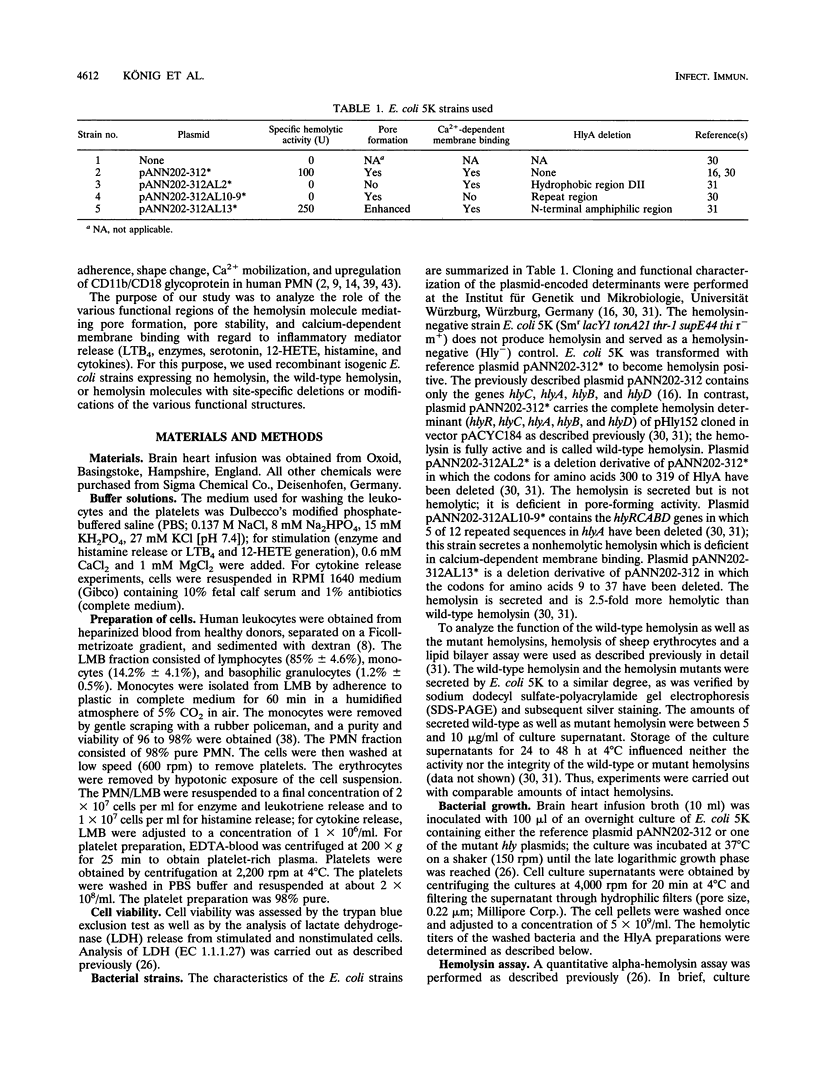
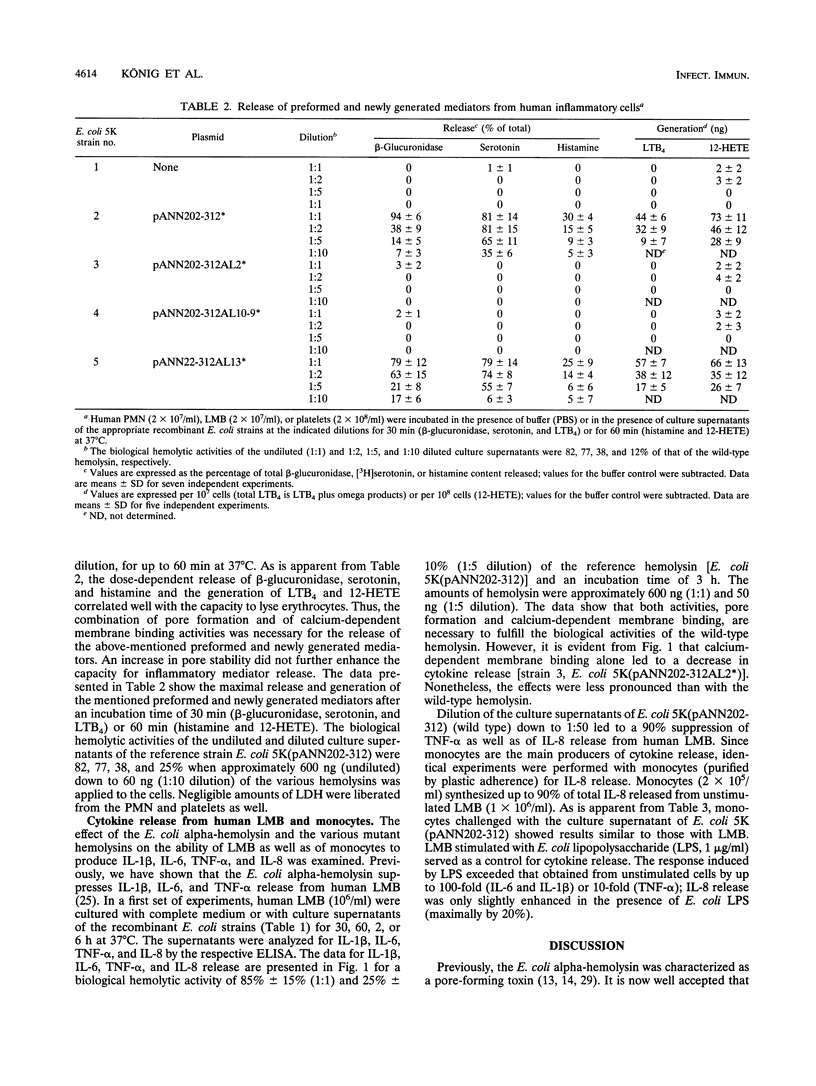
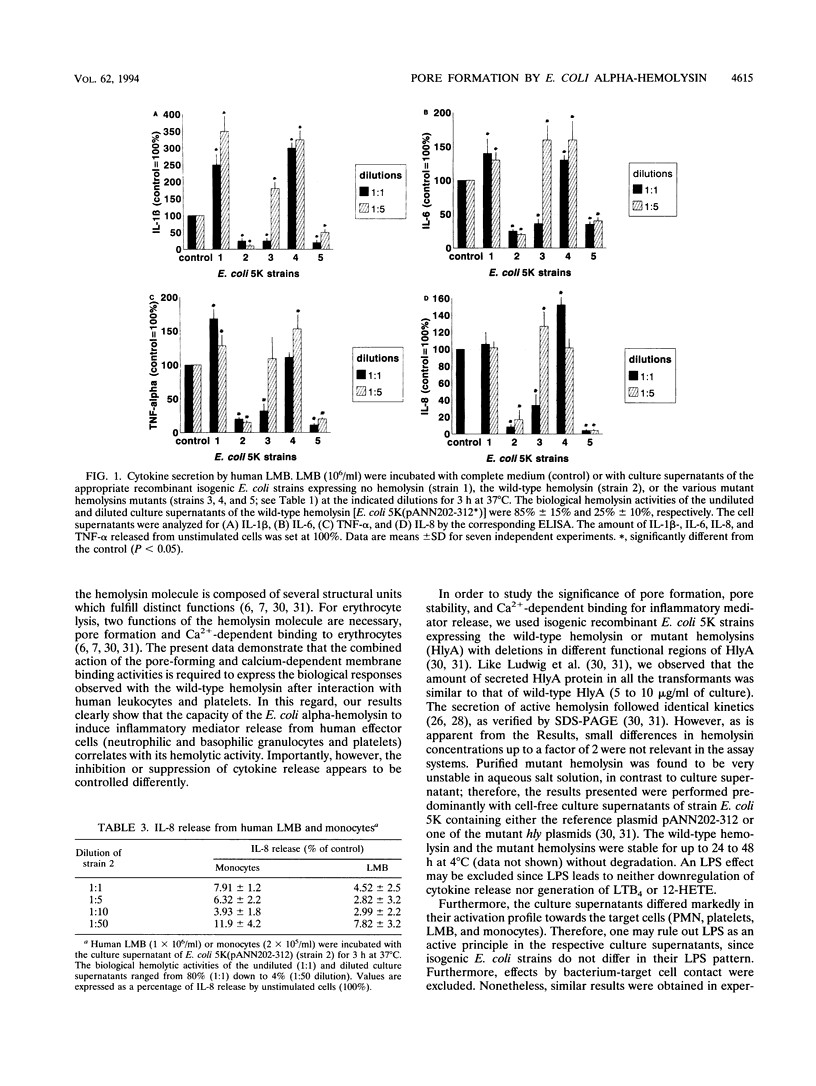
Abstract
The Escherichia coli alpha-hemolysin represents a potent stimulus for inflammatory mediator release (O2-, beta-glucuronidase release, and leukotriene generation) from human polymorphonuclear granulocytes, for histamine release from a suspension of human lymphocyte/monocyte basophil cells (LMB), and for serotonin release and 12-hydroxyeicosatetraenoic acid generation from human platelets. In contrast, the E. coli alpha-hemolysin leads to a downregulation of cytokine release (interleukin-1 beta [IL-1 beta], IL-6, and tumor necrosis factor alpha) from human LMB. Recently, it became apparent that the E. coli alpha-hemolysin is composed of several functional structures. We analyzed the role of pore formation, pore stability, and calcium-dependent membrane binding for inflammatory mediator release by using washed bacteria as well as culture supernatants of isogenic recombinant E. coli strains expressing no hemolysin (Hly-), the wild-type hemolysin (Hly+), or hemolysin molecules deficient or modulated in defined functions (pore formation, calcium-dependent membrane binding, or pore stability). In human granulocytes and platelets, mutant hemolysin with enhanced pore stability did not lead to a further increase in induction; mutant hemolysin deficient in pore-forming activity or calcium-dependent membrane binding no longer induced leukotriene B4 generation or beta-glucuronidase release compared with the wild-type hemolysin. Similar results were obtained with regard to histamine release from human LMB. The induction of cytokine release from human LMB differed depending on the type of mutant E. coli alpha-hemolysin. The wild-type hemolysin, the mutant hemolysin with enhanced pore-forming activity, and, to a lesser degree, the mutant hemolysin deficient in pore-forming activity decreased cytokine release (IL-1 beta, IL-6, IL-8, and tumor necrosis factor) compared with untreated cells. In contrast, the mutant hemolysin deficient in calcium-dependent membrane binding led to an increase of up to 50% in cytokine release compared with that by unstimulated cells. Our results indicate that simultaneous expression of the pore-forming and calcium-dependent membrane-binding activities of the hemolysin molecule was necessary to obtain the full cellular inflammatory response pattern observed with the wild-type hemolysin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann H., Gauldie J. Regulation of hepatic acute phase plasma protein genes by hepatocyte stimulating factors and other mediators of inflammation. Mol Biol Med. 1990 Apr;7(2):147–159. [PubMed] [Google Scholar]
