Abstract
Pathogens are hypothesized to play an important role in the maintenance of tropical forest plant species richness. Notably, species richness may be promoted by incomplete filling of niche space due interactions of host populations with their pathogens. A potentially important group of pathogens are endophytic fungi, which asymptomatically colonize plants and are diverse and abundant in tropical ecosystems. Endophytes may alter competitive abilities of host individuals and improve host fitness under stress, but may also become pathogenic. Little is known of the impacts of endophytes on niche-space filling of their hosts.
Here we evaluate how a widespread fungal endophyte infecting a common tropical palm influences its recruitment and survival in natural ecosystems, and whether this impact is modulated by the abiotic environment, potentially constraining host niche-space filling. Iriartea deltoidea dominates many wet lowland Neotropical forests. Diplodia mutila is a common asymptomatic endophyte in mature plants; however, it causes disease in some seedlings. We investigated the effects of light availability on D. mutila disease expression.
We found I. deltoidea seedlings to preferentially occur under shady conditions. Correspondingly, we also found that high light triggers endophyte pathogenicity, while low light favors endosymbiotic development, constraining recruitment of endophyte-infested seedlings to shaded understory by reducing seedling survival in direct light. Pathogenicity of D. mutila under high light is proposed to result from light-induced production of H2O2 by the fungus, triggering hypersensitivity, cell death, and tissue necrosis in the palm. This is the first study to demonstrate that endophytes respond to abiotic factors to influence plant distributions in natural ecosystems; and the first to identify light as a factor influencing where an endophyte is placed on the endosymbiont–pathogen continuum. Our findings show that pathogens can indeed constrain niche-space filling of otherwise successful tropical plant species, providing unoccupied niche space for other species.
Introduction
Pathogens and other natural enemies have long been hypothesized to play an important role in the local maintenance of species diversity, especially in species-rich tropical forests [1], [2], [3]. Notably, it has recently been proposed that species richness may be promoted by incomplete filling of niche space due localized dynamic coevolutionary interactions of populations with their pathogens or other natural enemies [4]. However, direct empirical evidence for such host-pathogen interactions and their ecological importance remain scarce (e.g., [5], [6], [7]). A potentially important group of pathogens are endophytic fungi, which asymptomatically colonize plants [8] and are diverse and abundant in tropical ecosystems [9]. These organisms may be pathogenic and/or mutualistic, depending on the circumstances [10]. The variable virulence of the endophyte, the host defense response, and environmental conditions constitute the disease triangle [11], ‘or disease/mutualism triangle’, and could be influencing the where an endophyte is placed on the endosymbiont-pathogen continuum. This has never been investigated though. Suboptimal environmental conditions may stress the host defense status, resulting in disease [12]. Several studies have shown that under conditions of stress, inoculation of endophytes into plant tissues [13] often resulted in disease symptoms (necrosis or chlorosis) and/or growth inhibition of the host. Additionally, other studies have focused on evaluating how endophytes alter competitive abilities of host individuals and improve host fitness under abiotic or biotic stress [14], [15]. However, none have looked for or identified environmental factors that alter the behavior of endophytes in natural ecosystems, their relationships to hosts, and the ecological implications for the niche-space filling (spatial and environmental patterns of distribution and abundance) of host plant species.
The palm Iriartea deltoidea is one of the most dominant tree species in wet lowland and premontane tropical forests of western Amazonia [16], [17], [18] and the Chocó- and Central American region [19], [20]. In contrast to most large palms [21], this species does not depend on large forest gaps for recruitment [22], perhaps related to its peculiar growth strategy. This palm undergoes ontogenetic transitions in leaf morphology. Young seedlings produce 3 to 6 round, simple leaves. Subsequent leaves are longer and compound, carrying increasing numbers of pinnae [23]. However, the inordinate success of I. deltoidea in wet New World tropical forests remains an enigma and cannot be explained by morphological attributes such as fruit size or height [19], [24].
Diplodia mutila is an endophytic/pathogenic fungus infecting I. deltoidea [25], with approximately 88.4% of the adult palms being infected, 45.1% of newborn seedlings, and 63.2% of 1-year old seedlings [29]. Diplodia mutila and related species have been reported as endophytes or latent pathogens for several plant species worldwide [27], [28], [29]. The fungus is an asymptomatic endophyte in I deltoidea mature plants, and disease and mortality are expressed in some seedlings, while others remain disease free. The infection pattern of D. mutila does not correspond with the one described in the J-C hypothesis [1], [2]. Infected plants are found near and far away from parental trees [26]. Here we investigate if the widespread endophytic fungal pathogen D. mutila influences survival and recruitment patterns of its host plant the common tropical palm, I. deltoidea in natural ecosystems and whether this impact is modulated by the abiotic environment, potentially affecting host niche-space filling. Additionally we investigated whether D. mutila alters the competitive ability of I. deltoidea under biotic stress (insect attack).
Results
Light conditions for I. deltoidea seedlings
Seedlings of I. deltoidea preferentially occur under shady conditions. Extensive sampling at two sites in western Amazonia showed that more than 91% of I. deltoidea seedlings were found in understory conditions. The total number of seedlings in the Northeastern Peru transects was 660, 94% of these seedlings were located at understory conditions (canopy scope <5). The negative correlation between the number of I. deltoidea seedlings and canopy openness in the 102 transects (Spearman r = −0.117, P<0.05) was estimated using n = 280 5×5 m subplots with at least one I. deltoidea seedling. Statistical significance was assessed as a one-tailed test and correcting for spatial autocorrelation using Dutilleul's approach for computing the geographically effective degrees of freedom = 227 [30]. The total number of seedlings in the Southeastern Peru plots was 518 seedlings (less than 25 cm), 91% seedlings were located in dense understory (∼55–120±15 µmol m−2 s−1).
