Summary
CD4+ T cells with immune regulatory function can be either FOXP3+ or FOXP3−. We have previously shown that priming of naturally occurring TCR-peptide-reactive regulatory CD4+FOXP3− T cells (Treg) specifically controls Vβ8.2+CD4+ T cells mediating experimental autoimmune encephalomyelitis (EAE). However, the mechanism by which these Treg are primed to recognize their cognate antigenic determinant, which is derived from the TCRVβ8.2-chain, is not known. In this study we show that antigen presenting cells (APC) derived from splenocytes of naïve mice are able to stimulate cloned CD4+ Treg in the absence of exogenous antigen, and their stimulation capacity is augmented during EAE. Among the APC populations DC were the most efficient in stimulating the Treg. Stimulation of CD4+ Treg was dependent upon processing and presentation of TCR peptides from ingested Vβ8.2TCR+ CD4+ T cells. Additionally, dendritic cells pulsed with TCR peptide or apoptotic Vβ8.2+ T cells are able to prime Treg in vivo and mediate protection from disease in a CD8-dependent fashion. These data highlight a novel mechanism for the priming of CD4+ Treg by CD8α+ DC, and suggest a pathway that can be exploited to prime antigen-specific regulation of T cell-mediated inflammatory disease.
Keywords: Dendritic cells, TCR, EAE/MS, Antigen Presentation/Processing, Tolerance/Suppression/Anergy
Introduction
Suppression of autoaggressive T cell responses can be mediated by several subsets of lymphocytes; for example, CD4+CD25+FOXP3+, CD4+CD25+FOXP3−, CD8αα+TCRαβ+ and NKT subsets of T cells [1-5]. The priming of regulatory CD4+ T cells has been shown to play a central role in the down-regulation of experimental autoimmune diseases; including experimental autoimmune encephalomyelitis (EAE), murine inflammatory colitis, and diabetes in nonobese diabetic mice [2, 6-8]. Additionally regulatory CD4+ T cells have been isolated from humans and correlated with protection against autoimmune disease [7, 9-11]. Naturally occurring CD4+CD25+FOXP3+ Treg have received much attention, demonstrating regulatory function in humans and rodents [1]. Their growth and development is dependent on FOXP3 expression, IL-2 and TGF-β, but they do not produce IL-2 and reside in a hyporesponsive state. CD4+CD25+FOXP3+ Treg can mediate regulation in a cell contact dependent manner and involve cell surface molecules such CTLA-4 and TGF-β [12, 13]. In addition to naturally occurring populations, CD4+ Treg can also be induced. For example, IL-10-producing Tr1 cells and TGF-β-producing Th3 cells can be induced to mediate bystander suppression [7, 14].
We have previously characterized a distinct subset of naturally-induced regulatory CD4+ T cells that target autoaggressive Vβ8.2+ T cell responses for down-regulation and protect against autoimmune disease, such as EAE and collagen-induced arthritis [6, 15-17]. Treg cell lines and clones were successfully generated that displayed reactivity towards a peptide (B5) derived from the conserved framework 3 region of the TCR Vβ8.2 chain [6, 16, 17]. We used these T cell lines and clones throughout this study and will be referred to as CD4+ Treg in this manuscript [3]. We have shown that these Treg arise spontaneously during the recovery phase of MBP-induced EAE in the H-2u mouse [6] and during arthritis in the H-2q mouse [16]. Furthermore, clinical disease is exacerbated and recovery hindered after the depletion or inactivation of TCR peptide-reactive CD4+ Treg [17]. Additionally, we have shown CD4+ Treg function in unison with CD8αα+TCRαβ+ Treg, in a mechanism that results in the cytotoxic killing of disease-mediating Vβ8.2+ T cells [3, 15, 18, 19]. Upon activation, CD4+ Treg provide “help” for the CD8αα+TCRαβ+ Treg effector response to proceed [3]. However, little is known regarding how regulatory CD4+ T cells are naturally primed to initiate immunosuppression mechanisms. Here we delineate a novel mechanism involved in the priming of an antigen-specific CD4+ Treg population.
During active EAE an increased frequency of peripheral TCRVβ8.2+ T cells have been detected to be undergoing apoptotic cell death [20, 21]. Professional antigen presenting cells, such as dendritic cells (DC) and macrophages, are adept at ingesting apoptotic cells for both clearance purposes and the presentation of antigen material to the adaptive immune system [22]. It has been demonstrated that following ingestion of apoptotic B cells, DC can process and present antigens derived from the dying cell’s B cell receptor via MHC class II pathway to prime CD4+ T cells [23]. We have recently described a novel mechanism by which immature bone marrow-derived DC can ingest apoptotic Vβ8.2+ T cells, process antigen through the endosomal pathway, and present a Vβ8.2TCR-derived peptide to cross-prime the non-classical MHC class I molecule, Qa-1-restricted CD8αα+TCRαβ+ Treg [24]. Once primed, CD8αα+TCRαβ+ Treg target only activated Vβ8.2+ T cells for killing.
Here, we have examined whether a similar pathway involving dendritic cell presentation of TCR peptides operates in the priming of MHC Class II-restricted CD4+ Treg. We show that the splenocyte population in the H-2u mouse contains antigen presenting cells capable of specifically stimulating cloned antigen-reactive CD4+FOXP3− Treg. Our data indicate dendritic cells as the most potent APC for the activation of these Treg. DC pulsed with apoptotic Vβ8.2+ T cells prime a CD4+ Treg response in vivo and in vitro. Furthermore, adoptively transferred DC loaded with TCRVβ8.2 peptide protect H-2u mice from MBP-induced EAE. These data delineate a novel mechanism by which antigen-reactive CD4+ Treg are primed naturally to assist in the negative feedback immune regulation of T cell-mediated autoimmune disease. These findings also have implications in the design of dendritic cell-based therapies against inflammatory disease.