- Bazzoni F., Cassatella M. A., Rossi F., Ceska M., Dewald B., Baggiolini M. Phagocytosing neutrophils produce and release high amounts of the neutrophil-activating peptide 1/interleukin 8. J Exp Med. 1991 Mar 1;173(3):771–774. doi: 10.1084/jem.173.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R., Schmid A., Wagner W., Goebel W. Pore formation by the Escherichia coli hemolysin: evidence for an association-dissociation equilibrium of the pore-forming aggregates. Infect Immun. 1989 Mar;57(3):887–895. doi: 10.1128/iai.57.3.887-895.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Mackman N., Nicaud J. M., Holland I. B. Escherichia coli hemolysin may damage target cell membranes by generating transmembrane pores. Infect Immun. 1986 Apr;52(1):63–69. doi: 10.1128/iai.52.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Muhly M., Korom S., Schmidt G. Effects of Escherichia coli hemolysin on human monocytes. Cytocidal action and stimulation of interleukin 1 release. J Clin Invest. 1990 Jun;85(6):1746–1753. doi: 10.1172/JCI114631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm D. F., Welch R. A., Snyder I. S. Calcium is required for binding of Escherichia coli hemolysin (HlyA) to erythrocyte membranes. Infect Immun. 1990 Jun;58(6):1951–1958. doi: 10.1128/iai.58.6.1951-1958.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm D. F., Welch R. A., Snyder I. S. Domains of Escherichia coli hemolysin (HlyA) involved in binding of calcium and erythrocyte membranes. Infect Immun. 1990 Jun;58(6):1959–1964. doi: 10.1128/iai.58.6.1959-1964.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brom J., König W. Cytokine-induced (interleukins-3, -6 and -8 and tumour necrosis factor-beta) activation and deactivation of human neutrophils. Immunology. 1992 Feb;75(2):281–285. [PMC free article] [PubMed] [Google Scholar]
- Bøyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol. 1976 Jun;Suppl 5:9–15. [PubMed] [Google Scholar]
- Castell J. V., Gómez-Lechón M. J., David M., Andus T., Geiger T., Trullenque R., Fabra R., Heinrich P. C. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett. 1989 Jan 2;242(2):237–239. doi: 10.1016/0014-5793(89)80476-4. [DOI] [PubMed] [Google Scholar]
