Abstract
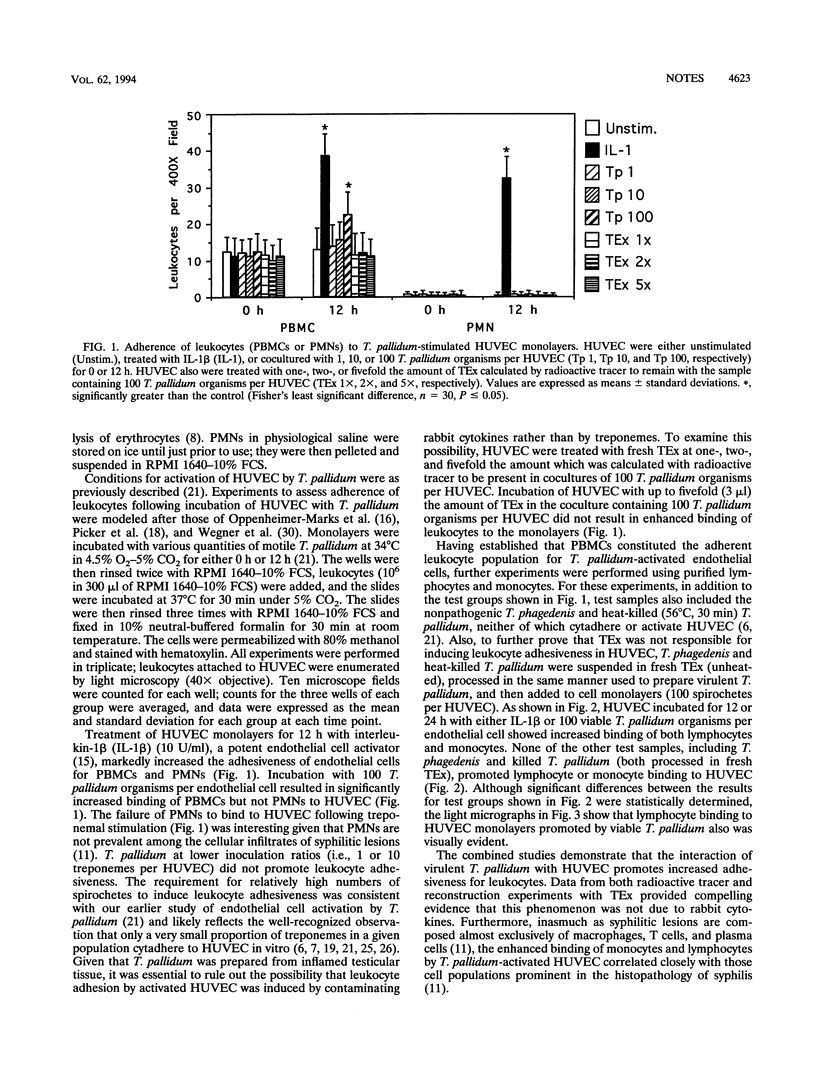
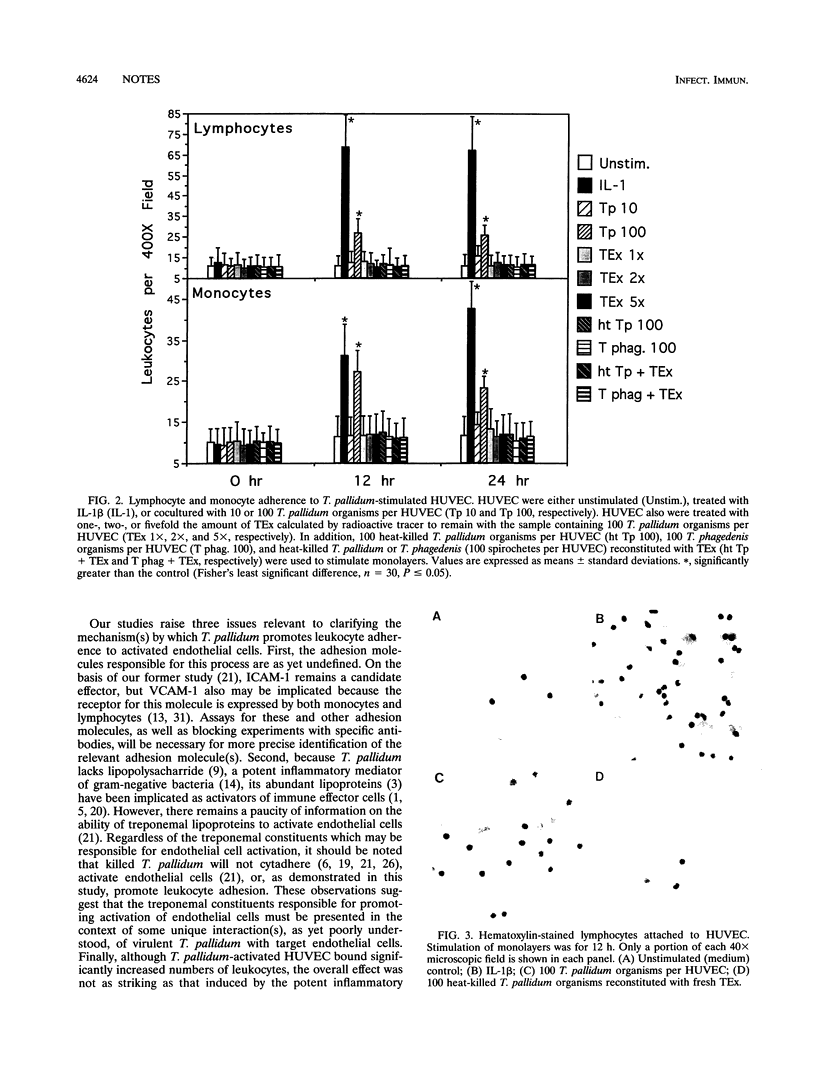
Perivasculitis and endothelial cell abnormalities are characteristic histopathologic features of syphilis, a sexually transmitted disease caused by Treponema pallidum. To extend earlier studies demonstrating that T. pallidum activates endothelial cells, we now show that virulent T. pallidum, but not heat-killed T. pallidum or nonpathogenic Treponema phagedenis, promotes increased adherence of lymphocytes and monocytes to human umbilical vein endothelial cells. Lymphocytes and monocytes are the two cell types prominent in the histopathology of syphilis. Recognition that T. pallidum can stimulate endothelial cells to bind leukocytes provides important insights into the early mechanisms of syphilis immunopathogenesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akins D. R., Purcell B. K., Mitra M. M., Norgard M. V., Radolf J. D. Lipid modification of the 17-kilodalton membrane immunogen of Treponema pallidum determines macrophage activation as well as amphiphilicity. Infect Immun. 1993 Apr;61(4):1202–1210. doi: 10.1128/iai.61.4.1202-1210.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Peterson K. M., Baseman J. B. Affinities of Treponema pallidum for human lactoferrin and transferrin. Genitourin Med. 1988 Dec;64(6):359–363. doi: 10.1136/sti.64.6.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain N. R., Brandt M. E., Erwin A. L., Radolf J. D., Norgard M. V. Major integral membrane protein immunogens of Treponema pallidum are proteolipids. Infect Immun. 1989 Sep;57(9):2872–2877. doi: 10.1128/iai.57.9.2872-2877.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D. L., Chang P., McDowall A. W., Radolf J. D. The outer membrane, not a coat of host proteins, limits antigenicity of virulent Treponema pallidum. Infect Immun. 1992 Mar;60(3):1076–1083. doi: 10.1128/iai.60.3.1076-1083.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeOgny L., Pramanik B. C., Arndt L. L., Jones J. D., Rush J., Slaughter C. A., Radolf J. D., Norgard M. V. Solid-phase synthesis of biologically active lipopeptides as analogs for spirochetal lipoproteins. Pept Res. 1994 Mar-Apr;7(2):91–97. [PubMed] [Google Scholar]
