Abstract
Chlamydophila (Cp.) psittaci, the causative agent of psittacosis in birds and humans, is the most important zoonotic pathogen of the family Chlamydiaceae. These obligate intracellular bacteria are distinguished by a unique biphasic developmental cycle, which includes proliferation in a membrane-bound compartment termed inclusion. All Chlamydiaceae spp. possess a coding capacity for core components of a Type III secretion apparatus, which mediates specific delivery of anti-host effector proteins either into the chlamydial inclusion membrane or into the cytoplasm of target eukaryotic cells. Here we describe the interaction between Type III-secreted protein IncA of Cp. psittaci and host protein G3BP1 in a yeast two-hybrid system. In GST-pull down and co-immunoprecipitation experiments both in vitro and in vivo interaction between full-length IncA and G3BP1 were shown. Using fluorescence microscopy, the localization of G3BP1 near the inclusion membrane of Cp. psittaci-infected Hep-2 cells was demonstrated. Notably, infection of Hep-2 cells with Cp. psittaci and overexpression of IncA in HEK293 cells led to a decrease in c-Myc protein concentration. This effect could be ascribed to the interaction between IncA and G3BP1 since overexpression of an IncA mutant construct disabled to interact with G3BP1 failed to reduce c-Myc concentration. We hypothesize that lowering the host cell c-Myc protein concentration may be part of a strategy employed by Cp. psittaci to avoid apoptosis and scale down host cell proliferation.
Introduction
The avian and human pathogen Cp. psittaci is the causative agent of psittacosis and represents the most important animal chlamydiosis of zoonotic character [1]. In addition, recent surveys showed that Cp. psittaci can also be found in non-avian domestic animals and wildlife [2], [3]. All members of the family Chlamydiaceae are obligate intracellular parasites that develop in a host cell within an inclusion, i.e. a membrane-bound compartment that does not fuse with lysosomes [4]. The membrane of the inclusion is initially formed by invagination of the plasma membrane and pinching off of a vesicle containing the infectious form of the bacterium, the elementary body (EB). Thereafter, EBs differentiate into non-infectious but metabolically active reticulate bodies (RB), which proliferate within the expanding inclusion, giving rise to 1000 or more progeny per host cell. The developmental cycle ends after 2–3 days depending on the strain, when RBs transform back into EBs and are released into the extracellular medium [5]. During this unique biphasic developmental cycle replicating bacteria acquire energy and biosynthetic precursors from the infected cell. Furthermore, chlamydiae modulate cellular functions such as apoptotic programs and immune response [6], [7]. Studies on inhibitors of bacterial protein synthesis suggest that modulation of the host cell functions requires the activity of chlamydial proteins. All Chlamydiaceae ssp. possess genes encoding core components of a Type III Secretion (TTS) apparatus [8], a protein transport system used by Gram-negative bacteria to translocate proteins into the cytoplasm of the host cell. Therefore, it is commonly accepted that chlamydial effector proteins are targeted by the TTS to the inclusion membrane. The first set of chlamydial effector proteins identified was a family of integral inclusion membrane (Inc) proteins that share one remarkable feature, i.e. they possess a very large (50–80 amino acids) bilobed hydrophobic domain, a secondary structure motif predictive of protein localization to the chlamydial inclusion membrane [9], [10]. The first member of the family of Inc proteins identified, IncA, is the one that has attracted most of the attention. First cloned in Cp. caviae it has known homologs in Chlamydia (C.) trachomatis, Cp. pneumoniae, Cp. felis, C. muridarum, Cp. abortus and Cp. psittaci. The level of sequence similarity among the homologs is low, and antibodies against IncA do not cross-react with other chlamydial species. Furthermore, in all IncA proteins identified so far, SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) – like motifs were identified [5]. These motifs allow interactions with several host SNARE proteins, which are essential for membrane fusion [11], [12]. In addition to the bilobed hydrophobic domain Inc proteins, such as IncA and IncG, harbor domains exposed to the cytoplasmic side of chlamydial inclusion where they mediate interactions with eukaryotic host proteins such as Rab GTPases [13], [14], 14-3-3β protein [15], and Act1 [16]. Thus, Inc proteins are probably central regulators of pathogen-host interactions.
Ras-GTPase activating protein SH3 domain binding protein 1 (G3BP1) was initially identified as an ubiquitously expressed cytosolic 68 kDa protein that co-immunoprecipitates with Ras-GTPase-activating protein (GAP). The G3BP1 cDNA revealed that G3BP1 is a 466-amino-acid protein that shares several features with heterogeneous nuclear RNA-binding proteins, including RNA recognition motifs (RRM) RNP1 and RNP2, an RG-rich domain and acidic sequences [17]. G3BP1 colocalizes and physically interacts with GAP at the plasma membrane of serum-stimulated but not quiescent Chinese hamster lung fibroblasts. In quiescent cells, G3BP1 was hyperphosphorylated on serine residues and harbors a phosphorylation-dependent RNase activity which specifically cleaves the 3′-untranslated region of human c-myc mRNA [18]. In addition of its role in Ras-GAP signalling and its function as a phosphorylation-dependent RNase several other putative biological activities of G3BP1 were suggested, i.e. involvement in NFκB and IκB nucleo-cytoplasmic equilibrium, interactions with ubiquitin-specific proteases and participation in stress-granule formation (reviewed in [19]). Moreover, the G3Bp family of proteins is evolutionarily conserved throughout eukaryota [19].