Results
Antigen presenting cells derived from the spleen of naïve H-2u mice can activate cloned CD4+ Treg
Spontaneous expansion of I-Au-restricted CD4+ Treg during recovery from MBPAc1-9-induced EAE [6] suggest that antigen-presenting cells may be presenting TCR-derived antigens. First, we determined whether the splenocyte population in the naïve B10.PL (H-2u) mouse contained APC that could stimulate the conserved FR-3 region TCRVβ8.2-peptide-reactive, I-Au-restricted CD4+ Treg clone B5.2 [6]. B5.2 CD4+ T cell clones were incubated in vitro with an increasing number (10 – 1000 × 103) of irradiated splenocytes from naïve B10.PL mice, and proliferation was measured after 72 hours incubation (Fig.1a). In parallel we analyzed the response of the CD4+ T cell clone (B4.2) that is reactive to another conserved region peptide, B4, from the TCRVβ8.2 chain. B4-reactive CD4+ T cells do not spontaneously expand during EAE disease and do not regulate EAE upon adoptive transfer [6]. In addition, L-cell transfectants expressing the I-Au Class II MHC molecules were used in the place of splenocytes to control for non-specific I-Au -reactivity. Data presented in Fig1A. show that co-culture with high numbers of irradiated splenocytes (0.1 - 1 × 106) induces significant proliferation in the B5.2 CD4+ T cells. Specificity of the B5.2 T cell response was confirmed by the failure of the B4.2 CD4+ T cell clone to proliferate. Neither clone proliferated on incubation with the I-Au-expressing L-cell transfectants. These transfectants express functional I-Au molecules as is evidenced by their ability to stimulate B5.2 T cell clones (Stimulation index from 8.5 to 11.2) upon exogenous addition of peptide B5 to the co-culture [Data not shown and 25]. Results suggest that the TCR peptide determinant within B5, but not B4, is being naturally presented by APC in the splenocyte population.
Figure 1. Stimulation of the CD4+ Treg clone B5.2 by syngenic antigen presenting cells isolated from naïve mice, and from mice with ongoing EAE.
(A) 20 × 103 TCR peptide-B5-reactive (B5.2) or TCR peptide-B4-reactive (B4.2) T cell clones were co-cultured with the indicated numbers of irradiated splenocytes (Spl) derived from naïve B10.PL mice or with L-cell transfectants expressing I-Au (L cell). T-cell proliferation was measured by 3H-thymidine incorporation (counts per minute (CPM)) after 72 h. (B) APC, including macrophages (Mφ), B cells and DC, were purified from the spleens of naïve mice. (C) DC purified from the draining lymph nodes (DLN) of naïve mice or those displaying EAE. Purified APC (both (B) and (C)) were co-cultured with 20 × 103 B5.2 T cell clones at the indicated concentrations. IFN-γ levels in the culture supernatants were measured by ELISA at 48 hrs. Data show mean ± SD (n=3) and are representative of four independent experiments.
Next we identified the APC population that was most efficient in stimulating the B5.2 CD4+ T cell clone. B cells, macrophages and dendritic cells were enriched from spleens derived from naïve B10.PL mice using magnetic beads. For examining the B5.2 T cell stimulation by isolated APC subsets, analysis of IFN-γ-secretion was performed as it was found to be more sensitive than a proliferation readout. The enriched APC populations (1 - 100 × 103) were co-cultured with the B5.2 CD4+ T cell clone. Fig1B. shows that dendritic cells were the most efficient stimulators of the B5.2 CD4+ T cells, with significant IFN-γ production (850 pg/ml) detected at a concentration of 30 × 103 DC/well. It is also clear that at higher numbers, macrophages could stimulate the B5.2 CD4+ T cell clones. However, as macrophages were enriched using anti-11b beads, it was possible that CD11b+ myeloid DC were contaminating the macrophage population and stimulating the B5.2 CD4+ Treg. However, since only a minor population (less than 5 %) of purified CD11b+ cells were CD11c+ it is likely that macrophages are also able to stimulate CD4+ Treg, albeit less efficiently. B cells could not stimulate B5.2 CD4+ T cell clones. These data identify DC as the most likely candidate for the physiological processing and presentation of TCR-derived peptide, and priming TCR-reactive CD4+ Treg in vivo.
Stimulation of CD4+ Treg is augmented if dendritic cells are derived from the draining cervical lymph nodes during active disease
Large numbers of Vβ8.2+ T cells undergo apoptosis in the CNS during the course of EAE [20]. This suggests that an enhanced number of apoptotic Vβ8.2+ T cells will be engulfed by the DC in an inflammatory environment, leading to increased TCR-peptide display. If this were true, it predicts that stimulation of the CD4+ Treg would be augmented by APC derived from the CNS-draining cervical lymph nodes of mice with ongoing EAE in comparison to healthy mice. To examine this hypothesis, DC were isolated form the cervical draining lymph nodes (DLN) of mice with ongoing EAE and from healthy naïve mice. Fig.1C. demonstrates that DLN DC derived from animals with active disease, but not from healthy naïve mice, stimulated B5.2 CD4+ T cell clones. As expected, DLN DC from EAE mice did not stimulate B4.2 CD4+ T cell clones, suggesting this effect is not due to non-specific stimulation owing to an inflammatory environment (data not shown). These results suggest DC are able to engulf apoptotic Vβ8.2+CD4+ T cells in the CNS, and present Vβ8.2TCR-derived peptides in the context of MHC class II molecules in draining cervical lymph nodes during EAE. In summary, data presented in Fig.1 suggest that dendritic cells are involved in the natural priming of TCR peptide-reactive CD4+ Treg during active EAE.
