Abstract
Background
Dental caries is the single most prevalent and costly infectious disease worldwide, affecting more than 90% of the population in the U.S. The development of dental cavities requires the colonization of the tooth surface by acid-producing bacteria, such as Streptococcus mutans. Saliva bicarbonate constitutes the main buffering system which neutralizes the pH fall generated by the plaque bacteria during sugar metabolism. We found that the saliva pH is severely decreased in a mouse model of cystic fibrosis disease (CF). Given the close relationship between pH and caries development, we hypothesized that caries incidence might be elevated in the mouse CF model.
Methodology/Principal Findings
We induced carious lesions in CF and wildtype mice by infecting their oral cavity with S. mutans, a well-studied cariogenic bacterium. After infection, the mice were fed a high-sucrose diet for 5 weeks (diet 2000). The mice were then euthanized and their jaws removed for caries scoring and bacterial counting. A dramatic increase in caries and severity of lesions scores were apparent in CF mice compared to their wildtype littermates. The elevated incidence of carious lesions correlated with a striking increase in the S. mutans viable population in dental plaque (20-fold increase in CF vs. wildtype mice; p value<0.003; t test). We also found that the pilocarpine-stimulated saliva bicarbonate concentration was significantly reduced in CF mice (16±2 mM vs. 31±2 mM, CF and wildtype mice, respectively; p value<0.01; t test).
Conclusions/Significance
Considering that bicarbonate is the most important pH buffering system in saliva, and the adherence and survival of aciduric bacteria such as S. mutans are enhanced at low pH values, we speculate that the decrease in the bicarbonate content and pH buffering of the saliva is at least partially responsible for the increased severity of lesions observed in the CF mouse.
Introduction
Dental caries is the single most prevalent and costly infectious disease worldwide, affecting more than 90% of the population in the U.S. [1]. The development of dental cavities requires the colonization of the tooth surface by acid-producing bacteria, such as Streptococcus mutans, in conjunction with the frequent ingestion of a cariogenic high-sucrose diet, the substrate for acid and glucan production by organisms. The elevated amounts of acid and glucans modulate the establishment of cariogenic organisms within tightly adherent biofilms known as dental plaque. Numerous host-derived and dietary factors in saliva also affect the pathogenesis of this multifactorial disease [2]. A direct association between incidence of carious lesions and decreased saliva production is well documented [3], [4], [5], but the role of specific salivary constituents in the pathogenesis of dental caries is not well-understood, particularly in diseases such as cystic fibrosis (CF).
Cystic fibrosis is the most common genetic disease in Caucasians, occurring in approximately one out of 3,200 live births [6], and is generally associated with alterations in saliva composition [7], [8], [9], [10]. CF is caused by mutations of the CFTR (cystic fibrosis transmembrane conductance regulator) gene. CFTR is highly expressed in salivary glands [11], [12], [13], [14], [15], but the reported effects of CF on salivary gland function [6], [7], [8], [16], [17] and the incidence of dental cavities are inconsistent [16], [17], [18], [19], [20], [21]. The basis for these discrepancies is unknown, but many of these studies were performed before it was routine to determine the nature of the CF mutation, which relates to the severity of disease, and when patients rarely survived to adulthood because the treatment of CF was largely ineffective. Moreover, CF patients consume potentially anti-cariogenic foods such casein-containing dairy products [22], [23], [24] and are typically treated with wide-spectrum antibiotics, which alter the oral flora and likely mask the relationship of CF and caries production [16], [17].
To gain insight into the relationship between cystic fibrosis and incidence of dental caries we induced carious lesions in CF mice and their wildtype littermates. A dramatic increase in cavity formation and severity of lesions was apparent on both smooth and sulcal tooth surfaces in CF mice. The elevated incidence of carious lesions correlated with a dramatic increase in the S. mutans viable population in the plaque of CF mice, and a decrease in saliva pH and HCO3 - levels (associated with buffering capacity).