In this study, we describe the interaction between the Type III-secreted protein IncA of Cp. psittaci [20] and the host protein G3BP1 in a yeast two-hybrid system. While there is a growing list of publications dealing with interactions between Incs from C. trachomatis and Cp. pneumoniae, this is, to our knowledge, the first example of a documented interaction between an Inc protein from a zoonotic chlamydia and a host protein. In GST-pull down and co-immunoprecipitation experiments, both in vitro and in vivo interaction between full-length IncA and G3BP1 could be shown. Using fluorescence microscopy the localization of G3BP1 near the inclusion membrane of Cp. psittaci-infected Hep-2 cells was demonstrated. Finally, infection of Hep-2 cells with Cp. psittaci and overexpression of IncA in HEK293 cells led to a decrease in c-Myc protein concentration, but not at mRNA level. This effect could be ascribed to the interaction between IncA and G3BP1 since overexpression of an IncA mutant construct disabled to interact with G3BP1 did not cause a decrease in c-Myc concentration. Additionally, siRNA mediated knock-down of G3BP1 in Hep-2 cells had the same influence on c-Myc protein level as overexpression of IncA.
Results
IncA of Cp. psittaci and the host protein G3BP1 interact in a yeast two-hybrid system
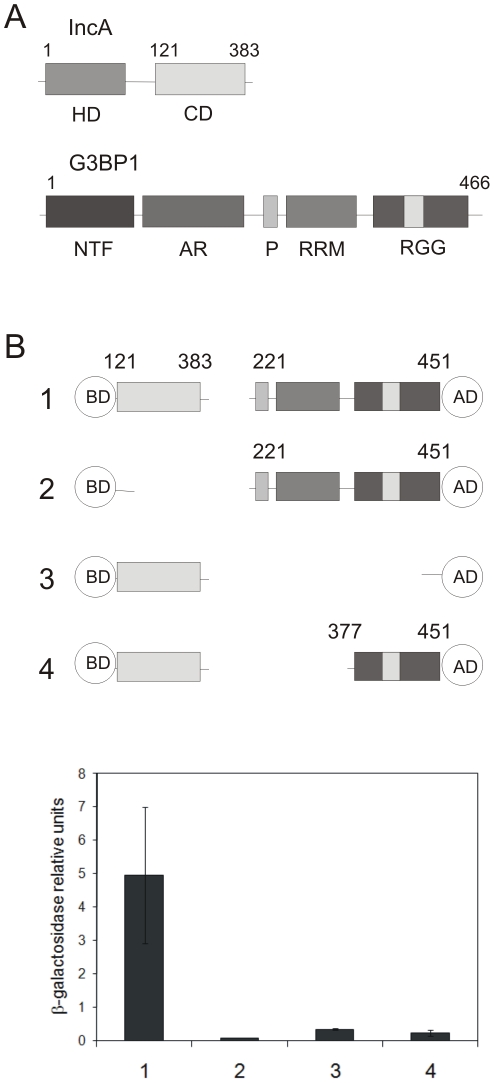
To gain insights into pathogenicity mechanisms of zoonotic Chlamydiaceae, we chose the cytoplasmic domain (aa 121-383) of the inclusion membrane protein IncA from Cp. psittaci DC15 (Fig. 1A) as a bait in a yeast two-hybrid screen for specific interactions with target eukaryotic proteins. The localization of IncA from Cp. psittaci in the inclusion membrane of infected cells was shown very recently by fluorescence microscopy and immuno electron microscopy [20]. Expression of the cytoplasmic domain of IncA from Cp. psittaci fused to the GAL4 DNA-Binding domain (BD) in pGBKT7 (CLONTECH) in Sacharomyces (S.) cerevisiae Y190 (CLONTECH) did not activate any of the reporter genes (HIS3, lacZ) (Fig. 1B). Therefore this strain was mated with S. cerevisiae Y187 expressing the HeLa cDNA MATCHMAKER 3 library in the activation domain (AD) vector pGADT7-Rec (CLONTECH). HeLa cDNA-expressing plasmids from clones growing on high stringency medium were isolated and retransformed into competent yeast expressing BD-IncA (aa 121-383). In the case of positive retransformation HeLa cDNA-expressing plasmids were recovered from these strains, propagated in Echerichia (E.) coli and sequenced to identify coding sequences. One isolate contained in frame coding sequence for the C-terminal part (aa 221-451) of human G3BP1 (Fig. 1A). To verify the validity of the interaction S. cerevisiae Y190 expressing pGBKT7-IncA (aa 121-383) or only the GAL4 BD were co-transformed with the G3BP1 HeLa-library plasmid and the interaction was quantitatively assessed by β-galactosidase (β-gal) activity assays (Fig. 1B). Compared with the IncA-BD and pGADT7 (column 3) and pGBKT7 and AD-G3BP1 (aa 221-451) (column 2) vector controls, β-gal activity was significantly induced in S. cerevisiae Y190 expressing pGBKT7-IncA (aa 121-383) and AD-G3BP1 (aa 221-451) (column 1). The G3BP1 HeLa-library fragment encludes the characteristic RNA recognition motifs of G3BP1 (Fig. 1A). To further narrow down the interaction surface between IncA and G3BP1 we deleted the RRM resulting in an AD-G3BP1 (aa 377-451) construct (Fig. 1B). As shown in Figure 1B, column 4, deletion of the RRM led to the complete loss of interaction with IncA (aa 121-383) in the yeast two-hybrid system. Taken together, these data indicate that the cytoplasmic domain of Cp. psittaci IncA specifically interacts with the C-terminal part of human G3BP1 in the yeast two-hybrid system.