High light availability increases plant disease development
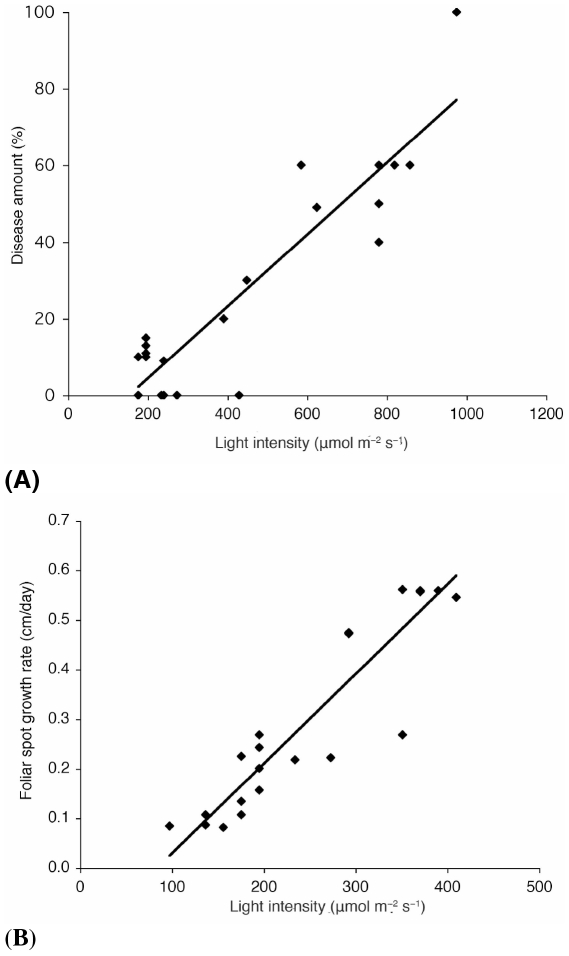
We found that D. mutila is beneficial to I. deltoidea under understory conditions, but strongly reduces I. deltoidea's capacity for recruitment in high-light forest gaps. Additionally, the foliar necrotic spot symptoms produced by D. mutila appeared more frequently in seedlings and juveniles that grew in gaps or diffuse open canopy conditions: Plants with visible symptoms caused by D. mutila received significantly higher illumination, 408.3±17.3 µmol m−2 s−1 than plants with no visible symptoms receiving lower illumination, 208.2±6.1 µmol m−2 s−1, (mean ± SE, t test, n = 808, P<0.0001). Disease development was faster and more lethal in seedlings with two leaves or less when exposed to higher light conditions (F 1,22 = 55.4, P = 0.0001, r2 = 0.73) (Fig. 1A). These results are consistent with the landscape recruitment patterns of I. deltoidea mentioned above. Ontogenic or age-related resistance may be responsible for differences in disease expression between seedlings in different stages of development [31]. An additional experiment showed that pathogenicity of D. mutila increased with light availability. We inoculated 22 healthy 6-month old I. deltoidea seedlings (no foliar spots or insect marks) with D. mutila, following inoculation procedures from previous studies [25]. Foliar spots produced by D. mutila had a higher growth rate and mortality was greater and faster at higher light availability (F 1,22 = 93.26, P = 0.0001, r 2 = 0.816) (Fig. 1B).
Figure 1. Higher light intensities increased disease development produced by Diplodia mutila.
(A) For young seedlings with 2 leaves or less there was a significant interaction between amount of infection (% of D. mutila foliar spots in Iriartea deltoidea leaves) and light level (F 1,22 = 55.4, P = 0.0001**, r 2 = 0.73). (B) The diametric growth rate of the foliar spots produced by D. mutila was higher at higher light conditions (ANOVA, F 1,22 = 93.26, P = 0.0001**, r 2 = 0.816).
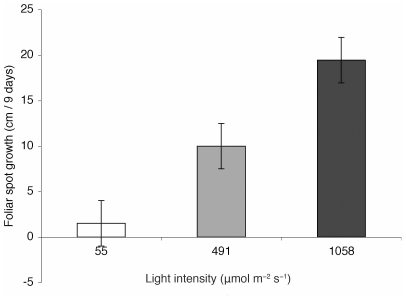
Using transplant experiments we demonstrated that increased light availability switched the endosymbiotic phase of the fungus to its pathogenic phase. In a greenhouse experiment diametric growth rate of foliar spots produced by D. mutila was higher in full sun conditions (mean 19.5±2.5 SE cm/day) than in reduced light (10.0±2.5 cm/day) and full shaded conditions (0.52±2.5 cm/day; analysis of variance (ANOVA), F 3,30 = 12.62, P = 0.0001; Fig. 2). Diplodia mutila-induced seedling mortality in plants exposed to full sun was 80% after ten days. Seedlings under shaded conditions had 10% mortality and seedlings in the greenhouse had 40% mortality.