- Fitzgerald T. J., Miller J. N., Sykes J. A. Treponema pallidum (Nichols strain) in tissue cultures: cellular attachment, entry, and survival. Infect Immun. 1975 May;11(5):1133–1140. doi: 10.1128/iai.11.5.1133-1140.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy P. H., Jr, Levin J. Lack of endotoxin in Borrelia hispanica and Treponema pallidum. Proc Soc Exp Biol Med. 1983 Oct;174(1):47–52. doi: 10.3181/00379727-174-41702. [DOI] [PubMed] [Google Scholar]
- Marlin S. D., Springer T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987 Dec 4;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- McEver R. P. Selectins: novel receptors that mediate leukocyte adhesion during inflammation. Thromb Haemost. 1991 Mar 4;65(3):223–228. [PubMed] [Google Scholar]
- Morrison D. C., Ulevitch R. J. The effects of bacterial endotoxins on host mediation systems. A review. Am J Pathol. 1978 Nov;93(2):526–618. [PMC free article] [PubMed] [Google Scholar]
- Moser R., Schleiffenbaum B., Groscurth P., Fehr J. Interleukin 1 and tumor necrosis factor stimulate human vascular endothelial cells to promote transendothelial neutrophil passage. J Clin Invest. 1989 Feb;83(2):444–455. doi: 10.1172/JCI113903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer-Marks N., Davis L. S., Lipsky P. E. Human T lymphocyte adhesion to endothelial cells and transendothelial migration. Alteration of receptor use relates to the activation status of both the T cell and the endothelial cell. J Immunol. 1990 Jul 1;145(1):140–148. [PubMed] [Google Scholar]
- Osborn L. Leukocyte adhesion to endothelium in inflammation. Cell. 1990 Jul 13;62(1):3–6. doi: 10.1016/0092-8674(90)90230-c. [DOI] [PubMed] [Google Scholar]
- Picker L. J., Kishimoto T. K., Smith C. W., Warnock R. A., Butcher E. C. ELAM-1 is an adhesion molecule for skin-homing T cells. Nature. 1991 Feb 28;349(6312):796–799. doi: 10.1038/349796a0. [DOI] [PubMed] [Google Scholar]
- Quist E. E., Repesh L. A., Zeleznikar R., Fitzgerald T. J. Interaction of Treponema pallidum with isolated rabbit capillary tissues. Br J Vener Dis. 1983 Feb;59(1):11–20. doi: 10.1136/sti.59.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf J. D., Norgard M. V., Brandt M. E., Isaacs R. D., Thompson P. A., Beutler B. Lipoproteins of Borrelia burgdorferi and Treponema pallidum activate cachectin/tumor necrosis factor synthesis. Analysis using a CAT reporter construct. J Immunol. 1991 Sep 15;147(6):1968–1974. [PubMed] [Google Scholar]