Results
Elevated incidence and severity of carious lesions in the ΔF508 mouse CF model
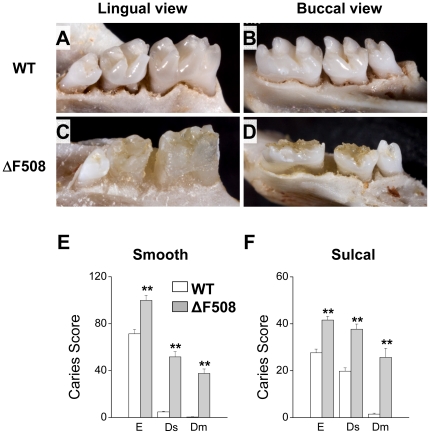
Of the more than 1,500 known disease-causing CFTR mutations, the most common mutation (∼90% of mutations) is a deletion of phenylalanine 508 (the ΔF508 mutation) [6]. To directly test the association between CF and dental caries we used the ΔF508 mouse CF model, which reproduces many of the defects observed in human disease [25]. A dramatic increase in number and severity of carious lesions was clearly evident after only five weeks exposure to a cariogenic diet (Figure 1). This was most apparent on the lingual and occlusal smooth surfaces where the enamel layer of the first and second mandibular and maxillary molars was nearly completely destroyed (compare mandibular molars of wildtype to ΔF508 mice, Figures 1A & 1B to 1C & 1D, respectively). Note that the relatively small third molars were essentially free of carious lesions. Mouse third molars typically erupt into the oral cavity 24 to 36 days postnatal [26], [27], after the cariogenic diet was introduced. The incidence and severity scores of the carious lesions for the first and second mandibular and maxillary molars of animals like those shown in Figures 1A-D are summarized in Figures 1E & 1F. The incidence of smooth surface and sulcal caries were elevated in mutant ΔF508 mice, while the increase in the severity of smooth surface and sulcal caries was especially dramatic (Figures 1E & 1F, respectively). Scores for carious enamel involvement are expressed as E, while severities of carious lesions, based on degree of dentin involvement, are expressed as Ds and Dm (slight and moderate dentin involvement, respectively) [28].
Figure 1. Elevated incidence and severity of carious lesions in ΔF508 mutant mice.
Panels A, B, C and D. Lingual (panels A and C) and buccal (panels B and D) views of representative mandibular jaws from wildtype (WT, panels A and B) and mutant mice (ΔF508, panels C and D). Panels E and F. Smooth and sulcal caries and severity of lesions. Evaluations of carious enamel involvement in submandibular and maxillary first and second molars are expressed as E, while severities of carious lesions, based on degree of dentin involvement, are expressed as Ds and Dm (slight and moderate dentin involvement, respectively) [28]. Open and filled bars correspond to WT (n = 13) and ΔF508 (n = 7) mice, respectively. Values are given as the mean ± S.E.M. ** p<0.01, t test.
Number of viable S. mutans are dramatically increased in the ΔF508 mouse CF model
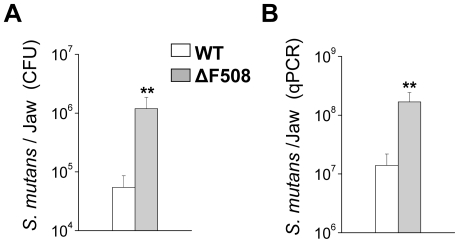
The higher number and severity of carious lesions observed in the ΔF508 mice suggest that the colonization of the tooth surface by acid-producing bacteria may have been enhanced in the CF mouse model. S. mutans is a critical microorganism associated with the pathogenesis of dental caries [2]. Figure 2A shows that the number of S. mutans colony forming units was ∼20 fold higher in the ΔF508 mice, correlating with the enhanced frequency and severity of caries lesions in these animals. Quantitative real-time PCR confirmed that the increase in the number of the caries-causing S. mutans population was more than an order of magnitude greater in the oral cavity of ΔF508 mice (Figure 2B).
Figure 2. Number of viable S. mutans cells were dramatically increased in ΔF508 mutant mice.
Bacterial counts in the lower jaws were evaluated by two independent techniques. Panel A. Numbers of colony forming units (CFU) were calculated by plating bacterial suspensions in MSB plates and counting using a grid plate system. Panel B. S. mutans in the mandibular jaws were also estimated using quantitative real-time PCR. Open and filled bars correspond to wildtype (WT, n = 9) and mutant (ΔF508, n = 5) mice, respectively. Values are given as the mean ± S.E.M. ** p<0.02, t test.