Figure 2. Increased light availability switched the endosymbiotic phase of D mutila to its pathogenic phase.
Young seedlings that were colonized with endophytic Diplodia mutila showed faster growth rates of diameter of foliar spots (cm) caused by the pathogenic phase of D. mutila at higher light intensities (∼1058±23 µmol m−2 s−1) than seedlings under shaded conditions (∼55±15 µmol m−2 s−1) (n = 30, t test, P = 0.0001**). There were also significant differences of foliar spot growth rates among plants growing in the greenhouse (∼491±34 µmol m−2 s−1) and plants growing under shaded conditions (n = 30, t test, P = 0.024*). Foliar spot growth rates among plants growing in the greenhouse were lower than plants growing under high light intensities (n = 30, t test, P = 0.013*), (Tukey Kramer HSD test, ANOVA, F 3,30 = 12.62, P = 0.0001**).
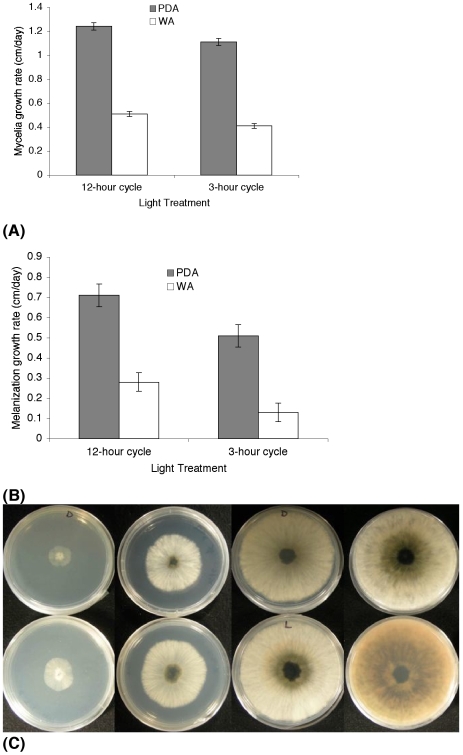
Laboratory assays showed that fungal growth (measured as diameter of mycelial colonies or as density of mycelium comprising colony) was greater when a 12-hr alternating light-dark cycle was provided than when periods of light were restricted to 3 hours. On Water Agar medium (WA) the average growth rate per day of the colony mycelium for five days was higher under a 12-hour light cycle (mean 0.52±0.03 SE cm/day) than under a 3-hour light cycle (0.38±0.03 cm/day, t test, n = 12, P<0.004). On Potato Dextrose Agar medium (PDA) the average growth rate per day of the colony mycelium was faster and also higher under the longer light period (mean 1.25±0.01 SE cm/day), compared to 1.11±0.11 cm/day for the 3-hour photoperiod (t test, n = 12, P<0.018) and the mycelium was notably denser with more aerial mycelium (Fig. 3A). We recorded greater melanization of mycelium in colonies exposed to the longer light period. This was especially evident in colonies grown on PDA: Colonies grown in PDA under the 12-hour light cycle had significantly faster growth of the central melanized area (mean 0.71±0.05 SE cm/day) than colonies exposed to the 3-hour light treatment (0.5±0.05 cm/day, t test, n = 12, P<0.022; Fig. 3B, 3D). The absorption spectrum of methanolic extracts of D.mutila cultures showed a characteristic peak in the UV region of 200–260 nm [32]. HPLC chromatograms of methanolic extracts of light and dark grown culture extracts showed that light grown cultures have at least twice the amount of melanin than dark grown cultures. Melanization of mycelium has been linked to enhanced virulence in numerous plant and animal pathogenic fungi [33]. Similar significant results were obtained for colonies growing in WA medium (Fig. 3B).
Figure 3. Fungal growth variation of Diplodia mutila under different light treatments and two different media.
(A) Mycelial radial growth of Diplodia mutila on Potato Dextrose Agar (PDA) was faster under a 12-hour cycle than the 3-hour cycle: 1.25 (±0.03) cm/day vs. 1.11 (±0.03) cm./day (n = 12, t test, P>0.018*). On Water Agar (WA) the average radial growth rate per day of the colony mycelium was 0.51 cm/day under a 12-hour light cycle; while under a 3-hour light cycle the average growth rate of the colony mycelium was significantly lower, at 0.41 cm/day after 7 days (n = 12, t test, P>0.004*). (B) Colonies grown in PDA under the 12-hour light cycle had a more rapid melanization of the central area of the colony (∼0.71 cm/day) than colonies exposed to 3-hours of light (∼0.5 cm/day) (n = 12, t test, P>0.022*). Similar results were obtained for colonies growing in WA (n = 12, t test, P>0.026*). (C) Melanization of colonies of D. mutila growing in PDA observed on 4 days (from left to right). Rate of melanization was reduced in the 3-hour cycle treatment (above). Faster melanization was observed in cultures maintained in a 12-hour light cycle (below).
Colonies grown on PDA under high light conditions showed higher hydrogen peroxide production than those grown under low light conditions as evidenced by staining using the DAB/peroxidase H2O2 assay (Fig. 4). Hydrogen peroxide has been shown to be a signal molecule for plants that triggers hypersensitivity cell and tissue death [34].
Figure 4. In vitro hydrogen peroxide production of Diplodia mutila on Potato Dextrose Agar (PDA).