Figure 1. IncA of Cp. psittaci interacts with human G3BP1.
(A) Schematic structures of IncA of Cp. psittaci and human G3BP1. Abbreviations: HD, hydrophobic domain; CD, cytoplasmic domain; NTF2, Nuclear Transport Factor 2-like domain; AR, acid rich region; P, proline rich motif; RRM, RNA recognition motif; RGG, arginine-glycine rich box. (B) Specific interaction of the IncA-CD fused to GAL4 DNA-binding domain (BD) with C-terminal part (aa 221-451) of G3BP1 fused to GAL4 activation domain (AD) in yeast. Interactions were quantitatively assessed by β-galactosidase liquid culture activity assays. β-galactosidase values represent the mean numbers and standard deviations of three independent experiments.
Full-length proteins IncA of Cp. psittaci and human G3BP1 interact in vitro and in vivo in HEK293 cells
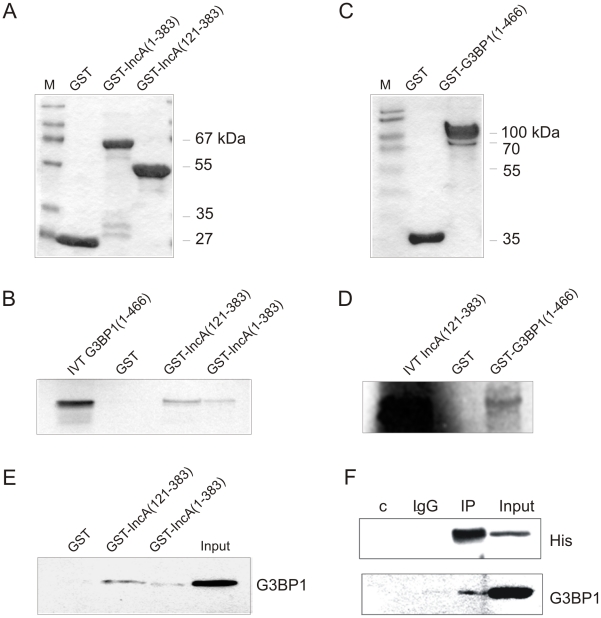
Next we performed GST pull-down assays to examine whether IncA and G3BP1 directly interact in vitro. Glutathione Sepharose beads bound to GST-tagged full-length IncA, GST-IncA (aa 121-383) or GST alone (Fig. 2A) were incubated with [35S]methionine-labeled full-length G3BP1, processed as described in Materials and Methods section and analyzed by autoradiography. As shown in Figure 2B, full-length G3BP1 bound specifically to both the GST-IncA (aa 121-383) and the GST-IncA full-length matrix, but no binding was detected using GST as a control, suggesting a direct physical interaction between IncA of Cp. psittaci and human G3BP1. In the reversed experiment IncA (aa 121-383) bound specifically to the GST-G3BP1 (aa 1-466) matrix (Fig. 2C and D). In a second approach, we performed a GST pull-down experiment with Hep-2 cell lysates instead of [35S]methionine-labeled full-length G3BP1. The associated proteins were pelleted by centrifugation, washed and analyzed for full-length G3BP1 by Western blot analysis (Fig. 2E). Endogenous G3BP1 from Hep-2 cell lysate exclusively bound to GST-IncA (aa 121-383) and GST-IncA and did not interact with GST alone. Finally, in order to show that IncA and G3BP1 also interact in vivo, HEK293 cells were transfected with a His-tagged IncA mammalian expression construct. To get a stronger expression of the heterologous protein the codon usage of the IncA gene of Cp. psittaci was adapted to human cells. As shown in Figure 2E we were able to co-immunoprecipitate endogenous G3BP1 with His-tagged IncA using an anti-His antibody. No binding was obtained using pre-immune serum or Protein G-Sepharose beads only as negative controls. Consequently, our interaction studies showed that IncA and G3BP1 interact in vitro and in vivo. The cytoplasmic domain of IncA was proved sufficient to bind the full-length G3BP1.
Figure 2. Full-length IncA of Cp. psittaci and G3BP1 proteins interact in vitro and in vivo.