Saliva pH and bicarbonate concentration are dramatically reduced in the ΔF508 mouse CF model
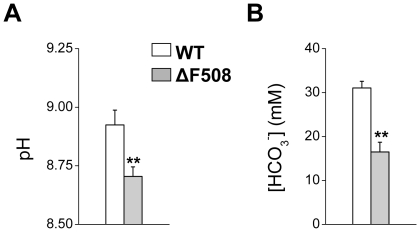
S. mutans is unusual in that its tight glucan-dependent adherence, growth and survival on tooth surfaces prefer a more acidic pH environment than most other oral microorganisms, consistent with its role in cavity formation [2]. Using the ΔF508 mouse cystic fibrosis model, we previously found that the CFTR channel mediates Cl− reabsorption by salivary gland ducts [11]. We also noted that the pH of submandibular saliva was decreased in the ΔF508 mouse, suggesting that CFTR mutations may compromise the HCO3 − secretion mechanism. HCO3 − is the most important pH buffering system in saliva. Consequently, if the ΔF508 mutation reduces secretion of bicarbonate in saliva, its pH would be expected to decrease as well. We found that the pH decreased in the whole saliva of ΔF508 mice (Figure 3A), and this acidification correlated with a dramatic, nearly 50% decrease in the HCO3 − concentration (Figure 3B) in the mutant mice.
Figure 3. Saliva pH and bicarbonate concentration were dramatically reduced in ΔF508 mutant mice.
Secretion was stimulated in non-infected mice by intraperitoneal injection of pilocarpine HCl (10 mg/kg). Panel A. pH value was measured immediately after saliva collection using a pH-sensitive electrode. Panel B. Bicarbonate was measured using an enzymatic-based colorimetric kit. Open and filled bars correspond to wildtype (WT, n = 7) and mutant (ΔF508, n = 6) mice, respectively. Values are given as the mean ± S.E.M. ** p<0.01, t test.
Discussion
Saliva is a fluid secreted primarily by the three major salivary glands, i.e. parotid, submandibular and sublingual glands. Human salivary glands typically secrete 0.5–1 liter of saliva per day in response to sympathetic and parasympathetic stimulation [29]. Fluid and electrolyte secretion involves two stages: the secretory endpiece secretes an isotonic NaCl-rich, plasma-like fluid (stage 1) which is subsequently modified as it passes through the ductal network (stage 2). Most of the NaCl is reabsorbed in the ducts, and importantly, KHCO3 is secreted. HCO3 − ions play a major role in buffering the pH of saliva.
Salivary glands express CFTR, an anion channel gated by an increase in intracellular cAMP. CFTR channels have been postulated to be involved in both acinar (stage 1) and ductal (stage 2) functions [15], [30]. Using the ΔF508 mouse model of cystic fibrosis, we found that the CFTR channel mediates Cl− reabsorption by salivary gland ducts but fluid secretion was normal [11]. We also noted that the submandibular saliva pH in ΔF508 mice was decreased compared to their wildtype littermates. In the present study, the pH of whole saliva, which is most relevant to caries formation and progress, was also significantly reduced in the ΔF508 mouse. However, the pH of whole saliva was higher than observed previously in submandibular saliva [11]. The difference in the saliva pH between the two studies is likely the consequence of the different stimulation protocol (carbachol/isopreterenol-stimulated vs. pilocarpine-stimulated), different type of saliva collected (submandibular ductal saliva vs. whole saliva), and/or the difference in flow rate between ex vivo and in vivo approaches. Related to this last point, HCO3 − secretion is flow rate dependent.
The lower saliva pH observed in the ΔF508 mouse suggests that the ΔF508 CFTR mutation compromises the HCO3 − secretion mechanism. Consistent with this prediction, we found that the bicarbonate concentration of pilocarpine-stimulated whole saliva was severely reduced in the ΔF508 mice. HCO3 − is the most important pH buffering system in saliva, while adherence and survival of many oral bacteria are dependent on pH. Consequently, we hypothesized that S. mutans colonization and prevalence of carious lesions may be enhanced in the ΔF508 mouse model of cystic fibrosis. We found that the incidence of both smooth and sulcal surface caries of mandibular and maxillary molars was significantly elevated in the ΔF508 mouse. The severity of carious lesions was also dramatically elevated, increasing in most cases by an order of magnitude for both smooth and sulcal surfaces. This remarkable increase in the severity of carious lesions in the ΔF508 mouse in just five weeks exposure to a cariogenic diet is noteworthy in that it is comparable to that seen in mice that had been desalivated [28]. Nevertheless, the elevated incidence and severity of dental caries was not related to a decrease in saliva by itself because the fluid secretion volume was essentially unchanged in the ΔF508 mouse [11]. Thus, the ΔF508 CFTR mutation appears to decrease HCO3 − secretion in salivary glands, reducing the buffering capacity and pH of saliva. These phenomena would greatly affect the ability of saliva to reduce the adverse effects of acid production by S. mutans and other acidogenic bacteria, and thereby increase the extent of acidification within the dental plaque on the tooth surface. The persistence of this aciduric environment in the plaque's matrix leads to selection and establishment of highly acid-tolerant (and acidogenic) organisms such as S. mutans, and the acidic pH at plaque-tooth interface results in dissolution of enamel [2].