(A) Hydrogen peroxide (orange pigmentation) accumulation in culture grown under light at 30°C treatment. (B) Culture grown under dark at 30°C treatment, showing no or minimal accumulation of hydrogen peroxide.
Distribution of seedlings affected by biotic stress (stem borers)
We found that stem borers (order Coleoptera) are the main mortality cause of I. deltoidea seedlings. After 50 days, 9% of the marked plants had died. Stem borers were the cause of 73% of the total palm mortality after 50 days, while D. mutila (in its pathogenic phase) caused just 2% of the mortality. After 150 days, stem borers induced 43% of mortality, while D. mutila (pathogenic phase) was responsible for a mere 0.1% of the mortality. We did not find any relationship between light availability and endophyte presence.
The proportion of plants affected by stem borers within the first 2.5 m near the parental tree was significantly higher than proportions in the other 4 annuli far away from the parental tree, in the first census: After ∼7 days, stem-borer infection was 8%±0.01% (mean ± SE) in the inner annulus, and 6%±0.01%, 4%±0.01%, 3%±0.01%, and 3%±0.01% in the outer annuli (going outwards) (One-way ANOVA, F4,50 = 2.65, P>0.045*). After ∼50 days, the same numbers were 10%±0.01%, 4%±0.01%, 1%±0.01%, 2%±0.01%, and 0.7%±0.01% (One-way ANOVA, F4,50 = 4.28, P>0.0051*).
Protection from insect pests
We found evidence that D. mutila benefits its host plants by enhancing resistance to herbivory by some insects. Field surveys in the ten surveyed plots, showed that insect herbivory (stem borers) decreased with increasing incidence of D. mutila infection. Plots with few D. mutila-infested I. deltoidea plants had higher incidence of stem-borer mortality, whereas plots with higher incidence of plants colonized by D. mutila had lower rates of stem-borer- induced mortality (ANOVA, F 1, 10 = 18.49, P = 0.0026, r2 = 0.69). Additional feeding experiments employing I. deltoidea fruits and PDA media (Potato Dextrose Agar) colonized by D. mutila showed that adults of the beetle Coccotrypes sp., and two unidentified species of larvae of the order Coleoptera avoided consumption of fruits and PDA colonized by D. mutila. Beetles consistently preferred PDA and avoided D. mutila infested PDA, mean 4.8 beetles ±0.14 SE versus 1.4 beetles±0.14 (Repeated measures ANOVA, F4, 456 = 160.13, P = 0.0001**). Similar results were also obtained when the experiment was performed using Coccotrypes sp. adults and I. deltoidea fruits instead of PDA.
Discussion
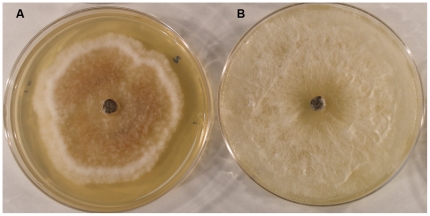
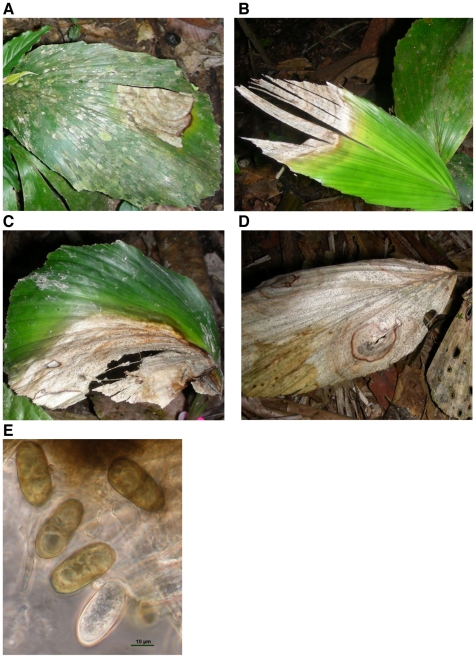
Diplodia mutila is frequently an asymptomatic endophyte in seeds, fruits, leaves of healthy juvenile and mature plants of I. deltoidea (88.4% infection of adult plants [29]) but can also act as a pathogen, causing mortality in young I. deltoidea seedlings after 5 to 16 days of infection and producing foliar spots in adult plants [25] (Fig. 5A–D). In the pathogenic phase, D. mutila forms pycnidia, flask-shaped asexual structures that exude masses of uni-cellular to bi-cellular, hyaline to brown conidia [35] (Fig. 5E). In its endophytic phase the fungus exists only as mycelium within tissues of the host's leaves, stems and seeds [8]. An endophyte life cycle that includes both vertical transmission through host seeds and infective spread using fungal spores (horizontal transmission) should provide enhanced dispersal potential over fungal life cycles that involve only a single transmission pathway.
Figure 5. Foliar spots in Iriartea deltoidea caused by Diplodia mutila, at different infection stages.
(A) Leaf spot infection for a plant with 2 leaves and one spot covering less than 20% of the leaf (B) A plant with two leaves and with a spot covering ∼40% of one leaf (C) A plant with two leaves and with the two foliar spots covering 50% of both leaves (D) Foliar spots covering the entire plant, representing 100% of infection. These plants died after 15 to 31 days. (E) Diplodia mutila pycnidia produced slowly maturing, non-striate, brown, 1-septate conidia measuring 26–28×15–20 µm. Liquid conidial darkening and septation was recorded to take place after discharge.