Dendritic cells pulsed with apoptotic Vβ8.2+ T cells can stimulate TCR peptide-reactive CD4+ Treg
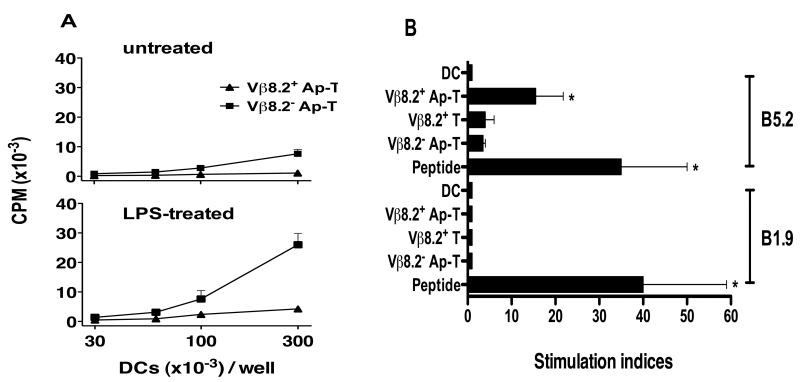
The above results suggested that DC may capture Vβ8.2+ CD4+ T cells and present TCR-derived peptides to the CD4+ Treg population. Previous studies have demonstrated that DC can phagocytose apoptotic cells, and process and present peptides derived from the ingested cells in the context of MHC class I and II molecules [23, 26]. We have recently demonstrated for the first time that DC can ingest apoptotic Vβ8.2+ T cells and stimulate CD8αα+TCRαβ+ Treg that recognize a Class Ib-binding Vβ8.2TCR-derived peptide [24]. However, it is not known whether the presentation of TCR-derived antigens from apoptotic T cells can also occur via the MHC class II presentation pathway. Thus to determine whether DC that have captured apoptotic Vβ8.2+ T cells could prime CD4+ Treg we set up the following in vitro assay. First we induced apoptosis in Vβ8.2+ or Vβ8.2− CD4+ T cell clones by anti-Fas antibody treatment or by UV irradiation [24]. Apoptosis was confirmed by annexin V staining and DNA ladder fragmentation, as described in the materials and methods section. Immature bone marrow-derived DC were pulsed with apoptotic T cells. Before assay, DC were removed from any T cells that had not been captured by selecting for CD11c expression, and treated for 12 hrs with 1 μg/ml of LPS or left untreated. LPS was used as we had previously demonstrated that Toll-like receptor (TLR) activation augmented the DC’s priming ability of CD8αα+TCRαβ+ Treg [24]. DC were then co-cultured with 2 × 104 B5.2 T cells. Fig.2A. (top panel) shows that untreated DC pulsed with Vβ8.2+ apoptotic T cells, but not Vβ8.2− apoptotic T cells, could weakly stimulate CD4+ Treg (B5.2), and stimulation was significantly augmented when LPS-treated DC were used (bottom panel). To determine whether this stimulation was specific, another I-Au-restricted CD4+ T cell clone (B1.9) reactive to a non-TCR antigen (MBPAc1-9) was cultured under the same conditions; LPS-treated DC pulsed with peptide, apoptotic Vβ8.2+ (Vβ8.2+ Ap-T), non-apoptotic Vβ8.2+ (Vβ8.2+ T) or apoptotic Vβ8.2− (Vβ8.2− Ap-T) T cells. Data presented in Fig.2B. shows that stimulation of CD4+ Treg was specific and dependent on DC being pulsed with Vβ8.2+ T cells undergoing cell death.
Figure 2. Dendritic cells loaded with apoptotic TCRVβ8.2+, but not TCRVβ8.2− T cells stimulate CD4+ Treg.
(A) 1 × 106 DC were pulsed for 8 hrs with 1 × 106 apoptotic (Ap-T) T cell clones (TCRVβ8.2+ or TCRVβ8.2−). CD11c+ DC were selected and either treated for 12 hrs with LPS (1 μg/ml) or left untreated, and then co-cultured with B5.2 T cell clones (2 × 104). T cell proliferation was measured by 3H-thymidine incorporation after 72 h. (B) DC were pulsed with either Vβ8.2+ or Vβ8.2− Ap-T, as in (A), cognate peptides (10 μg/ml), non-apoptotic TCRVβ8.2+ T cells (Vβ8.2+ T) or were left unpulsed. DC were then treated with LPS (1 μg/ml) and 3 × 104 of the cells were cultured with 2 × 104 CD4+ responder T cells. B1.9 CD4+ T cell clones reactive to MBP peptide Ac1-9 were used as responder T cell controls. The results are presented as stimulation indices(3H-thymidine incorporation CPM in test cultures / 3H-thymidine incorporation CPM in DC only control well). Data presented from one representative experiment of a total of four. *p<0.05 (comparison of stimulation indices between DC only and the highlighted (*) condition, using the Mann-Whitney test).
In summary, these data suggest that dendritic cells are capturing apoptotic Vβ8.2+ T cells and processing and presenting Vβ8.2TCR-derived peptide to CD4+ Treg in a stimulatory manner.
Inhibiting antigen presenting function of DC abrogates stimulation of CD4+ Treg
Next we determined whether DC were processing and presenting the TCR-derived antigenic determinants from the apoptotic T cell, or whether CD4+ Treg stimulation involved direct presentation of non-processed antigens attached to the DC cell surface. We examined the antigen presenting function of DC with respect to the stimulation of CD4+ Treg following gluturaldehyde-mediated cell membrane fixing, or endosomal inhibition using Concanamycin A. Fig.3. shows that fixing the DC’s cell membrane inhibited their ability to stimulate B5.2 CD4+ T cell clones by approximately 80% compared to non-fixed control. Additionally, DC-treatment with the endosomal protease inhibitor concanamycin A (50nM) resulted in around 80% inhibition of B5.2 CD4+ T cell clone stimulation compared to control conditions. Importantly, neither treatment caused DC cell death as confirmed by trypan blue exclusion. Additionally, treated DC could still efficiently present MHC class II peptide, MBP Ac1-9 that directly binds to cell surface I-Au (data not shown). In summary these data confirm that the dendritic cells must engulf the apoptotic T cell, then process the TCR-derived antigenic determinants via the endosomal pathway before presenting them to CD4+ Treg.
Figure 3. Inhibition of CD4+ Treg stimulation following blockade of antigen presentation function of dendritic cells.
CD11c+ DC (1 × 106) were either fixed with 0.05% glutaraldehyde or left untreated, or treated with 50 nM concanamycin A (CMA) or DMSO (vehicle) as a control. DC were then pulsed with TCRVβ8.2+ or TCRVβ8.2− apoptotic T cells (2 × 106) and 30 × 103 of the pulsed DC were then co-cultured with 2 × 104 B5.2 T-cell clones. T-cell proliferation was measured by 3H-thymidine incorporation after 72 h. Proliferation is presented as stimulation indices (3H-thymidine incorporation CPM in test cultures containing DC pulsed with TCRVβ8.2+ apoptotic T cells / 3H-thymidine incorporation CPM control cultures containing DC pulsed with TCRVβ8.2− apoptotic T cells). Data plotted as mean SI ± SD. Background CPM counts for cultures containing DC pulsed with TCRVβ8.2− apoptotic T cells were between 70 – 798 CPM. Data presented from one representative experiment of a total of two. *p<0.05, Mann-Whitney test.
Dendritic cells pulsed with apoptotic CD4+ T cells or B5 can prime CD4+ Treg in vivo
First we demonstrated that CD4+ T cells that could mediate protection against EAE could be generated after vaccination with apoptotic Vβ8.2+ T cells (Table 1). H-2u mice were injected i.p with 5 × 106 apoptotic Vβ8.2+ T cells or Vβ8.2− T cells. 7-10 days later CD4+ T cells were isolated from the spleen and stimulated in vitro with 40 μg peptide B5 for 72 hours, CD4+ T cells were then harvested and 4-5 × 106 cells transferred i.p into naïve WT or CD8−/− recipients. Recipient mice were challenged with MBPAc1-9/CFA/PTx and EAE was monitored. Table 1 demonstrates that WT recipient mice that received CD4+ T cells from donors that had been immunized with Vβ8.2+ apoptotic T cells and not Vβ8.2− apoptotic T cells were protected from EAE. However, CD8-deficient recipients of CD4+ T cells derived from mice immunized with either apoptotic Vβ8.2+ or Vβ8.2− T cells were not protected. These results indicate that TCR B5-reactive CD4+Treg function in a CD8-dependent fashion to control EAE in H-2u mice [3,15-19, 30].