Another possible mechanism for the elevated incidence and severity of lesions in the CF mouse is that the ΔF508 mutation might alter the composition of the tooth enamel. However, Bronckers et al. found that the ameloblasts of molars were not structurally affected in mice lacking Cftr [31]. Indeed, Gawenis et al. failed to detect changes in the ion composition of the molars of these mice [32]. No lesions were detected in the mouse incisors probably because they erupt continuously. Consequently, considering that HCO3 − is the most important pH buffering system in saliva, and tight adherence and survival of aciduric bacteria such as S. mutans are enhanced at low pH values, the decrease in the HCO3 − content and pH buffering of the saliva is most likely to be at least partially responsible for the severity of lesions observed in the CF mouse. Importantly, this is the first genetic model to demonstrate a clear relationship between saliva composition and the incidence of carious lesions. Accordingly, the ΔF508 CF mouse paves the way for future studies to evaluate the complex, multifactorial relationship between host genetic factors and the pathogenesis of dental caries. The insight gained from such studies may ultimately lead to the generation of cost effective preventive agents for dental caries, the most common and costly oral infectious disease worldwide.
Materials and Methods
General Methods
Breeding mice were housed in micro-isolator cages with ad libitum access to laboratory chow and water during 12-hour light/dark cycles. Heterozygous ΔF508 Cftr (Cftr ΔF508/ΔF508) mice on a Black Swiss/129 SvJ hybrid background were bred to generate homozygous wildtype and ΔF508 Cftr animals of either sex. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Experimental protocol #98-232 was approved by the University of Rochester Animal Resources Committee. All surgery was performed under chloral hydrate anesthesia, and all efforts were made to minimize suffering. Reagents were obtained from Sigma (St. Louis, MO) unless otherwise specified.
Caries Studies
Male and female breeder pairs were separated prior to birth of the pups. Pups were inoculated by swabbing the oral cavity on 3 consecutive days starting 19 days after birth with S. mutans cultures (strain UA159; ATCC 700610), a well-characterized cariogenic bacterium [28]. After weaning at 22 days of age, both homozygous wildtype and ΔF508 pups were fed Diet 2000 and GoLYTELY (Braintree Laboratories, MA), an oral osmotic laxative used to increase the survival of ΔF508 mice [33]. An oral swab was obtained from pups one and four days after the final inoculation. The swab was plated on selective media to verify implantation by S. mutans (MSB plates, Mitis salivarius + bacitracin). All wildtype and ΔF508 animals were successfully infected by S. mutans. After five weeks on Diet 2000, mice were killed by CO2 asphyxiation. Caries scores were performed as previously described on both mandibular and maxillary first and second molar teeth [28].
In a pilot study, we tested three variables: 1) are CF mice and their control littermates susceptible to infection by S. mutans; 2) because CF mice display intestinal absorption defects, we also tested if the cariogenic diet (diet 2000) affects CF mouse viability; and 3) is gross caries seen after a short exposure to the cariogenic diet. Mice were infected with S. mutans for three consecutive days, as described above, and then exposed over a 13 day period to the cariogenic diet. The pilot study confirmed that all four CF and four wildtype mice were infected by S. mutans and that diet 2000 did not affect the viability of the CF mice. At the end of the 13 day exposure to the cariogenic diet, photos were taken of control and CF jaws. Note that smooth surface caries is not visible in the mandibular jaws from either the control or CF mice (Figure S1). This latter result is consistent with the current understanding that dental caries is a diet bacterial disease; i.e. caries doesn't occur in the absence of appropriate infection and dietary challenge over time. Our pilot study indicates that this is also true in the CF mouse model.