(A) Purified GST, GST-IncA (aa 1-338) and GST-IncA (aa 121-383) or (C) GST and GST-G3BP1 (aa1-466) stained with Coomassie. (B) Results of GST pull-down experiments performed with IncA full-length and truncation clone fused to GST and [35S]-methionine-labeled full-length G3BP1 or (E) with Hep-2 cell lysate instead of labeled G3BP1. (D) Results of GST pull-down experiment performed with full-length G3BP1 fused to GST and [35S]-methionine-labeled IncA (aa 121-383). GST served as control. IVT: 10 µl of the in vitro translation product. Input: 25% (75 µg protein) of Hep-2 cell lysate used for the interaction assay. (F) Whole cell lysate of HEK293 cells transfected with a His-tagged codon-adapted IncA mammalian expression construct was subjected to co-immunoprecipitation experiments using anti-His antibody. Co-immunoprecipitated endogenous G3BP1 was detected with a mouse anti-G3BP1 monoclonal antibody. Controls were beads alone (c) or pre-immune serum (lgG). Input: 10% (100 µg) of the protein used for immunoprecipitation.
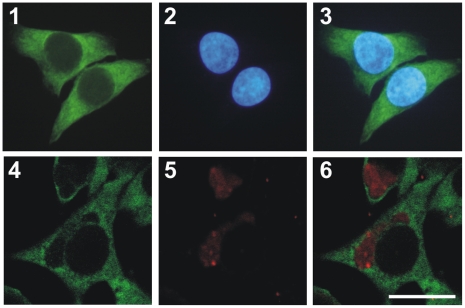
G3BP1 is accumulated around the chlamydial inclusion in Cp. psittaci-infected Hep-2 cells
As IncA of Cp. psittaci is localized on the membrane of the chlamydial inclusion [20], interaction with G3BP1 should lead to accumulation of G3BP1 in the vicinity of the inclusion. To test this assumption, we performed immunolocalization studies of endogenous G3BP1 in Cp. psittaci-infected and non-infected Hep-2 cells using polyclonal rabbit anti-G3BP1 antibody (Fig. 3). In non-infected Hep-2 cells, G3BP1 showed a uniformly cytoplasmic localization (Fig. 3, 1– 3). In Cp. psittaci-infected Hep-2 cells, a fraction of G3BP1 was found to be concentrated around the chlamydial inclusion as shown in Fig. 3 (4–6, inclusion in red). An alternative approach, the transfection of infected Hep-2 cells with a DsRed-G3BP1 expression vector, gave disappointing results because overexpression of G3BP1 induced assembly of stress granules [21], [22] which prevented any further study (data not shown). In summary, the immunolocalization data show an accumulation of G3BP1 around chlamydial inclusions which is due if IncA and G3BP1 interact.
Figure 3. G3BP1 is accumulated around the chlamydial inclusion in Cp. psittaci infected Hep-2 cells.
Hep-2 cells were infected with Cp. psittaci at a MOI of 3, and at 48 h postinfection the cells were fixed and stained with anti-chlamydial LPS (red), anti-G3BP1 (green) antibodies and DAPI (blue, nuclei), and viewed under a fluorescence (1–3) or a confocal laser-scanning microscope (4–6). Non-infected cells served as control (1–3). The anti-G3BP1 (1 and 4) and anti-LPS (5) or DAPI (2) signals were merged (3 and 6). A fraction of endogenous G3BP1 is concentrated around chlamydial inclusions (6). Bar = 20 µm.
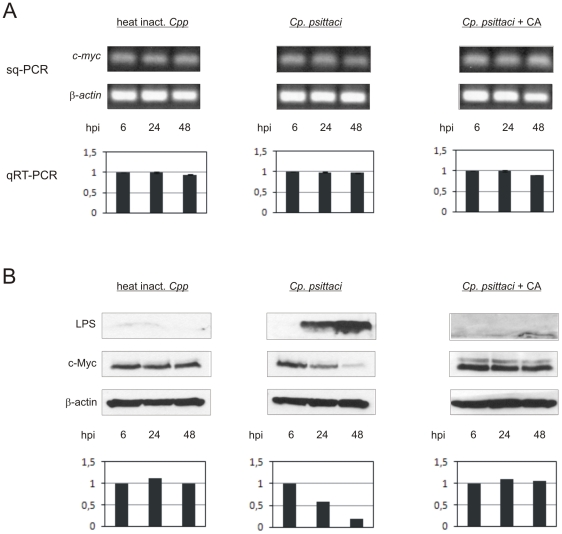
Infection of Hep-2 cells with Cp. psittaci leads to a decrease in c-Myc protein concentration, but does not affect c-myc mRNA concentration
As it is known that G3BP1 harbors a phosphorylation-dependent RNase activity which specifically cleaves the 3′-untranslated region of human c-myc mRNA [18], we wondered whether changes in c-myc mRNA and/or protein concentration would be detectable in Cp. psittaci-infected Hep-2 cells. For this purpose, Hep-2 cells were infected with Cp. psittaci at a MOI of 3, with heat-inactivated Cp. psittaci, or chloramphenicol-treated after infection as controls. Total RNA was isolated at different time points of infection and reverse transcribed into cDNA. Expression levels of c-myc mRNA and. β-actin as a control were estimated by semi-quantitative PCR (Fig. 4A, above) and by quantitative Real-Time PCR using GAPDH for standardization (Fig. 4A, below). In both experiments no differences in c-myc mRNA levels during the course of infection were detectable in comparison with the control (Fig. 4A). But to our surprise, the c-Myc protein concentration significantly declined in Cp. psittaci infected cells with progressive infection under the same experimental conditions (Fig. 4B, middle column). No changes in c-Myc protein concentration were detected in Hep-2 cells treated with heat-inactivated Cp. psittaci (Fig. 4B, left column). This inhibitory effect required bacterial protein synthesis as the level of c-Myc protein was nearly unchanged in Cp. psittaci-infected cells treated with chloramphenicol (Fig. 4B, right column). Protein levels of c-Myc were standardized to β-actin (Fig. 4B, below).These findings suggest that infection of Hep-2 cells with Cp. psittaci leads to a decrease in the c-Myc protein concentration of the host cell.