Table 1.
Wild type recipients of CD4+ T cells isolated from mice immunized with Vβ8.2+ apoptotic T cells are protected from EAE.
| Adoptive transfer | EAE incidence | Average disease score (Individual score) |
|---|---|---|
| WT Recipients | ||
| PBS/None | 4/5 | 2.4 (5,3,3,1,0) |
| CD4+ (ApoVβ8.2+ vaccinated) | 1/7 | 0.3 (1,0,0,0,0,0,0) |
| CD4+ (ApoVβ8.2− vaccinated) | 7/8 | 3.0 (5,5,4,4,3,2,1,0) |
|
| ||
| CD8−/− Recipients | ||
| PBS/None | 5/5 | 4.2 (5,5,4,4,3) |
| CD4+ (ApoVβ8.2+ vaccinated) | 8/8 | 3.6 (5,5,4,4,3,3,3,2) |
| CD4+ (ApoVβ8.2− vaccinated) | 7/8 | 3.5 (5,5,5,4,4,4,1,0) |
Next we determined whether DC that have captured apoptotic Vβ8.2+ T cells could prime B5-reactive CD4+ Treg in vivo. To do this, DC were either left unpulsed, pulsed with peptide B5 (10 μg/ml) or Vβ8.2+ Ap-T cells (2-3 × 106). DC populations were selected on CD11c expression, LPS-treated (1 μg/ml) and 1 × 106 DC were injected i.p. After 5 days spleens were harvested, and antigen recall responses of the splenocyte population were analyzed using IFN-γ ELISPOT assays. Fig.4A. shows a significantly higher (p < 0.05) number of splenocytes secreting IFN-γ on recall response to TCR peptide B5 (10 μg/ml) was associated with the transfer of DC pulsed with Vβ8.2+ Ap-T cells or TCR peptide B5, compared to DC only transfer.
Figure 4. In vivo priming of TCRVβ8.2-peptide-B5-reactive T cells by dendritic cells pulsed with TCRVβ8.2+ apoptotic T cells.
(A) CD11c+ DC (1 × 106) pulsed in vitro with TCRVβ8.2+ apoptotic T cells (2 × 106), B5 peptide (10 μg/ml) or left unpulsed were selected based on CD11c expression. 1 × 106 of the selected DC were injected i.p and, 5 days after injection, spleens were harvested and splenocyte IFN-γ recall responses to B1 control or B5 antigen (10 μg/ml) were measured by ELISPOT. *p<0.05, Mann-Whitney test. (B) CD8αhigh or CD8αlow splenic DC were pulsed with 10 μg/ml B5 peptide. 0.5 × 106 pulsed DC were injected i.p per mouse. On day 10 after injection, DLN were harvested from mice that received CD8αhigh (□) or CD8αlow (▲) DC, and proliferation responses to B1 (control), B5 antigens (10 μg/ml) or 1μg/ml Concalavin A (ConA) were determined by 3H-thymidine incorporation after 72 h. Each symbol represents mean CPM count per experiment. Horizontal line represents the average CPM ± SD from 5 – 7 independent experiments. * p = 0.0140, Mann-Whitney test.
Furthermore, we determined the subtype of DC that was most efficient for the priming of B5-reactive CD4+ Treg. T-helper 1 and 2 responses have been shown to be associated with CD8α+ or CD8α− DC, respectively [27, 28]. Previously we demonstrated in the H-2u mouse that effective CD4+ Treg-mediated regulation is dependent on the generation of a Th-1-type response to TCR peptide B5 [3, 29]. We sought to determine whether CD8α+ or CD8α− dendritic cells could effectively prime CD4+ Treg responses. DC were isolated on CD11c expression from the spleen of naïve mice, and FACS sorted into CD8αhigh and CD8αlow populations. Sorted DC were then pulsed with peptide B5 (10 μg/ml), and injected i.p into B10.PL mice (0.5 × 106 cells / mouse). 10 days later, draining lymph node cells were collected and recall responses to antigen B5 determined in a proliferation assay. Fig.4B. shows that injection of CD8αhigh DC was associated with a significantly higher (p = 0.0140) recall response to peptide B5 compared to those injected with CD8αlow DC. Thus, the ability to effectively prime CD4+ Treg resides within the CD8αhigh DC population.
Transfer of DC pulsed with TCR peptide B5 efficiently blocks MBPAc1-9-induced EAE in H-2u mice
The data above indicate that DC pulsed either with TCR peptide B5 or apoptotic Vβ8.2+ T cells can stimulate CD4+ Treg both in vitro and in vivo. We have recently demonstrated that DC pulsed with apoptotic Vβ8.2+ T cells protect against EAE [24]. Here, we determined whether DC carrying peptide B5 were efficient in mediating protection from EAE. Day -1, mice were injected i.p with 0.5 × 106 bone marrow-derived DC were pulsed with either 10 μg/ml of TCR peptide B5 (group one) or the control B1 peptide (group two). A third group of mice were injected with PBS only. Day 0, mice were challenged with MPBAc1-9/CFA/PTx and EAE was monitored. Injection of DC pulsed with peptide B5 was associated with significant protection from EAE compared to mice injected with B1-pulsed DC or PBS only (Fig.5). The disease scores of mice treated with B5-pulsed DC were significantly lower (p < 0.0001) than mice treated with B1-pulsed DC. Collectively, these data demonstrate that DC loaded with TCR peptide B5 activate CD4+ Treg, resulting in protection against MBP-induced EAE disease.
Figure 5. Vaccination with dendritic cells pulsed with TCR peptide B5, but not B1 prevents EAE.
Bone marrow-derived DC were pulsed with 10 μg/ml of either peptide B1, B5 or PBS. 0.5 × 106 peptide-pulsed DC were injected i.p per mouse. 24 hrs later, the mice were challenged with MBP/CFA/PTx for the induction of EAE and disease symptoms were monitored for 30 days (as described in materials and methods section). Results represent the average disease scores ± SD of three independent experiments (7 mice in each group). *p<0.0001, Mann-Whitney test between mice treated with DC pulsed with B5 and those with B1.