We found that high light triggers pathogenicity of the fungus, while low light favors endosymbiotic development, constraining recruitment of endophyte-infested seedlings to the shaded understory by limiting survival of seedlings in direct light, i.e., constraining the niche-space filling [4] of the otherwise highly successful I. deltoidea. In vitro studies show that light induces the fungus to produce melanin and secrete hydrogen peroxide. Hydrogen peroxide is a plant signal molecule that induces hypersensitivity cell death response in plants [34]. In alternating light/dark cycle experiments, radial growth of D. mutila was also enhanced by longer periods of light, and faster growth may contribute to disease expression by the fungus.
Laboratory experiments indicate that mycelial melanin and hydrogen peroxide production increase with light exposure. Melanin correlates with increased production of reactive oxygen species (ros) in pathogens and is often associated with increased virulence to hosts. Melanin is generally believed to be an antioxidant defense in fungal pathogens [32], and appears be produced in cultures of D. mutila that show enhanced hydrogen peroxide production. We suggest that higher light intensity increases fungus virulence to plants by triggering the hypersensitivity cell death response in plants [34]. An explanation for the relationship between light and H2O2 production may be found in the glyoxylate cycle, where H2O2 is produced by the fungus. The activity of the glyoxylate cycle is known to be enhanced by exposure to light [36].
Regardless of the precise explanation, it is apparent that D. mutila-colonized I. deltoidea seedlings survive better under closed canopy conditions due to the effect of light in triggering the pathogenic phase of the endophyte. When these plants become older seedlings, the pathogen does not seem to affect plant performance even at high light availability and, additionally, may confer other advantages to these plants, i.e., defensive mutualism [37]. Endophytes in many plants have been shown to provide hosts with increased herbivore and/or environmental stress resistance [14], [15], [38]. In the case of the D. mutila-palm association we found that infection correlated with reductions in the insect herbivory (stem borer attack) on the palm and thus probably improves host fitness. This effect is all the more important since stem borers were the main mortality agent for I. deltoidea seedlings, causing much more mortality than pathogenic D. mutila infections. Importantly, mortality rates after 50 and 150 days were higher next to fruiting trees and mostly produced by stem borers. Most young seedlings infested with stem borers were located in the vicinity of the fruiting adults, under closed canopy conditions. Hereby, the stem borer attack pattern follows the Janzen-Connell (J–C) hypothesis, which predicts high mortality of seeds and juvenile plants near parent trees, a process that facilitates the establishment of heterospecifics and therefore promote maintenance of plant diversity [1], [2]. Hereby, D. mutila should reduce negative density—dependence in the recruitment of I. deltoidea. Adding complexity to this finding, the case of D. mutila furthermore demonstrates that the environment can drastically modulate how an endosymbiotic fungus affects fitness of its host.
While pathogens and other natural enemies may potentially play crucial roles in driving diversification dynamics and maintaining species diversity [1], [2], [3], [4], we still have a very limited understanding of the actual roles played by pathogens in natural ecosystems. Our findings show that host plant characteristics such as age, light-dependent pathogenicity and virulence of an endophyte-pathogen (i.e., D. mutila) are intrinsically connected, influencing patterns of host seedling survival, potentially driving small-scale host species distribution patterns as well as its abundance at larger scales. Notably, D. mutila limits niche-space filling in an otherwise highly successful plant species, providing niche space for other species [4]. The resistance to insect predators such as stem and seed borers conferred by D. mutila may allow I. deltoidea to escape the otherwise potentially high intraspecific density- and distance-dependent mortality [1], [2], [3] and recruit - in the shady understory - near adult trees. Hence, while D. mutila constrains the niche-space filling of I. deltoidea, it may at the same time also contribute to this species' extraordinary abundance in many Neotropical wet forests [16], [17], [18], [19], [20]. It seems clear that patterns of plant abundance and the mechanisms maintaining tropical forest biodiversity may be the result of a more complex interplay between the abiotic environment and biotic interactions than previously thought, with pathogens playing an important role. However, much more research is needed before we can fully evaluate the extent to which microorganisms influence plant populations and community assembly in tropical ecosystems.
Materials and Methods
Demographic censuses
In Northeastern Peru [cf. 39] we arbitrarily placed 102 transects (5×500 m, divided in 5×5 m subunits) located in mature primary tropical rain forest within 300 km of Iquitos, Peru (excluding transects located in secondary forests, white sand soils, steep topographical conditions and human disturbed forests). Sites in Southeastern Peru were located at Cocha Cashu, (CCBS) [40] and Los Amigos, (LABS) [41]. Ten plots were established in May 2007, in primary floodplain forest, with similar floristic composition and topographic characteristics. Five of plots were located at CCBS and five at LABS. Nine plots measured 900 m2 and one plot at CCBS measured 2.25 ha. In each plot all I. deltoidea plants were tagged with numbered plastic tags and mapped in an X - Y coordinate system. The total number of plants located in the ten plots was 1068: 63 fruiting adults, 518 seedlings and 487 were considered juveniles-adults (non-fruiting). We measured height of the tallest photosynthetic leaf (cm) and number of leaves and diameter of foliar spots caused by D. mutila (cm) for all seedlings. The amount of disease was calculated by dividing the diameter of the foliar spot by the diameter of the affected leaf and expressed as ‘% disease’. Disease development over a period of 150 days was calculated by subtracting the % disease in the initial estimate from the final estimate.