Figure 4. Infection of Hep-2 cells with Cp. psittaci leads to a decrease in c-Myc protein concentration.
Hep-2 cells were infected with Cp. psittaci at a MOI of 3, with heat-inactivated Cp. psittaci or chloramphenicol-treated after infection as controls, and at different hours postinfection (hpi) cells were lysed for RNA or protein analysis. (A, above) Semi-quantitative (sq) PCR shows the unchanged expression of c-myc mRNA in infected (middle column), infected and chloramphenicol-treated (right) and control (left) Hep-2 cells. β-actin served as an internal control gene. (A, below) Quantitative Real-Time (qRT) PCR. c-myc levels were standardized on GAPDH. Experiments were performed three times in duplicate. (B) Immunoblots of whole cell lysates of Cp. psittaci infected (middle column), infected and chloramphenicol-treated (right) and control (left) cells probed with anti-LPS, anti-c-Myc and anti-β-actin antibodies. Bar diagrams below the immunoblots represent a quantification of c-Myc protein related to β-actin protein concentration. Experiments were performed three times.
The decrease in c-Myc concentration could be ascribed to the interaction between IncA and G3BP1
To find out whether the decrease in c-Myc protein concentration during Cp. psittaci infection of mammalian cells is somehow connected with chlamydial IncA, we transfected HEK293 cells with a mammalian expression vector containing the HA-tagged codon-adapted cytoplasmic domain of IncA (aa 121-383) from Cp. psittaci, or the vector only as a control. Overexpression of this IncA fragment in HEK293 cells led to a decrease of c-Myc protein concentration in the same manner as in Cp. psittaci-infection of Hep-2 cells (Fig. 5C, lanes 1 and 2). This prompted us to hypothesize that the decrease in c-Myc protein concentration was caused by the recruitment of G3BP1 to the cytoplasmic portion of Cp. psittaci IncA. To verify this hypothesis we generated a mutant of IncA (aa 121-383) that was no longer able to interact with G3BP1. Mutagenesis as performed in S. cerevisiae using error-prone PCR (see Materials and Methods section) resulted in an IncA (aa 121-383) double mutant (N159S; S237G), which failed to interact with G3BP1 in the yeast two-hybrid system (Fig. 5A and B, column 3). The following overexpression of this mutant in HEK293 cells had no effect on c-Myc protein concentration (Fig. 5C, column 3). Assuming that recruitment of G3BP1 to IncA on the surface of inclusion is responsible for decrease in c-Myc protein concentration, siRNA-mediated knockdown of G3BP1 should have a similar effect. We therefore treated Hep-2 cells with a pool of four siRNAs specific for G3BP1 and as a control with non-targeting siRNAs and analyzed the G3BP1 and c-Myc protein concentrations by Western blot (Fig. 5D). As expected, downregulation of G3BP1 at approximately 50% led to a corresponding downregulation of c-Myc whereas the treatment of cells with control siRNA had no effect (Fig. 5D). As in the case of infection of Hep-2 cells by Cp. psittaci the c-myc mRNA level remained unchanged. We therefore conclude that the decrease in c-Myc protein concentration resulting from IncA (aa 121-383) in HEK293 cells and most likely also from infection of Hep-2 cells with Cp. psittaci can be ascribed to the interaction between IncA and G3BP1.
Figure 5. The decrease in c-Myc protein concentration can be ascribed to the interaction between IncA and G3BP1.