Discussion
It has been widely demonstrated that CD4+ T cells with regulatory function can be harnessed to protect against inflammatory diseases. However, pathways leading to the priming or activation of antigen-specific CD4+ Treg have yet to be fully defined. Here the mechanism for the natural priming of antigen-specific CD4+FOXP3− Treg to a defined self-antigen derived from the conserved framework 3 region of the TCR is presented. This mechanism of CD4+ Treg priming is dependent on antigen presenting cells engulfing apoptotic Vβ8.2+CD4+ T cells, and processing and presenting a conserved TCR-derived antigenic determinant to the CD4+ Treg population. Notably, DC activation is required for optimal priming of the Treg and CD8α+ DC seem to be most efficient in this priming.
It was indicated by earlier studies that the regulatory CD4+ and CD8+ T cells that suppressed the anti-MBP response in humans and mice were recognizing antigenic determinants associated with the disease-mediating CD4+ T cell population [30-34]. However, due to the lack of knowledge concerning the exact antigenic determinants recognized on the disease mediating cells, the unknown role of antigen presenting cells, and the paucity of defined CD4+ and CD8+ Treg clones, the mechanism of natural Treg priming had not been delineated. Studies presented here show that the naturally occurring TCR-peptide-reactive CD4+ Treg were stimulated upon co-culture with large numbers of irradiated spleen cells form naïve H-2u mice (Fig 1.). Stimulation of Vβ8.2 TCR peptide-reactive CD4+ Treg, but not irrelevant CD4+ T cells, indicated that APC (especially DC) within the splenocyte population present a MHC class II-associated TCR peptide. We have recently delineated the mechanism by which dendritic cells acquire TCR antigenic determinants from Vβ8.2+ T cells and present another TCR-derived antigenic determinant in the context of the non-classical MHC class I molecule Qa-1 to novel subset of CD8αα+TCRαβ+ Treg [24]. As Vβ8.2TCR peptide-reactive CD4+ and CD8αα+TCRαβ Treg work in unison to down-regulate the Vβ8.2+ T cell response [3, 15, 30], it is not surprising that DC are able to process and present different TCR-derived peptides in the context of Class II and Class Ib MHC molecules.
Dendritic cells have previously been reported to be important in the induction of Treg [35-38]. It is clear that the maturation state of the DC is a crucial determining factor in the induction of Treg in the periphery. On one hand, by providing only partial or negative (e.g CTLA-4) co-stimulatory signals or secreting immunosuppressive cytokines (e.g IL-10, TGF-β), immature DC can be good inducers of T cell tolerance and certain types of Treg. Jonuleit and colleagues demonstrated IL-10-dependent generation of Tr-1 cells in vitro using immature DC [36]. On the other hand, peripheral expansion of CD4+ Treg may be dependent on optimal co-stimulatory signals from the mature dendritic cell. Yamazaki and colleagues reported in vivo expansion of CD4+CD25+ Treg require DC-T cell contact and B7 co-stimulation from the DC [37]. Here we show that the DC’s in vivo and in vitro stimulatory ability is associated with both the maturation state and subset of DC. In line with the results presented here, CD8α+ DC have previously been reported to be superior to CD8α− DC in the induction of Foxp3+ CD4+ Treg [28]. Data from our laboratory and others have shown that the CD8α+ DC population produces Type-1 cytokines and preferentially primes Th-1 responses to peptide [unpublished data, 27]. Consistent with earlier studies, TCR-reactive CD4+FOXP3− Treg are most efficiently primed by the Th-1-priming CD8α+ DC population. These studies suggest a Th-1 like milieu is essential for successful priming of the TCR-based negative feedback mechanism and protection from EAE [29,30]. Thus our working model of regulation predicts that CD4+ and CD8αα+TCRαβ Treg are primed within the Th-1 inflammatory milieu associated with active EAE.
Furthermore, dendritic cells that have captured Vβ8.2+ T cells can activate TCR peptide-reactive CD4+ Treg and stimulation is augmented when the DC have been treated with the TLR4-agonist LPS (Fig. 2). Additionally, stimulation of the CD4+ Treg is enhanced using DC isolated from mice with active EAE compared with DC from naïve mice (Fig. 1). Inflammatory mediators induce the DC maturation process, this results in the remodeling of endosomal compartments, relocation of MHC class II molecules from the late endosomal compartments to the cell surface, and upregulation of costimulatory molecules. Together these events augment the DC’s stimulatory capacity. Our data suggest that during inflammatory conditions such as active EAE there is optimal priming of the CD4+ and CD8αα+TCRαβ Treg. Importantly, engulfment of apoptotic T cells does not activate the DC with respect to up-regulation of co-stimulatory and MHC molecules [24]. Thus we predict under steady-state conditions DC that capture the small number of Vβ8.2+ T cells undergoing apoptotic cell death may not stimulate an efficient Treg response. This may be an important mechanism by which the negative feedback regulation, based upon TCR as the target molecule, ensures productive immunity against pathogens.
Through the use of antigen processing and presentation inhibitors we have investigated the dendritic cell antigen presentation pathways for TCR-peptides derived from apoptotic T cells. Presentation of exogenous antigen by both non-classical MHC class I molecules and classical MHC class II molecules requires antigen entry into the endosomal pathway [39, 40]. In agreement with this, we demonstrated that an endosomal pathway operates in the presentation of TCR-peptides associated with I-Au and Qa-1 molecules to CD4+ and CD8αα+TCRαβ+ Treg, respectively (Fig. 3 and [24]). We have yet to determine whether CD4+ and CD8αα+TCRαβ+ Treg are primed by the same DC. We do, however, think this is the case due to the shared endosomal pathway and for the following reasons: (i) DC engulf Vβ8.2+ apoptotic T cells containing both the cognate CD4+ and CD8αα+TCRαβ+ Treg antigenic determinants; (ii) DC are adept at presenting antigen in the context of both MHC class II and non-classical class I molecules; (iii) CD4+ T cells can license DC, e.g by CD40L-CD40 interactions, to stimulate a CD8+ T cell response [41]; and (iv) CD4+ T cells may provide help through the secretion of cytokines that act directly on proximal CD8+ T cells [42].