Distribution of seedlings affected by biotic stress (stem borers)
Plants damaged and killed by epicotyl borers, such as caterpillars, beetle larvae or crickets, were considered as “damaged by stem borers”. Plants located in the Southeastern Peru plots were monitored for presence/absence of D. mutila and stem borers, three times after initial establishment (7, 50 and 150 days). In each plot, the minimum distances from all seedlings to the nearest I. deltoidea fruiting plant were computed using the coordinates of the labeled plant under consideration and the coordinates of the nearest fruiting tree within the plot. We surveyed seedlings in 5 concentric 2.5 m annuli centered on a focal fruiting tree. The number of seedlings affected by stem borers and D. mutila was tallied for each 2.5 m annulus and then divided by the total number of plants located in the selected annulus to yield proportions. One-way ANOVA was used to compare diseases and mortality proportions among plots for each distance annulus (Tukey's HSD used to contrast means).
Light availability measurement
In Northeastern Peru light availability was measured using the canopy scope methodology [42]. In Southeastern Peru light availability was estimated above the tallest photosynthetic leaf of each I. deltoidea seedling, using the average value of light intensity over the leaf with a light meter (Environmental Concepts Plant Light Intensity Meters, LIM2500, USA). The average value was obtained from three measurements over each plant at 6 am, 12 pm and 5 pm for three consecutive days.
Transplant experiments
We transplanted 30 I. deltoidea seedlings from one plot at Cocha Cashu where adults, juveniles, seeds and fruits were colonized by D. mutila. Ten seedlings were transplanted to shade conditions, ∼55±15 µmol m−2 s−1, ten to a reduced light environment in a greenhouse, ∼491±34 µmol m−2 s−1, and ten to full sun exposure, ∼1058±23 µmol m−2 s−1. All seedlings had two leaves and did not have any visible disease symptoms produced by D. mutila or any other foliar spot. Light availability was measured three times a day (6 am, 12 pm and 5 pm) for a period of ten days and all disease symptoms and insect damage were recorded and measured daily. The average daily temperature in the understory and full sun conditions was 23±3°C and 26±5°C in the greenhouse.
In vitro deterrence experiment
On December 2007, ∼370 Coccotrypes sp. beetles and larvae were extracted from more than 100 fruits and seeds of I. deltoidea. In a Petri plate (60×15 mm, Fisher Scientific Co. Canada) we placed two 1-cm2 of PDA (Potato Dextrose Agar) and two 1-cm2 of PDA infested with D. mutila, covered with squares of non-acidic paper to simulate dark conditions found inside seeds and fruits. PDA was replaced everyday for the duration of the experiment to avoid contamination of non-infested PDA by D. mutila. We set up 12 repetitions following this procedure. Six to ten beetles were released in the each Petri plate and monitored daily for 8 to 12 days.
Laboratory assays
To assess the effect of light on the fungus, laboratory observations were made on mycelial growth in Water Agar (WA) and Potato Dextrose Agar (PDA). Two photoperiod treatments were employed for five days with six D. mutila samples per treatment. The first treatment consisted of 12-hour cycle of darkness and 12-hour cycle of white light (fluorescent, 100±10 µmol m−2 s−1), while the second consisted of 21-hour cycle of darkness and 3-hour cycle of light for five days, (6 repetitions per treatment, constant temperature for all treatments was 24°C).
Analysis of methanolic extracts of D. mutila for in vitro production of melanin
Methanolic extracts of culture discs from D. mutila grown at room temperature and 30°C in dark and light conditions were analyzed by HPLC [34]. Fungal cultures were extracted in methanol 80% at day 10 of the experiment. Samples were concentrated using a rotovapour (model 11, Buchi, Flawil, Switzerland) at 45°C and dried in speed vacuum (Savant PSD 2010 Speed Vac Concentrator, Thermo). Dried samples were dissolved in 1 ml of methanol 80% with add of vortex and sonication, and filtered using 0.45 µm Nylon (CostarR) filters held in a 2.0 ml polypropylene tube and centrifuged at 5000 rpm for 45 sec before analysis. Sample analysis was conducted with HPLC (Waters, Milford, MA) using a C 18 Luna column (4.6×150 mm; particle size 5 µm; Phenomenex, Torrance, CA). Samples (20 µl) were injected into the column, and gradient elution was used for fractional separation with two solvents at a flow of 1 mL/minute, Solvent A consisted of 10% Methanol in H2O adjusted pH 3.5 with formic acid0. Solvent B consisted of 20% water (pH 3.5), 20% methanol and 60% acetronitrile. The gradient consisted of 0 minute at 100% A, followed by 5 minutes at 85% A, 10 minutes at 80% A, 20 minutes at 75% A, 25 minutes at 73% A27 minutes at 60% A, 30 minutes at 60% A, 35 minutes at 50% A, 40 minutes at 10% A, 45 minutes at 0% A, and 50 minutes at 100% A and holding at 100% A for a final 55 minutes. Five minutes of equilibration at 100% A was performed before and after each injection.