(A) Schematic structure of the Cp. psittaci IncA (aa 121-383) double mutant (N159S; S237G) which is no longer able to interact with G3BP1 in the yeast two-hybrid system. Mutagenesis was performed in S. cerevisiae using error-prone PCR. (B) Yeast two-hybrid interaction assay of mutated IncA with G3BP1. Interactions were quantitatively assessed by β-galactosidase liquid culture activity assays. β-galactosidase values represent the mean numbers and standard deviations of three independent experiments. (1) GAL4 BD-IncA (aa 121-383)-EGFP + pGADT7 (negative control), (2) GAL4 BD-IncA (aa 121-383)-EGFP + GAL4 AD-G3BP1 (aa 221-451) (positive control), (3) GAL4 BD-IncA (aa 121-383, N159S; S237G)-EGFP + GAL4 AD-G3BP1 (aa 221-451). (C) Immunoblots of whole HEK293 cell lysates transfected with pcDNA3 (1), pcDNA3-HA-IncA (aa 121-383) (2) or pcDNA3-HA-IncA (aa 121-383, N159S; S237G) (3) and probed with anti-HA, anti-c-Myc and anti-β-actin antibodies. Bar diagram below the immunoblots represent a quantification of c-Myc protein concentration related to β-actin. Experiments were performed in triplicate. (D) Immunoblots of whole Hep-2 cells untreated (1), transfected with non-targeting siRNA (2) or transfected with ON-TARGET G3BP1 siRNA pool and probed with anti-G3BP1, anti-c-Myc and anti-β-actin antibodies. Bar diagrams show knock-down rate of G3BP1 and c-Myc standardized to β-actin and the c-myc mRNA concentrations estimated by qRT-PCR. Experiments were performed in triplicate.
Discussion
All Chlamydiaceae express a TTS apparatus [8] capable of mediating specific delivery of anti-host effector proteins either into the chlamydial inclusion membrane or into the cytoplasm of target eukaryotic cells [10], [7]. Prominent examples of the first group of anti-host effector proteins are the Inc proteins. IncA was the first member of the Inc protein family to be identified, and it is still the only member of the Inc family for which a function has been proposed, namely involvement in the homotypic fusion of inclusions in C. trachomatis. In this process, the already mentioned SNARE-like motif within the cytoplasmic domain of IncA may play a role [5]. Despite the low sequence similarity among IncA proteins of the various Chlamydiaceae spp., conservation of biochemical properties of IncA proteins from Cp. caviae and C. trachomatis was found [5]. Both proteins self-associate to form multimers. When artificially expressed by the host cell, they localize to the endoplasmatic reticulum and completely inhibit concomitant inclusion development when additionally infected with Cp. caviae or C. trachomatis.
While the present knowledge on IncA functionality almost entirely refers to the human pathogens C. trachomatis and Cp. pneumoniae, we focused on the IncA protein of the zoonotic agent Cp. psittaci using a yeast two-hybrid screen to search for interacting host proteins that could indicate further functions of this protein. Having identified human G3BP1 as an interaction partner of IncA of Cp. psittaci in yeast, we conducted GST pull-down and co-immunoprecipitation experiments that confirmed the interaction between both proteins in vitro as well as in vivo in HEK293 cells transfected with an IncA-expression construct. Moreover, an accumulation of G3BP1 around the chlamydial inclusion was shown in Cp. psittaci-infected Hep-2 cells by means of fluorescence microscopy.
Consequently, the question of functional relevance of the IncA-G3BP1 interaction arose. As shown by Gallouzi et al., G3BP1 is hyperphosphorylated on serine residues and harbors a phosphorylation-dependent RNase activity that specifically cleaves the 3′-untranslated region of human c-myc mRNA [18]. Rather unexpectedly, our finding that c-myc mRNA concentrations were practically unchanged in the course of Cp. psittaci infection of Hep-2 cells was in contrast to the significant decrease in c-Myc protein. Overexpression of IncA in HEK293 cells caused the same effect, whereas an IncA mutant disabled to interact with G3BP1 in yeast did not cause a decrease in c-Myc protein concentration. Moreover, siRNA-mediated knockdown of G3BP1 in Hep-2 cells also downregulated c-Myc. This suggests that the effect could be ascribed to the interaction between IncA and G3BP1. Absence of the possibility to modify Chlamydia experimentally precluded us from proving this hypothesis in more detail. We have currently no definitive explanation for a connection between IncA-G3BP1 interaction and c-Myc concentration. An intriguing possibility is that interaction between these proteins disrupts a still unknown c-Myc-stabilizing interaction of G3BP1 with other host proteins thereby rendering c-Myc susceptible to degrading activity. An example for such a protein-stabilizing interaction of G3BP1 is the complex with USP10, a ubiquitin-specific protease, described for human [23] and yeast cells [24]. USP10 removes ubiquitin from proteins to which it is attached, leading to stabilization of the protein. At least for S. cerevisiae it was shown that USP10 requires an additional protein, i.e. G3BP1, to form an active de-ubiquitination complex that cleaves ubiquitin from specific substrates [24]. Whether the USP10 – G3BP1 complex is relevant for the stabilization of c-Myc protein in human cells remains to be elucidated.