We have shown that injection of dendritic cells pulsed with Vβ8.2+ apoptotic T cells or TCR peptide B5 prime CD4+ Treg in vivo (Fig. 4), and that DC loaded with B5 can protect from EAE disease (Fig. 5). Data presented here and in other studies demonstrate that DC are the most efficacious APC for inducing optimal T cell responses [43]. DC can migrate to lymphoid organs, process and present antigens from multiple sources by both MHC class I and class II pathways, cross-present non-replicating antigens, and be manipulated to induce immunogenic or tolerogenic responses. However, to date immunotherapeutic studies that have attempted to harness the immuno-modulating ability of the DC, either by targeting antigens to the DC in vivo or by adoptive transfer of antigen-loaded DC, have demonstrated minimal clinical efficacy [44]. One major hindrance has been the lack of knowledge of the specific antigen targets. Here we have delineated the mechanism by which defined antigens are presented to a characterized CD4+ Treg population. Our data clearly show that disease-causing CD4+ T cells can be used to pulse DC’s for efficient in vivo priming of appropriate CD4+ as well as CD8+ Treg populations and subsequent regulation of autoimmune disease. Thus, in this defined system we have an excellent opportunity to study the optimal way to manipulate DC therapy to induce optimal priming of the T cells involved in regulation of an autoimmune disease. In addition, our data suggest a DC-based immune intervention strategy for the induction of negative feedback regulation of T cell-mediated inflammatory autoimmune disease.
Materials and Methods
Mice
B10.PL and PL/J H-2u mice were purchased from Jackson Laboratory (Bar Harbor, ME). CD8−/− PL/J mice were kindly provided by Dr. Tak Mak [45]. Mice were kept under pathogen-free conditions in our own colony at the Torrey Pines Institute for Molecular Studies (TPIMS, San Diego, CA). Female mice, aged-matched at 8-16 weeks, were used in the described experiments. Treatment of animals was in compliance with federal and institutional guidelines, and approved by the TPIMS institute animal care and use committee.
T cell lines and clones
T cell lines reactive to TCR peptides B1, B4 or B5, or MBP Ac1-9 were generated from naive B10.PL mice by stimulating splenocytes with peptide (40 μg/ml) in RPMI 1640 media containing 10% FBS [6]. CD4+ T cell clones were isolated from peptide-reactive T cell lines by the technique of two sequential limiting dilution clonings at 0.2 cells per well (as previously described, [6]). T cell line and clone cultures were maintained by the addition of rIL-2 (10 U/ml) every 3 days, and stimulated with TCR peptide and irradiated autologous spleen cells (2–5 × 106 spleen cells/well) in alternate weekly cycles. L-cell-transfectants expressing-I-Au MHC molecules were used as described earlier [25].
Peptides
TCR peptides were synthesized by S. Horvath (California Institute of Technology, Pasadena, CA) using a solid phase technique on a peptide synthesizer (430A; Applied Biosystems) and purified on a reverse phase column by HPLC, as described earlier [46]. TCR Vβ8.2 chain peptides are as follows (single-letter amino acid code): B1, aa 1–30(L): EAAVTQSPRNKVAVTGGKVTLSCNQTNNHNL; B4, aa 61-90: PDGYKASRPSQENFSLILELATPSQTSVYF and B5, aa 76–101: LILELATPSQTSVYFCASGDAGGGYE. Myelin basic protein peptide: MBPAc1-9 (AcASQKRPSQR), was purchased from Macromolecular Resources, Colorado State University.
Induction of EAE and clinical evaluation
For induction of EAE, mice were immunized s.c. with MBPAc1-9 emulsified in CFA, and i.p. with 0.15 μg of pertussis toxin (PTx; List Biological Laboratories) in PBS. 48 hrs later mice were injected with 0.15 μg PTx in PBS. Mice were observed daily for the clinical appearance of EAE. Disease severity was scored on a 5-point scale [6]: 1, Flaccid tail; 2, hind limb weakness; 3, hind limb paralysis; 4, whole body paralysis; 5, death.
Dendritic cell generation
Murine DC were derived from tibias and femurs by flushing out the bone marrow (BM) with RPMI 1640 medium. Red blood cells were lysed, and BM was cultured in 24-well plates at 1 × 106 cells/ml in complete medium containing 10 ng/ml IL-4 and 25 ng/ml GM-CSF for 5-7 days [24]. The medium was refreshed on day 3 and day 5. For some experiments DC were fixed by suspending the cells at 2 × 106/ml in PBS containing 0.05% glutaraldehyde for 30 secs at 37°C. 0.2 M of Lysine was added to stop the reaction. Recombinant IL-4 and GM-CSF were both purchased from Peprotech.
Microbead selection of antigen presenting cells and T cells
Subsets of APC were isolated from the spleen and DLN of naïve mice, and mice during active EAE, by positive selection using Microbeads conjugated to antibodies against cell surface markers. For isolation of B cells, anti-CD45R (B220); DC, anti-CD11c (N418); Macrophages, anti-CD11b (Mac-1); Thelper cells, anti-CD4 (L3T4) conjugated beads were used to manufacturers’ instructions (Miltenyi Biotec).
ELISA
IFN-γ levels in the supernatants from T cell assays were measured by a sandwich ELISA [19]. Briefly, 96-well MaxiSorp F96 plates (Nunc) were coated with anti-IFN-γ capture antibody overnight (O/N) at 4°C. Plates blocked with PBS containing 10% FBS before 50 μl supernatants were added, and the incubated O/N at 4°C.
After extensive washing, plates were incubated with a biotinylated anti-IFN-γ detection antibody. Plates were developed using avidin-peroxidase and 2-2′-azino-bis(3-ethyl-benzthiazoline-6-sulfonic acid) substrate (Sigma-Aldrich). OD405 was measured, and cytokine levels determined against a recombinant protein standard. All antibodies were purchased form BD Pharmingen.
ELISPOT
IFN-γ–producing cells were enumerated from splenocyte populations isolated from immunized mice by cellular ELISPOT assay [47]. Briefly, splenocytes (5 × 106 cells/ml) were cultured for 48 hr in 24-well plates either with B5, B1 (10 μg/ml) or medium alone. Millititer HA nitrocellulose plates (Millipore) were coated overnight at 4°C with anti–IFN-γ. After blocking coated plates, antigen-stimulated cells were added at graded concentrations for 24 hr at 37°C. The wells were then incubated with biotin-conjugated anti–IFN-γ mAbs followed by incubation with avidin peroxidase (Vector Laboratories). Spots were developed by the addition of 3-amino-9-ethylcarbazole substrate (Sigma-Aldrich) and counted using a computerized image analysis system (Ligh-tools Research) and the image analyzer program, NIH Image 1.61.