In vitro hydrogen peroxide production
Fungal cultures were grown on PDA medium and incubated in two different temperatures (25°C and 30°C) and light conditions (dark and light). Accumulation of hydrogen peroxide was evaluated after 5 days of growth following method described by Munkres (1990) [43]. In brief, hydrogen peroxide was indicated by the accumulation of a red precipitate after being flooded with 5 ml solution of 100 mM potassium phosphate buffer, pH 6.9, 2.5 mM diaminobenzidine tetrachloride and 5 purpurogallin units/ml of horseradish peroxidase (Type VI, Sigma Chemical Company, St. Louis, MO). Plates were incubated at 30°C in dark for one hour before the solution was discarded. Afterward, plates were inverted and incubated at 30°C in the dark for 24 h and then photographed.
Acknowledgments
We thank Manu National Park, Cocha Cashu and Los Amigos Biological Station staff, M. Bergen for advice in laboratory techniques, F. Cornejo and J. Huarancasi at Cocha Cashu, L. Noblick and Montgomery Botanical Garden for logistical support, J. Terborgh and J. Bunce for insightful comments.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: P. Álvarez-Loayza was supported by Rutgers University Fellowship, Pre-Dissertation Travel Grant, and the Francis J. Bossuyt Fellowship from the Organization for Tropical Studies, and Amazon Conservation Association (ACA). This research was also financially supported by the New Jersey Agricultural Experiment Station, the Danish Natural Science Research Council (grants 272-06-0476 and 272-07-0242) and the European Community FP7 programme PALMS project (grant agreement 212631). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Janzen D. Herbivores and the number of tree species in tropical forests. American Naturalist. 1970;104:501–529. [Google Scholar]
- 2.Connell JH. On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forest trees. In: Boer PJD, Gradwell GR, editors. Dynamics of numbers in populations. Wageningen, Netherlands: Centre for Agricultural Publication and Documentation; 1971. pp. 298–312. [Google Scholar]
- 3.Peters HA. Neighbour-regulated mortality: the influence of positive and negative density dependence on tree populations in species-rich tropical forests. Ecology Letters, 2003;6:757–765. [Google Scholar]
- 4.Ricklefs RE. Evolutionary diversification, coevolution between populations and their antagonists, and the filling of niche space. Proceedings of the National Academy of Sciences USA. 2010;107:1265–1272. doi: 10.1073/pnas.0913626107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell T, Freckleton RP, Lewis OT. Plant pathogens drive density-dependent seedling mortality in a tropical tree. Ecology Letters. 2006;9:569–574. doi: 10.1111/j.1461-0248.2006.00905.x. [DOI] [PubMed] [Google Scholar]
- 6.Hood LA, Swaine MD, Mason PA. The influence of spatial patterns of damping-off disease and arbuscular mycorrhizal colonization on tree seedling establishment in Ghanaian tropical forest soil. Journal of Ecology. 2004;92:816–823. [Google Scholar]
- 7.Packer A, Clay K. Soil pathogens and spatial patterns of seedling mortality in a temperate tree. Nature. 2000;404:278–281. doi: 10.1038/35005072. [DOI] [PubMed] [Google Scholar]
- 8.Petrini O. Taxonomy of endophytic fungi of aerial plant tissues. In: Fokkema NJ, van den Heuve J, editors. Microbiology of the Phyllosphere. Cambridge, UK: Cambridge University Press; 1986. pp. 175–187. [Google Scholar]
- 9.Arnold E, Maynard Z, Gilbert G, Coley PD, Kursar TA. Are tropical fungal endophytes hyperdiverse? Ecology Letters. 2000;3:267–274. [Google Scholar]
- 10.Schulz B, Boyle C. The endophytic continuum. Mycological Research. 2005;109:661–686. doi: 10.1017/s095375620500273x. [DOI] [PubMed] [Google Scholar]
- 11.Agrios GN. Plant Pathology: Elsevier Academic Press 2005 [Google Scholar]
- 12.Kuldau GA, Yates IE. Evidence for Fusarium endophytes in cultivated and wild plants. In: Bacon CW, White JF, editors. Microbial Endophytes. New York and Basel: Marcel Dekker; 2000. pp. 85–120. [Google Scholar]
- 13.Schulz B, Guske S, Dammann U, Boyle C. Endophyte host interactions II. Defining symbiosis of the endophyte-host interaction. Symbiosis. 1998;25:213–227. [Google Scholar]
- 14.Arnold AE, Mejía L, Kyllo D, Rojas E, Maynard Z, et al. Fungal endophytes limit pathogen damage in a tropical tree. Proceedings of the National Academy of Sciences USA. 2003;100:15649–15654. doi: 10.1073/pnas.2533483100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnold AE, Engelbrecht BMJ. Fungal endophytes nearly double minimum leaf conductance in seedlings of a tropical tree. Journal of Tropical Ecology. 2007;23:369–372. [Google Scholar]
- 16.Macía MJ, Svenning J-C. Oligarchic dominance in western Amazonian plant communities. Journal of Tropical Ecology. 2005;21:613–626. [Google Scholar]
- 17.Valencia R, Foster RB, Villa G, Condit R, Svenning J-C, et al. Tree species distributions and local habitat variation in the Amazon: large forest plot in eastern Ecuador. Journal of Ecology. 2004;92:214–229. [Google Scholar]