- Cavalieri S. J., Bohach G. A., Snyder I. S. Escherichia coli alpha-hemolysin: characteristics and probable role in pathogenicity. Microbiol Rev. 1984 Dec;48(4):326–343. doi: 10.1128/mr.48.4.326-343.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerami A., Beutler B. The role of cachectin/TNF in endotoxic shock and cachexia. Immunol Today. 1988 Jan;9(1):28–31. doi: 10.1016/0167-5699(88)91353-9. [DOI] [PubMed] [Google Scholar]
- Chakraborty T., Schmid A., Notermans S., Benz R. Aerolysin of Aeromonas sobria: evidence for formation of ion-permeable channels and comparison with alpha-toxin of Staphylococcus aureus. Infect Immun. 1990 Jul;58(7):2127–2132. doi: 10.1128/iai.58.7.2127-2132.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colditz I. G., Zwahlen R. D., Baggiolini M. Neutrophil accumulation and plasma leakage induced in vivo by neutrophil-activating peptide-1. J Leukoc Biol. 1990 Aug;48(2):129–137. doi: 10.1002/jlb.48.2.129. [DOI] [PubMed] [Google Scholar]
- Goebel W., Hedgpeth J. Cloning and functional characterization of the plasmid-encoded hemolysin determinant of Escherichia coli. J Bacteriol. 1982 Sep;151(3):1290–1298. doi: 10.1128/jb.151.3.1290-1298.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Woods J. M., Gorman R. R. Stimulation of human eosinophil and neutrophil polymorphonuclear leukocyte chemotaxis and random migration by 12-L-hydroxy-5,8,10,14-eicosatetraenoic acid. J Clin Invest. 1977 Jan;59(1):179–183. doi: 10.1172/JCI108617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimminger F., Sibelius U., Bhakdi S., Suttorp N., Seeger W. Escherichia coli hemolysin is a potent inductor of phosphoinositide hydrolysis and related metabolic responses in human neutrophils. J Clin Invest. 1991 Nov;88(5):1531–1539. doi: 10.1172/JCI115463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker J., Hof H., Emödy L., Goebel W. Influence of cloned Escherichia coli hemolysin genes, S-fimbriae and serum resistance on pathogenicity in different animal models. Microb Pathog. 1986 Dec;1(6):533–547. doi: 10.1016/0882-4010(86)90039-2. [DOI] [PubMed] [Google Scholar]
- Hirano T., Akira S., Taga T., Kishimoto T. Biological and clinical aspects of interleukin 6. Immunol Today. 1990 Dec;11(12):443–449. doi: 10.1016/0167-5699(90)90173-7. [DOI] [PubMed] [Google Scholar]