In vitro T cell stimulation assays
Immature bone marrow-derived DC (1 × 106) were pulsed with 1 × 106 apoptotic (Ap-T) or untreated (T) T cells, peptide (20 μg/ml) or PBS for 8-12 hrs. In most experiments DC were enriched by positive selection using anti-CD11c microbeads (Miltenyi) and treated with activation modulators (for example, LPS (Sigma)) for 4-12 hrs. CD11c+ DC were disabled (irradiated (3000 rads) or glutaraldehyde-fixed) and incubated with T responder cells (2 × 104/well) for the duration of the assay at 37°C in 5% CO2. For experiments analyzing the effect of antigen processing/presentation blockade, inhibitors were first added to bone marrow-derived DC for duration of 2 hrs - Concanamycin A (10-100 nM). DC were then washed and pulsed with peptide or apoptotic T cells for a total of 8 hrs (the final 4 hrs in the presence of LPS (1 μg/ml)). DC were positively selected using anti-CD11c microbeads (Miltenyi), and glutaraldehyde-fixed, before co-culturing with responder T cells. For IFN-γ secretion analysis, supernatants were harvested at 48 hrs. T cell proliferation was measured by 3H-thymidine incorporation at 72 hrs.
T cell apoptosis induction and flow cytometry
The desired number T cells were incubated in complete medium 4-12 hrs at 37°C in 5% CO2 in the presence of 5 μg/ml anti-Fas antibody (BD Pharmingen). To determine apoptosis induction 1 × 105 T cells in 100 μl buffer were stained with 10 μl/ml Annexin V FITC (BD Pharmingen). By flow cytometry apoptosis induction was confirmed using two parameters: (a) an anti-clockwise shift of the T cell population in the forward vs. side scatter dot plot, and (b) a significant right shift of the peak on the FL1 histogram axis - indicating Annexin V staining. DNA ladder fragmentation analysis was also used to confirm apoptosis.
Statistical Analysis
Data is expressed as mean ± SD for each group. Statistical differences between groups were evaluated using a Mann-Whitney test using GraphPad Prism 4.03 software. p < 0.05 was considered statistically significant.
Acknowledgements
We thank Dr. Randle Ware for critical reading of the manuscript and the members of the Laboratory of Autoimmunity for their help. This work was supported by the National Institutes of Health grant RO1AI052227, MSNRI and DNRG to V Kumar.
Footnotes
Conflict of Interest The authors have no conflicting financial interests.
References
- 1.Sakaguchi S, Wing K, Miyara M. Regulatory T cells - a brief history and perspective. Eur.J Immunol. 2007;37(Suppl 1):S116–S123. doi: 10.1002/eji.200737593. [DOI] [PubMed] [Google Scholar]
- 2.Shevach EM. Regulatory T cells in autoimmmunity*. Annu.Rev.Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 3.Kumar V. Homeostatic control of immunity by TCR peptide-specific Tregs. J Clin Invest. 2004;114:1222–1226. doi: 10.1172/JCI23166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379–1388. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arrenberg P, Halder R, Kumar V. Cross-regulation between distinct natural killer T cell subsets influences immune response to self and foreign antigens. J Cell Physiol. 2009;218:246–250. doi: 10.1002/jcp.21597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar V, Sercarz EE. The involvement of T cell receptor peptide-specific regulatory CD4+ T cells in recovery from antigen-induced autoimmune disease. J Exp.Med. 1993;178:909–916. doi: 10.1084/jem.178.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 8.Piccirillo CA, Tritt M, Sgouroudis E, Albanese A, Pyzik M, Hay V. Control of type 1 autoimmune diabetes by naturally occurring CD4+CD25+ regulatory T lymphocytes in neonatal NOD mice. Ann.N Y.Acad Sci. 2005;1051:72–87. doi: 10.1196/annals.1361.048. [DOI] [PubMed] [Google Scholar]
- 9.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp.Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp.Med. 2004;200:277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI. Defective suppressor function in CD4(+)CD25(+) T-cells from patients with type 1 diabetes. Diabetes. 2005;54:92–99. doi: 10.2337/diabetes.54.1.92. [DOI] [PubMed] [Google Scholar]
- 12.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp.Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp.Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiner HL, Friedman A, Miller A, Khoury SJ, al-Sabbagh A, Santos L, Sayegh M, Nussenblatt RB, Trentham DE, Hafler DA. Oral tolerance: immunologic mechanisms and treatment of animal and human organ-specific autoimmune diseases by oral administration of autoantigens. Annu.Rev.Immunol. 1994;12:809–837. doi: 10.1146/annurev.iy.12.040194.004113. [DOI] [PubMed] [Google Scholar]
- 15.Madakamutil LT, Maricic I, Sercarz E, Kumar V. Regulatory T cells control autoimmunity in vivo by inducing apoptotic depletion of activated pathogenic lymphocytes. J Immunol. 2003;170:2985–2992. doi: 10.4049/jimmunol.170.6.2985. [DOI] [PubMed] [Google Scholar]
- 16.Kumar V, Aziz F, Sercarz E, Miller A. Regulatory T cells specific for the same framework 3 region of the Vbeta8.2 chain are involved in the control of collagen II-induced arthritis and experimental autoimmune encephalomyelitis. J Exp.Med. 1997;185:1725–1733. doi: 10.1084/jem.185.10.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar V, Stellrecht K, Sercarz E. Inactivation of T cell receptor peptide-specific CD4 regulatory T cells induces chronic experimental autoimmune encephalomyelitis (EAE) J Exp.Med. 1996;184:1609–1617. doi: 10.1084/jem.184.5.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang X, Maricic I, Kumar V. Anti-TCR antibody treatment activates a novel population of nonintestinal CD8 alpha alpha+ TCR alpha beta+ regulatory T cells and prevents experimental autoimmune encephalomyelitis. J Immunol. 2007;178:6043–6050. doi: 10.4049/jimmunol.178.10.6043. [DOI] [PubMed] [Google Scholar]
- 19.Tang X, Maricic I, Purohit N, Bakamjian B, Reed-Loisel LM, Beeston T, Jensen P, Kumar V. Regulation of immunity by a novel population of Qa-1-restricted CD8alphaalpha+TCRalphabeta+ T cells. J Immunol. 2006;177:7645–7655. doi: 10.4049/jimmunol.177.11.7645. [DOI] [PubMed] [Google Scholar]