- 18.Pitman NCA, Terborgh JW, Silman MR, Nunez P, Neill DA, et al. Dominance and distribution of tree species in upper Amazonian Terra Firme forests. Ecology. 2001;82:2101–2117. [Google Scholar]
- 19.Clark DB, Palmer MW, Clark DA. Edaphic factors and the landscape-scale distributions of tropical rain forest trees. Ecology. 1999;80:2662–2675. [Google Scholar]
- 20.Wattenberg I, Breckle S-W. Tree species diversity of a premontane rain forest in the Cordillera de Tilaran, Costa Rica. Ecotropica. 1995;1:21–30. [Google Scholar]
- 21.Svenning J-C. On the role of microenvironmental heterogeneity in the ecology and diversification of neotropical rain forest palms (Arecaceae). Botanical Review. 2001;67:1–53. [Google Scholar]
- 22.Svenning JC. Recruitment of tall arborescent palms in the Yasuní National Park, Amazonian Ecuador: are large treefall gaps important? Journal of Tropical Ecology. 1999;15:355–366. [Google Scholar]
- 23.Terborgh J, Davenport L. Endogenous and exogenous control of leaf morphology in Iriartea deltoidea (Palmae). Journal of Tropical Ecology. 2001;17:695–703. [Google Scholar]
- 24.Grubb PJ, Jackson RV, Barberis IM, Bee JN, Coomesi DA, et al. Monocot leaves are eaten less than dicot leaves in tropical lowland rain forests: Correlations with toughness and leaf presentation. Annals of Botany: 2008;101:1379–1389. doi: 10.1093/aob/mcn047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Álvarez-Loayza P, White JF, Bergen M, Cadenas C. Diplodia mutila causing seedling mortality of Iriartea deltoidea palm trees. Plant Pathology. 2008;57:382. [Google Scholar]
- 26.Álvarez-Loayza P. New Brunswick: Rutgers University; 2009. The role of pathogens in determining plant recruitment and distribution patterns in a western Amazonian floodplain. Ph.D. Dissertation. [Google Scholar]
- 27.Slippers B, Wingfield MJ. Botryosphaeriaceae as endophytes and latent pathogens of woody plants: diversity, ecology and impact. Fungal Biology Reviews. 2007;21:90–106. [Google Scholar]
- 28.Crous PW, Slippers B, Wingfield M, Rheeder J, Marasas WFO, et al. Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology. 2006;55:235–253. doi: 10.3114/sim.55.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Damm U, Crous PW, Fourie PH. Botryosphaeriaceae as potential pathogens of Prunus species in South Africa, with descriptions of Diplodia africana and Lasiodiplodia plurivora sp. nov. Mycologia. 2007;99:664–680. doi: 10.3852/mycologia.99.5.664. [DOI] [PubMed] [Google Scholar]
- 30.Rangel TF, Diniz-Filho JAF, Bini LM. Towards an integrated computational tool for spatial analysis in macroecology and biogeography. Global Ecology and Biogeography. 2006;15:321–327. [Google Scholar]
- 31.Panter SN, Jones DA. Age-related resistance to plant pathogens. Advances in Botanical Research: Academic Press. 2002:251–280. [Google Scholar]
- 32.Ravishankar JP, Muruganandam V, Suryanarayaanan TS. Isolation and characterization of melanin from a marine fungus. Botanica Marina. 1995;38:413–416. [Google Scholar]
- 33.Jacobson ES. Pathogenic roles for fungal melanins. Clinical Microbiology Reviews. 2000;13:708–717. doi: 10.1128/cmr.13.4.708-717.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mittler R, Simon L, Lam E. Pathogen-induced programmed cell death. Journal of Cell Science. 1997;110:1333–1344. doi: 10.1242/jcs.110.11.1333. [DOI] [PubMed] [Google Scholar]
- 35.Sutton BC. Kew: UK Commonwealth Mycological Institute; 1980. The Coelomycetes.696 [Google Scholar]
- 36.Ma L, Li J, Qu L, Hager J, Chen Z, et al. Light control of Arabidopsis development entails coordinated regulation of genome expression and cellular pathways. The Plant Cell. 2001;13:2589–2607. doi: 10.1105/tpc.010229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clay K, Holah J. Fungal endophyte symbiosis and plant diversity in successional fields. Science. 1999;285:1742–1744. doi: 10.1126/science.285.5434.1742. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez RJ, Redman RS, Henson JM. The role of fungal symbioses in the adaptation of plants to high stress environments. Mitigation and Adaptation Strategies for Global Change. 2004;9:261–272. [Google Scholar]
- 39.Kristiansen T, Svenning J.-C, Grández C, Salo J, Balslev H. Commonness of Amazonian palm (Arecaceae) species: Cross-scale links and potential determinants. Acta Oecologica. 2009;35:554–562.33. [Google Scholar]
- 40.Terborgh J. Princeton: Princeton University Press; 1983. Five New World primates: a study in comparative ecology. [Google Scholar]
- 41.Pitman NCA. Puerto Maldonado, Peru: ACCA; 2007. An overview of the Los Amigos watershed, Madre de Dios, southeastern Peru. [Google Scholar]
- 42.Brown N, Jennings S, Wheeler P, Nabe-Nielsen J. An improved method for the rapid assessment of forest understorey light environments. Journal of Applied Ecology. 2000;37:1044–1053. [Google Scholar]
- 43.Munkres KD. Histochemical detection of superoxide radicals and hydrogen peroxide by Age-1 mutants of Neurospora. Fungal Genetics Newsletter. 1990;37:24. [Google Scholar]