To estimate the number of viable S. mutans cells, the mandibular jaw was aseptically dissected, transferred to 5.0 ml of sterile saline solution and sonicated as described previously [34]. The suspensions were serially diluted and plated on mitis salivarius agar plus bacitracin (for S. mutans counts) using an automated spiral plater (Eddy Jet, IUL Instruments, Neutec Group Inc.). Following incubation, the viable recovered cells were determined by counting colony forming units (cfu) by means of a grid plate system. In parallel, the microbial suspensions were examined using quantitative real-time PCR and propidium monoazide (PMA, Biotium Inc., Hayward, CA) to quantify only cells with intact membrane (viable cells) as detailed by Nocker et al. [35]. After PMA treatment, the genomic DNA of treated microbial suspensions was extracted and purified using the MasterPure DNA purification kit (Epicenter Technologies). Ten nanograms of genomic DNA per sample and negative controls (without DNA) were amplified by a MyiQ real-time PCR detection system with iQ SYBR Green supermix (Bio-Rad Laboratories, Inc., CA, USA) using S. mutans 16S rRNA specific primers. The primers were designed using Beacon Designer 2.0 software (Premier Biosoft International, Palo Alto, CA, USA). For S. mutans quantification, a standard curve based on S. mutans genome size (2.03 Mb) was used as described previously [36]. This standard curve was used to transform the critical threshold cycle (Ct) values to the relative number of S. mutans cells.
In Vivo Whole Saliva Collection
Wildtype and ΔF508 mutant animals were anesthetized with chloral hydrate (400 mg/kg body weight, i.p.). Prior to saliva collection a tracheal tube was placed to maintain a patent airway during stimulation. Secretion was initiated by the injection of the muscarinic agonist pilocarpine HCl (10 mg/kg, i.p.). Whole saliva was collected by gravity into 1.5 mL plastic eppendorf tubes.
Saliva Ion Composition
The concentration of bicarbonate was determined as described by the manufacturer (Diazyme Laboratories, Poway, CA). Saliva pH was measured immediately after collection with a pH-sensitive electrode (Thermo Scientific, Beverly, MA).
Statistical Analysis
Graphs showed in figures 1, 2 and 3 are presented as the mean ± S.E. and the statistical significance was determined using Student's t test with Origin 7.0 Software (OriginLab, Northampton, MA). p values of less than 0.05 were considered statistically significant.
Supporting Information
Pilot study for dental caries in wildtype and ΔF508 mice. Lingual (panels A and C) and buccal (panels B and D) views of representative mandibular jaws from wildtype (WT, panels A and B) and mutant mice (ΔF508, panels C and D) show that no visible lesions were observed after 13 days exposure to a cariogenic diet.
(TIF)
Acknowledgments
The authors wish to thank Laurie Koek and Yasna Jaramillo for technical assistance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was funded by grants to JEM from the National Institutes of Health (DE09692 and DE08921). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Beltran-Aguilar ED, Barker LK, Canto MT, Dye BA, Gooch BF, et al. Surveillance for dental caries, dental sealants, tooth retention, edentulism, and enamel fluorosis–United States, 1988-1994 and 1999-2002. MMWR Surveill Summ. 2005;54:1–43. [PubMed] [Google Scholar]
- 2.Marsh PD. Are dental diseases examples of ecological catastrophes? Microbiology. 2003;149:279–294. doi: 10.1099/mic.0.26082-0. [DOI] [PubMed] [Google Scholar]
- 3.Bowen WH, Pearson SK, Young DA. The effect of desalivation on coronal and root surface caries in rats. J Dent Res. 1988;67:21–23. doi: 10.1177/00220345880670010301. [DOI] [PubMed] [Google Scholar]
- 4.Edgar WM, Bowen WH, Cole MF. Development of rampant dental caries, and composition of plaque fluid and saliva in irradiated primates. J Oral Pathol. 1981;10:284–295. doi: 10.1111/j.1600-0714.1981.tb01275.x. [DOI] [PubMed] [Google Scholar]
