi: 10.1111/j.1365-2958.1993.tb01172.x. [DOI] [PubMed] [Google Scholar]
- Halavatkar H., Barrow P. A. The role of a 54-kb plasmid in the virulence of strains of Salmonella enteritidis of phage type 4 for chickens and mice. J Med Microbiol. 1993 Mar;38(3):171–176. doi: 10.1099/00222615-38-3-171. [DOI] [PubMed] [Google Scholar]
- Karthigasu K., Reade P. C., Jenkin C. R. The functional development of the reticulo-endothelial system. 3. The bactericidal capacity of fixed macrophages of foetal and neonatal chicks and rats. Immunology. 1965 Jul;9(1):67–73. [PMC free article] [PubMed] [Google Scholar]
- Manning E. J., Baird G. D., Jones P. W. The role of plasmid genes in the pathogenicity of Salmonella dublin. J Med Microbiol. 1986 May;21(3):239–243. doi: 10.1099/00222615-21-3-239. [DOI] [PubMed] [Google Scholar]
- Nnalue N. A., Shnyra A., Hultenby K., Lindberg A. A. Salmonella choleraesuis and Salmonella typhimurium associated with liver cells after intravenous inoculation of rats are localized mainly in Kupffer cells and multiply intracellularly. Infect Immun. 1992 Jul;60(7):2758–2768. doi: 10.1128/iai.60.7.2758-2768.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH H. W. Observations on experimental fowl typhoid. J Comp Pathol. 1955 Jan;65(1):37–54. doi: 10.1016/s0368-1742(55)80005-7. [DOI] [PubMed] [Google Scholar]
- SMITH H. W. THE IMMUNIZATION OF MICE, CALVES AND PIGS AGAINST SALMONELLA DUBLIN AND SALMONELLA CHOLERAE-SUIS INFECTIONS. J Hyg (Lond) 1965 Mar;63:117–135. doi: 10.1017/s0022172400045022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selander R. K., Smith N. H., Li J., Beltran P., Ferris K. E., Kopecko D. J., Rubin F. A. Molecular evolutionary genetics of the cattle-adapted serovar Salmonella dublin. J Bacteriol. 1992 Jun;174(11):3587–3592. doi: 10.1128/jb.174.11.3587-3592.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skamene E., Gros P., Forget A., Kongshavn P. A., St Charles C., Taylor B. A. Genetic regulation of resistance to intracellular pathogens. Nature. 1982 Jun 10;297(5866):506–509. doi: 10.1038/297506a0. [DOI] [PubMed] [Google Scholar]
- Small R. G., Sharp J. C. A milk-borne outbreak due to Salmonella dublin. J Hyg (Lond) 1979 Feb;82(1):95–100. doi: 10.1017/s0022172400025511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W., Halls S. The production of oedema disease and diarrhoea in weaned pigs by the oral administration of Escherichia coli: factors that influence the course of the experimental disease. J Med Microbiol. 1968 Aug;1(1):45–59. doi: 10.1099/00222615-1-1-45. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Halls S. The simultaneous oral administration of Salmonella dublin, S. typhimurium and S. choleraesuis to calves and other animals. J Med Microbiol. 1968 Nov;1(2):203–209. doi: 10.1099/00222615-1-2-203. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Huggins M. B. Acquisition of genes from an O18:K1:H7 ColV+ strain of Escherichia coli renders intracranially-inoculated E. coli K12 highly virulent for chickens, ducks and guinea-pigs but not mice. J Hyg (Lond) 1985 Oct;95(2):363–374. doi: 10.1017/s0022172400062781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W., Jones J. E. Observations on experimental oral infection with Salmonella dublin in calves and Salmonella choleraesuis in pigs. J Pathol Bacteriol. 1967 Jan;93(1):141–156. doi: 10.1002/path.1700930114. [DOI] [PubMed] [Google Scholar]
- Stone B. J., Garcia C. M., Badger J. L., Hassett T., Smith R. I., Miller V. L. Identification of novel loci affecting entry of Salmonella enteritidis into eukaryotic cells. J Bacteriol. 1992 Jun;174(12):3945–3952. doi: 10.1128/jb.174.12.3945-3952.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. N., Bied J. M., Munro J. S., Feldman R. A. Salmonella dublin infections in the United States, 1979-1980. J Infect Dis. 1982 Sep;146(3):322–327. doi: 10.1093/infdis/146.3.322. [DOI] [PubMed] [Google Scholar]
- Valtonen V. V. Mouse virulence of Salmonella strains: the effect of different smooth-type O side-chains. J Gen Microbiol. 1970 Dec;64(3):255–268. doi: 10.1099/00221287-64-3-255. [DOI] [PubMed] [Google Scholar]
- Williams Smith H., Tucker J. F. The virulence of salmonella strains for chickens: their excretion by infected chickens. J Hyg (Lond) 1980 Jun;84(3):479–488. doi: 10.1017/s0022172400027017. [DOI] [PMC free article] [PubMed] [Google Scholar]