The c-Myc transcription factor is a powerful regulator of cell growth, proliferation, differentiation and apoptosis [25], [26]. Given its potent effects on cell fate, it is not surprising that cells have employed sophisticated methods for ensuring proper c-Myc expression levels. The expression of c-Myc is regulated at every possible level: transcriptionally (initiation and elongation), post-transcriptionally (mRNA stability and translation) and post-translationally (protein stability) [27]. In 1994, the Eick and Hay laboratories provided evidence that c-Myc deregulation activates the tumor suppressor p53 and triggers apoptosis, a process, highly relevant for chlamydial development [28], [29]. Zindy and colleagues in the Roussel laboratory showed mechanistically that deregulated c-Myc upregulates Arf, which in turn activates p53 to regulate a cohort of target genes involved in apoptosis [30]. Afterwards several other c-Myc-induced pathways that contribute to the apoptotic response were described [31]. Obligate intracellular Chlamydiaceae depend on an intact host cell for replication and production of infective progeny. Therefore, they possess diverse strategies to prevent apoptotic cell death in the host [32]. One of these is the proteasomal degradation of pro-apoptotic BH3-only proteins by the chlamydial protease/proteasome-like activity factor (CPAF) [33]. Another successful mechanism is the upregulation of anti-apoptotic Bcl-2 like proteins such as Mcl-1 [34]. We suggest here another possible strategy employed by Cp. psittaci to overcome host cell apoptotic mechanisms, i.e. the inhibition of c-Myc-driven apoptotic events by lowering c-Myc protein concentration.
In addition to apoptosis regulation, another aspect of c-Myc effects on cell fate is highly relevant to chlamydial development, i.e. the regulation of cell cycle and, therefore, proliferation. c-Myc enables cell growth by providing the cell with an abundant supply of several classes of basic building blocks, as well as increasing cell metabolism and protein synthesis. Several c-Myc target genes are thought to have a role in this activity, including those associated with cellular metabolism, ribosomal and mitochondrial biogenesis, as well as protein and nucleic acid synthesis [27]. Furthermore, expression profiling data indicate that c-Myc has the capacity to both stimulate cell growth and abrogate cell cycle inhibitors, a powerful combination of functions that, when deregulated, may drive the limitless replicative potential characteristic of nearly all tumors [26]. Regarding Chlamydiaceae, it is widely accepted that they develop predominantely within terminally differentiated epithelial cells of mammalian mucosae and not within actively dividing cells [35]. Furthermore, studies on C. trachomatis showed that host cells infected with C. trachomatis undergo a lower rate of cell division than their uninfected counterparts [36], and that C. trachomatis infection inhibits host cell cytogenesis [37] and induces cleavage of the mitotic cyclin B1 [38]. We propose that the downregulation of c-Myc protein concentration during infection of Hep-2 cells with Cp. psittaci is an alternative way to reduce host cell proliferation and so to establish optimal conditions for development of this obligate intracellular microorganism within the host cell.
Materials and Methods
Cell culture and organisms
Hep-2 cells (ATCC-CCL23) were grown in DMEM medium with 4.5 g/l glucose (PAA) supplemented with 10% heat-inactivated fetal calf serum (FCS) and maintained at 37°C in an atmosphere of 5% CO2. Sub-confluent monolayers were inoculated with Cp. psittaci DC15 (bovine isolate, genotype A [39]) or heat-inactivated DC15 at a multiplicity of infection (MOI) of 3. After centrifugation at 2.000 rpm and 37°C for one hour they were maintained as described above. For inhibition of chlamydial protein synthesis cells were treated with 200 µg/ml chloramphenicol. HEK293 cells (ATCC-CRL1573) were cultivated in RPMI medium with L-glutamine (PAA) supplemented with 10% heat-inactivated FCS under mentioned conditions.
Mammalian expression constructs and transfections
To obtain an optimal expression of IncA in human cells full-length DNA sequence of IncA of Cp. psittaci was optimized for human codon usage (sequence available on demand), in vitro synthesized (GenScript) and cloned as an EcoRI-NotI-fragment into pcDNA3.1/myc-His A (Invitrogen). To express HA-tagged codon adapted cytoplasmic domain of IncA (aa 121-383) from Cp. psittaci in mammalian cells the corresponding DNA fragment was amplified by PCR using the following primers: forward-5′- GCGAATTCACCATGTACCCATACGATGTTCCAGATTACGCTACCCCCAGCCAGGTGGCCCGG-3′, reverse-5′-CGCCTCGAGTTACTGCTCGTAGATGGGGAAGCCCT-3′ and the full-length pcDNA3.1/myc-His A-construct (see above) or the mutated construct (see below) as template. EcoRI-XhoI-digested PCR fragments were transferred into pcDNA3 (Invitrogen). HEK293 cells were transfected with FuGENE® HD transfection reagent according to the manufacturer's instructions (Roche) and incubated for 24 hours.
For siRNA experiments Hep-2 cells were transfected with ON-TARGET plus Non-Targeting siRNA #1 or ON-TARGET plus SMART pool human G3BP1 using DharmaFECT 1 transfection reagent as described by the manufacturer (Thermo Scientific Dharmacon®), maintained for 72 hours and lysed as mentioned below.