- 5.Watson GE, Pearson SK, Falany JL, Culp DJ, Tabak LA, et al. The effect of chronic atropine treatment on salivary composition and caries in rats. J Dent Res. 1989;68:1739–1745. doi: 10.1177/00220345890680120401. [DOI] [PubMed] [Google Scholar]
- 6.Quinton PM. Physiological basis of cystic fibrosis: a historical perspective. Physiol Rev. 1999;79:S3–S22. doi: 10.1152/physrev.1999.79.1.S3. [DOI] [PubMed] [Google Scholar]
- 7.Blomfield J, Warton KL, Brown JM. Flow rate and inorganic components of submandibular saliva in cystic fibrosis. Arch Dis Child. 1973;48:267–274. doi: 10.1136/adc.48.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies H, Bagg J, Goodchild MC, McPherson MA. Defective regulation of electrolyte and protein secretion in submandibular saliva of cystic fibrosis patients. Acta Paediatr Scand. 1991;80:1094–1095. doi: 10.1111/j.1651-2227.1991.tb11789.x. [DOI] [PubMed] [Google Scholar]
- 9.Davies H, Bagg J, Muxworthy S, Goodchild MC, McPherson MA. Electrolyte concentrations in control and cystic fibrosis submandibular saliva. Biochem Soc Trans. 1990;18:447–448. doi: 10.1042/bst0180447a. [DOI] [PubMed] [Google Scholar]
- 10.Aps JK, Delanghe J, Martens LC. Salivary electrolyte concentrations are associated with cystic fibrosis transmembrane regulator genotypes. Clin Chem Lab Med. 2002;40:345–350. doi: 10.1515/CCLM.2002.055. [DOI] [PubMed] [Google Scholar]
- 11.Catalan MA, Nakamoto T, Gonzalez-Begne M, Camden JM, Wall SM, et al. Cftr and ENaC ion channels mediate NaCl absorption in the mouse submandibular gland. J Physiol. 2010;588:713–724. doi: 10.1113/jphysiol.2009.183541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinudom A, Komwatana P, Young JA, Cook DI. A forskolin-activated Cl- current in mouse mandibular duct cells. Am J Physiol. 1995;268:G806–812. doi: 10.1152/ajpgi.1995.268.5.G806. [DOI] [PubMed] [Google Scholar]
- 13.Lee MG, Choi JY, Luo X, Strickland E, Thomas PJ, et al. Cystic fibrosis transmembrane conductance regulator regulates luminal Cl-/HCO3- exchange in mouse submandibular and pancreatic ducts. J Biol Chem. 1999;274:14670–14677. doi: 10.1074/jbc.274.21.14670. [DOI] [PubMed] [Google Scholar]
- 14.Zeiher BG, Eichwald E, Zabner J, Smith JJ, Puga AP, et al. A mouse model for the delta F508 allele of cystic fibrosis. J Clin Invest. 1995;96:2051–2064. doi: 10.1172/JCI118253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeng W, Lee MG, Yan M, Diaz J, Benjamin I, et al. Immuno and functional characterization of CFTR in submandibular and pancreatic acinar and duct cells. Am J Physiol. 1997;273:C442–455. doi: 10.1152/ajpcell.1997.273.2.C442. [DOI] [PubMed] [Google Scholar]
- 16.Handelman SL, Mills JR, Hawes RR. Caries incidence in subjects receiving long term antibiotic therapy. J Oral Ther Pharmacol. 1966;2:338–345. [PubMed] [Google Scholar]
- 17.Jagels AE, Sweeney EA. Oral health of patients with cystic fibrosis and their siblings. J Dent Res. 1976;55:991–996. doi: 10.1177/00220345760550065101. [DOI] [PubMed] [Google Scholar]
- 18.Aps JK, Van Maele GO, Claeys G, Martens LC. Mutans streptococci, lactobacilli and caries experience in cystic fibrosis homozygotes, heterozygotes and healthy controls. Caries Res. 2001;35:407–411. doi: 10.1159/000047483. [DOI] [PubMed] [Google Scholar]
- 19.Aps JK, Van Maele GO, Martens LC. Caries experience and oral cleanliness in cystic fibrosis homozygotes and heterozygotes. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:560–563. doi: 10.1067/moe.2002.121280. [DOI] [PubMed] [Google Scholar]
- 20.Narang A, Maguire A, Nunn JH, Bush A. Oral health and related factors in cystic fibrosis and other chronic respiratory disorders. Arch Dis Child. 2003;88:702–707. doi: 10.1136/adc.88.8.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrazzano GF, Orlando S, Sangianantoni G, Cantile T, Ingenito A. Dental and periodontal health status in children affected by cystic fibrosis in a southern Italian region. Eur J Paediatr Dent. 2009;10:65–68. [PubMed] [Google Scholar]