- Riley B. S., Oppenheimer-Marks N., Hansen E. J., Radolf J. D., Norgard M. V. Virulent Treponema pallidum activates human vascular endothelial cells. J Infect Dis. 1992 Mar;165(3):484–493. doi: 10.1093/infdis/165.3.484. [DOI] [PubMed] [Google Scholar]
- Robertson S. M., Kettman J. R., Miller J. N., Norgard M. V. Murine monoclonal antibodies specific for virulent Treponema pallidum (Nichols). Infect Immun. 1982 Jun;36(3):1076–1085. doi: 10.1128/iai.36.3.1076-1085.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell S., Norris S. J. The biology, pathology, and immunology of syphilis. Int Rev Exp Pathol. 1983;24:203–276. [PubMed] [Google Scholar]
- Staggs T. M., Greer M. K., Baseman J. B., Holt S. C., Tryon V. V. Identification of lactoferrin-binding proteins from Treponema pallidum subspecies pallidum and Treponema denticola. Mol Microbiol. 1994 May;12(4):613–619. doi: 10.1111/j.1365-2958.1994.tb01048.x. [DOI] [PubMed] [Google Scholar]
- Thomas D. D., Fogelman A. M., Miller J. N., Lovett M. A. Interactions of Treponema pallidum with endothelial cell monolayers. Eur J Epidemiol. 1989 Mar;5(1):15–21. doi: 10.1007/BF00145039. [DOI] [PubMed] [Google Scholar]
- Thomas D. D., Navab M., Haake D. A., Fogelman A. M., Miller J. N., Lovett M. A. Treponema pallidum invades intercellular junctions of endothelial cell monolayers. Proc Natl Acad Sci U S A. 1988 May;85(10):3608–3612. doi: 10.1073/pnas.85.10.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Wallis W. J., Harlan J. M. Effector functions of endothelium in inflammatory and immunologic reactions. Pathol Immunopathol Res. 1986;5(2):73–103. doi: 10.1159/000157005. [DOI] [PubMed] [Google Scholar]
- Wegner C. D., Gundel R. H., Reilly P., Haynes N., Letts L. G., Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) in the pathogenesis of asthma. Science. 1990 Jan 26;247(4941):456–459. doi: 10.1126/science.1967851. [DOI] [PubMed] [Google Scholar]
- Williams T. J., Hellewell P. G. Endothelial cell biology. Adhesion molecules involved in the microvascular inflammatory response. Am Rev Respir Dis. 1992 Nov;146(5 Pt 2):S45–S50. doi: 10.1164/ajrccm/146.5_Pt_2.S45. [DOI] [PubMed] [Google Scholar]