- Hughes C., Hacker J., Roberts A., Goebel W. Hemolysin production as a virulence marker in symptomatic and asymptomatic urinary tract infections caused by Escherichia coli. Infect Immun. 1983 Feb;39(2):546–551. doi: 10.1128/iai.39.2.546-551.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen S. E., Mulcahy P. F., Louis C. F. Effect of Escherichia coli hemolysin on permeability of erythrocyte membranes to calcium. Toxicon. 1986;24(6):559–566. doi: 10.1016/0041-0101(86)90176-5. [DOI] [PubMed] [Google Scholar]
- Kraig E., Dailey T., Kolodrubetz D. Nucleotide sequence of the leukotoxin gene from Actinobacillus actinomycetemcomitans: homology to the alpha-hemolysin/leukotoxin gene family. Infect Immun. 1990 Apr;58(4):920–929. doi: 10.1128/iai.58.4.920-929.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König B., König W. Induction and suppression of cytokine release (tumour necrosis factor-alpha; interleukin-6, interleukin-1 beta) by Escherichia coli pathogenicity factors (adhesions, alpha-haemolysin). Immunology. 1993 Apr;78(4):526–533. [PMC free article] [PubMed] [Google Scholar]
- König B., König W. Roles of human peripheral blood leukocyte protein kinase C and G proteins in inflammatory mediator release by isogenic Escherichia coli strains. Infect Immun. 1991 Oct;59(10):3801–3810. doi: 10.1128/iai.59.10.3801-3810.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König B., König W., Scheffer J., Hacker J., Goebel W. Role of Escherichia coli alpha-hemolysin and bacterial adherence in infection: requirement for release of inflammatory mediators from granulocytes and mast cells. Infect Immun. 1986 Dec;54(3):886–892. doi: 10.1128/iai.54.3.886-892.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König B., König W. The role of the phosphatidylinositol turnover in 12-hydroxyeicosatetraenoic acid generation from human platelets by Escherichia coli alpha-haemolysin, thrombin and fluoride. Immunology. 1993 Dec;80(4):633–639. [PMC free article] [PubMed] [Google Scholar]
- König B., Schönfeld W., Scheffer J., König W. Signal transduction in human platelets and inflammatory mediator release induced by genetically cloned hemolysin-positive and -negative Escherichia coli strains. Infect Immun. 1990 Jun;58(6):1591–1599. doi: 10.1128/iai.58.6.1591-1599.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König W., Schönfeld W., Raulf M., Köller M., Knöller J., Scheffer J., Brom J. The neutrophil and leukotrienes--role in health and disease. Eicosanoids. 1990;3(1):1–22. [PubMed] [Google Scholar]
- Ludwig A., Jarchau T., Benz R., Goebel W. The repeat domain of Escherichia coli haemolysin (HlyA) is responsible for its Ca2+-dependent binding to erythrocytes. Mol Gen Genet. 1988 Nov;214(3):553–561. doi: 10.1007/BF00330494. [DOI] [PubMed] [Google Scholar]