- 20.Tabi Z, McCombe PA, Pender MP. Antigen-specific down-regulation of myelin basic protein-reactive T cells during spontaneous recovery from experimental autoimmune encephalomyelitis: further evidence of apoptotic deletion of autoreactive T cells in the central nervous system. Int.Immunol. 1995;7:967–973. doi: 10.1093/intimm/7.6.967. [DOI] [PubMed] [Google Scholar]
- 21.Schmied M, Breitschopf H, Gold R, Zischler H, Rothe G, Wekerle H, Lassmann H. Apoptosis of T lymphocytes in experimental autoimmune encephalomyelitis. Evidence for programmed cell death as a mechanism to control inflammation in the brain. Am J Pathol. 1993;143:446–452. [PMC free article] [PubMed] [Google Scholar]
- 22.Peng Y, Martin DA, Kenkel J, Zhang K, Ogden CA, Elkon KB. Innate and adaptive immune response to apoptotic cells. J Autoimmun. 2007;29:303–309. doi: 10.1016/j.jaut.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inaba K, Turley S, Yamaide F, Iyoda T, Mahnke K, Inaba M, Pack M, Subklewe M, Sauter B, Sheff D, Albert M, Bhardwaj N, Mellman I, Steinman RM. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J Exp.Med. 1998;188:2163–2173. doi: 10.1084/jem.188.11.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith TR, Tang X, Maricic I, Garcia Z, Fanchiang S, Kumar V. Dendritic cells use endocytic pathway for cross-priming class Ib MHC-restricted CD8alphalpha+TCRalphabeta+ T cells with regulatory properties. J. Immunol. 2009;182:6959–68. doi: 10.4049/jimmunol.0900316. [DOI] [PubMed] [Google Scholar]
- 25.Kumar V, Tabibiazar R, Geysen HM, Sercarz E. Immunodominant framework region 3 peptide from TCR V beta 8.2 chain controls murine experimental autoimmune encephalomyelitis. J Immunol. 1995;154:1941–1950. [PubMed] [Google Scholar]
- 26.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 27.Yasumi T, Katamura K, Okafuji I, Yoshioka T, Meguro TA, Nishikomori R, Kusunoki T, Heike T, Nakahata T. Limited ability of antigen-specific Th1 responses to inhibit Th2 cell development in vivo. J Immunol. 2005;174:1325–1331. doi: 10.4049/jimmunol.174.3.1325. [DOI] [PubMed] [Google Scholar]
- 28.Maldonado-Lopez R, De ST, Michel P, Godfroid J, Pajak B, Heirman C, Thielemans K, Leo O, Urbain J, Moser M. CD8alpha+ and CD8alpha− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp.Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braciak TA, Pedersen B, Chin J, Hsiao C, Ward ES, Maricic I, Jahng A, Graham FL, Gauldie J, Sercarz EE, Kumar V. Protection against experimental autoimmune encephalomyelitis generated by a recombinant adenovirus vector expressing the V beta 8.2 TCR is disrupted by coadministration with vectors expressing either IL-4 or -10. J Immunol. 2003;170:765–774. doi: 10.4049/jimmunol.170.2.765. [DOI] [PubMed] [Google Scholar]
- 30.Smith TR, Kumar V. Revival of CD8(+) Treg-mediated suppression. Trends Immunol. 2008;29:337–342. doi: 10.1016/j.it.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Lider O, Reshef T, Beraud E, Ben-Nun A, Cohen IR. Anti-idiotypic network induced by T cell vaccination against experimental autoimmune encephalomyelitis. Science. 1988;239:181–183. doi: 10.1126/science.2447648. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Medaer R, Stinissen P, Hafler D, Raus J. MHC-restricted depletion of human myelin basic protein-reactive T cells by T cell vaccination. Science. 1993;261:1451–1454. doi: 10.1126/science.7690157. [DOI] [PubMed] [Google Scholar]
- 33.Vandenbark AA, Hashim G, Offner H. Immunization with a synthetic T-cell receptor V-region peptide protects against experimental autoimmune encephalomyelitis. Nature. 1989;341:541–544. doi: 10.1038/341541a0. [DOI] [PubMed] [Google Scholar]
- 34.Howell MD, Winters ST, Olee T, Powell HC, Carlo DJ, Brostoff SW. Vaccination against experimental allergic encephalomyelitis with T cell receptor peptides. Science. 1989;246:668–670. doi: 10.1126/science.2814489. [DOI] [PubMed] [Google Scholar]
- 35.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci U S A. 2002;99:351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp.Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamazaki S, Iyoda T, Tarbell K, Olson K, Velinzon K, Inaba K, Steinman RM. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J Exp.Med. 2003;198:235–247. doi: 10.1084/jem.20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L, Pino-Lagos K, de V, V, Guleria I, Sayegh MH, Noelle RJ. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc Natl Acad Sci U S A. 2008;105:9331–9336. doi: 10.1073/pnas.0710441105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moody DB, Porcelli SA. Intracellular pathways of CD1 antigen presentation. Nat Rev.Immunol. 2003;3:11–22. doi: 10.1038/nri979. [DOI] [PubMed] [Google Scholar]
- 40.Roy KC, Maricic I, Khurana A, Smith TR, Halder RC, Kumar V. Involvement of secretory and endosomal compartments in presentation of an exogenous self-glycolipid to type II NKT cells. J Immunol. 2008;180:2942–2950. doi: 10.4049/jimmunol.180.5.2942. [DOI] [PubMed] [Google Scholar]
- 41.Bevan MJ. Helping the CD8(+) T-cell response. Nat Rev.Immunol. 2004;4:595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 42.Wilson EB, Livingstone AM. Cutting edge: CD4+ T cell-derived IL-2 is essential for help-dependent primary CD8+ T cell responses. J Immunol. 2008;181:7445–7448. doi: 10.4049/jimmunol.181.11.7445. [DOI] [PubMed] [Google Scholar]
- 43.Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp.Med. 2000;191:411–416. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 45.Koh DR, Fung-Leung WP, Ho A, Gray D, cha-Orbea H, Mak TW. Less mortality but more relapses in experimental allergic encephalomyelitis in CD8−/− mice. Science. 1992;256:1210–1213. doi: 10.1126/science.256.5060.1210. [DOI] [PubMed] [Google Scholar]
- 46.Clark-Lewis I, Aebersold R, Ziltener H, Schrader JW, Hood LE, Kent SB. Automated chemical synthesis of a protein growth factor for hemopoietic cells, interleukin-3. Science. 1986;231:134–139. doi: 10.1126/science.3079915. [DOI] [PubMed] [Google Scholar]
- 47.Kumar V, Bhardwaj V, Soares L, Alexander J, Sette A, Sercarz E. Major histocompatibility complex binding affinity of an antigenic determinant is crucial for the differential secretion of interleukin 4/5 or interferon gamma by T cells. Proc Natl Acad Sci U S A. 1995;92:9510–9514. doi: 10.1073/pnas.92.21.9510. [DOI] [PMC free article] [PubMed] [Google Scholar]