- 22.Durie PR, Pencharz PB. Cystic fibrosis: nutrition. Br Med Bull. 1992;48:823–846. doi: 10.1093/oxfordjournals.bmb.a072580. [DOI] [PubMed] [Google Scholar]
- 23.Guggenheim B, Schmid R, Aeschlimann JM, Berrocal R, Neeser JR. Powdered milk micellar casein prevents oral colonization by Streptococcus sobrinus and dental caries in rats: a basis for the caries-protective effect of dairy products. Caries Res. 1999;33:446–454. doi: 10.1159/000016550. [DOI] [PubMed] [Google Scholar]
- 24.Reynolds EC, Cain CJ, Webber FL, Black CL, Riley PF, et al. Anticariogenicity of calcium phosphate complexes of tryptic casein phosphopeptides in the rat. J Dent Res. 1995;74:1272–1279. doi: 10.1177/00220345950740060601. [DOI] [PubMed] [Google Scholar]
- 25.Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, et al. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257:1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- 26.Zou SJ, D'Souza RN, Ahlberg T, Bronckers AL. Tooth eruption and cementum formation in the Runx2/Cbfa1 heterozygous mouse. Arch Oral Biol. 2003;48:673–677. doi: 10.1016/s0003-9969(03)00135-3. [DOI] [PubMed] [Google Scholar]
- 27.Kristenova-Cermakova P, Peterka M, Lisi S, Lesot H, Peterkova R. Postnatal lower jaw dentition in different phenotypes of tabby mice. Connect Tissue Res. 2002;43:283–288. doi: 10.1080/03008200290000727. [DOI] [PubMed] [Google Scholar]
- 28.Culp DJ, Quivey RQ, Bowen WH, Fallon MA, Pearson SK, et al. A mouse caries model and evaluation of aqp5-/- knockout mice. Caries Res. 2005;39:448–454. doi: 10.1159/000088179. [DOI] [PubMed] [Google Scholar]
- 29.Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol. 2005;67:445–469. doi: 10.1146/annurev.physiol.67.041703.084745. [DOI] [PubMed] [Google Scholar]
- 30.Ishibashi K, Okamura K, Yamazaki J. Involvement of apical P2Y2 receptor-regulated CFTR activity in muscarinic stimulation of Cl(-) reabsorption in rat submandibular gland. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1729–1736. doi: 10.1152/ajpregu.00758.2007. [DOI] [PubMed] [Google Scholar]
- 31.Bronckers A, Kalogeraki L, Jorna HJ, Wilke M, Bervoets TJ, et al. The cystic fibrosis transmembrane conductance regulator (CFTR) is expressed in maturation stage ameloblasts, odontoblasts and bone cells. Bone. 2010;46:1188–1196. doi: 10.1016/j.bone.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gawenis LR, Spencer P, Hillman LS, Harline MC, Morris JS, et al. Mineral content of calcified tissues in cystic fibrosis mice. Biol Trace Elem Res. 2001;83:69–81. doi: 10.1385/BTER:83:1:69. [DOI] [PubMed] [Google Scholar]
- 33.Clarke LL, Gawenis LR, Franklin CL, Harline MC. Increased survival of CFTR knockout mice with an oral osmotic laxative. Lab Anim Sci. 1996;46:612–618. [PubMed] [Google Scholar]
- 34.Koo H, Schobel B, Scott-Anne K, Watson G, Bowen WH, et al. Apigenin and tt-farnesol with fluoride effects on S. mutans biofilms and dental caries. J Dent Res. 2005;84:1016–1020. doi: 10.1177/154405910508401109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nocker A, Sossa-Fernandez P, Burr MD, Camper AK. Use of propidium monoazide for live/dead distinction in microbial ecology. Appl Environ Microbiol. 2007;73:5111–5117. doi: 10.1128/AEM.02987-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dolezel J, Bartos J, Voglmayr H, Greilhuber J. Nuclear DNA content and genome size of trout and human. Cytometry A. 2003;51:127–128. doi: 10.1002/cyto.a.10013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pilot study for dental caries in wildtype and ΔF508 mice. Lingual (panels A and C) and buccal (panels B and D) views of representative mandibular jaws from wildtype (WT, panels A and B) and mutant mice (ΔF508, panels C and D) show that no visible lesions were observed after 13 days exposure to a cariogenic diet.
(TIF)