- Ludwig A., Schmid A., Benz R., Goebel W. Mutations affecting pore formation by haemolysin from Escherichia coli. Mol Gen Genet. 1991 Apr;226(1-2):198–208. doi: 10.1007/BF00273604. [DOI] [PubMed] [Google Scholar]
- Marre R., Hacker J., Henkel W., Goebel W. Contribution of cloned virulence factors from uropathogenic Escherichia coli strains to nephropathogenicity in an experimental rat pyelonephritis model. Infect Immun. 1986 Dec;54(3):761–767. doi: 10.1128/iai.54.3.761-767.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer J., Vosbeck K., König W. Induction of inflammatory mediators from human polymorphonuclear granulocytes and rat mast cells by haemolysin-positive and -negative E. coli strains with different adhesins. Immunology. 1986 Dec;59(4):541–548. [PMC free article] [PubMed] [Google Scholar]
- Spector A. A., Gordon J. A., Moore S. A. Hydroxyeicosatetraenoic acids (HETEs). Prog Lipid Res. 1988;27(4):271–323. doi: 10.1016/0163-7827(88)90009-4. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987 Dec 17;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Tucker S. B., Pierre R. V., Jordon R. E. Rapid identification of monocytes in a mixed mononuclear cell preparation. J Immunol Methods. 1977;14(3-4):267–269. doi: 10.1016/0022-1759(77)90137-5. [DOI] [PubMed] [Google Scholar]
- Van Zee K. J., DeForge L. E., Fischer E., Marano M. A., Kenney J. S., Remick D. G., Lowry S. F., Moldawer L. L. IL-8 in septic shock, endotoxemia, and after IL-1 administration. J Immunol. 1991 May 15;146(10):3478–3482. [PubMed] [Google Scholar]
- Waage A., Brandtzaeg P., Halstensen A., Kierulf P., Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989 Jan 1;169(1):333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waage A., Halstensen A., Espevik T. Association between tumour necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet. 1987 Feb 14;1(8529):355–357. doi: 10.1016/s0140-6736(87)91728-4. [DOI] [PubMed] [Google Scholar]
- Welch R. A., Dellinger E. P., Minshew B., Falkow S. Haemolysin contributes to virulence of extra-intestinal E. coli infections. Nature. 1981 Dec 17;294(5842):665–667. doi: 10.1038/294665a0. [DOI] [PubMed] [Google Scholar]
- Willems J., Joniau M., Cinque S., van Damme J. Human granulocyte chemotactic peptide (IL-8) as a specific neutrophil degranulator: comparison with other monokines. Immunology. 1989 Aug;67(4):540–542. [PMC free article] [PubMed] [Google Scholar]