Co-immunoprecipitation
HEK293 cells were transfected with pcDNA3.1/myc-His full-length codon-adapted IncA expression construct and maintained for 24 hours. Co-immunoprecipitation was performed with µMACS Protein G microbeads as described by the manufacturer (Miltenyi Biotec). Transfected HEK293 cells were washed twice with cold PBS, harvested and resuspended in low-salt lysis buffer. Cell lysate was incubated with monoclonal mouse anti-His antibody (Roche) and µMACS Protein G microbeads on ice. After 45 minutes magnetic separation using microcolumns and the µMACS separator was performed. Samples were extensively washed and co-immunoprecipitated proteins were eluted from the column with SDS gel loading buffer.
Immunoblotting
For immunoblotting of co-immunoprecipitated proteins, samples were loaded on a 10% SDS-PAGE and transferred to a nitrocellulose membrane (Whatman). Membranes were blocked with 5% skim milk in TBST for 1 h and incubated overnight at 4°C with mouse anti-His (Roche) or mouse anti-G3BP1 (Santa Cruz Biotechnology) primary antibodies. After washing three times with TBST membranes were incubated with horseradish peroxidase-conjugated goat anti-mouse secondary antibody (Dianova). For detection of protein bands ECL Plus Western Blotting Detection Reagent (GE Healthcare) was used as recommended. For Western blot analyses of infection experiments, Hep-2 cells were collected at different time points, washed twice with cold PBS and lysed using RIPA-buffer (150 mM NaCl, 65 mM Tris-HCl pH 7.5, 1% sodium deoxycholate, 1% Triton-X 100, 0.1% SDS). For detection of protein bands mouse anti-c-Myc (Santa Cruz Biotechnology), mouse anti-β-actin (Sigma Aldrich) and mouse anti-chlamydial LPS (AbD Serotec) primary antibodies and goat anti-mouse secondary antibody (Dianova) were used. Hep-2 cells treated with siRNA and HEK293 cells transfected with HA-tagged IncA expression vectors were treated equally. Detection occurred with monoclonal mouse anti-HA, mouse anti-G3BP1 and mouse anti-c-Myc (all obtained from Santa Cruz Biotechnology) primary antibodies and secondary anti-mouse antibody (Dianova).
Immunofluorescence and confocal microscopy
Hep-2 cells grown on coverslips were transfected with DsRED-G3BP1 expression construct (obtained from Zhi-Min Yuan, Harvard School of Public Health, Boston, USA) or DsRed vector. After 24 hours cells were infected with Cp. psittaci DC15 at a MOI of 3 and incubated for additional 48 hours. Infected and uninfected Hep-2 cells were washed with PBS and fixed with 3,8% paraformaldehyde. For neutralization cells were washed twice with 0,1 M glycin/PBS followed by permeabilization in 0,5% NP-40/PBS for 10 min. Then cells were blocked with PBS containing 0,1% NP-40 and 5% FCS overnight at 4°C. Immunostaining of endogenous G3BP1 occurred with polyclonal rabbit anti-G3BP1 antibody (Abcam), inclusions were visualized using mouse anti-chlamydial LPS antibody (AbD Serotec) by incubation for 1, 5 h at room temperature. After washing four times with 0,1% NP-40/PBS secondary FITC-conjugated goat anti-rabbit (Dianova) and RRX-conjugated goat anti-mouse (Dianova) were incubated for 1 h. All samples were additionally stained with 0,1 µg/ml DAPI, extensively washed with 0,1% NP-40/PBS, PBS and A. bidest. and mounted with MountFluor (Pro Taqs). Images of infected and immunostained Hep-2 cells were collected with a BX51 fluorescence microscope (Olympus) or an Axiovert 200 M/LSM 510 META laser scanning confocal microscope (Carl Zeiss, Jena) according to [40].
RNA isolation, semi-quantitative PCR and qRT-PCR
Hep-2 cells were infected as described above and collected at different time points. For RNA purification RNeasy Mini Kit and QIAshredder (Qiagen) were used according to manufacturer's instructions. For DNA digestion samples were treated with RQ1 RNase-free DNase (Promega) and purified with RNA Clean and Concentrator (Zymo Research) according to usage informations. Reverse transcription was done using M-MLV Reverse Transcriptase RNase H Minus (Promega), 0, 5 µg RNA, 1 µg oligo(dT)12-18 (Roth), 10 mM of each dNTP, 5 µl of 5× reaction buffer, 40 U of RiboLockTM RNase inhibitor (Fermentas) and 100 U M-MLV RT (H-) in a total volume of 25 µl according to manufacturer's instructions. With all RNA samples control reactions without reverse transcriptase during production of the cDNA which was subsequently used in the PCR were performed. For semi-quantitative PCR with 1 U Pwo-DNA-Polymerase (Peqlab), 1 µl of cDNA sample, 10 mM of each dNTP, 5 µl of 10× Pwo-reaction buffer and 10 µM of according primers were used in a total volume of 50 µl. Primer set: c-myc for: 5′-GCCAGAGGAGGAACGAGCT-3′ and c-myc rev: 5′-GGGCCTTTTCATTGTTTTCCA-3′; β-actin for: 5′-CTGGAACGGTGAAGGTGACA-3′ and β-actin rev: 5′-AAGGGACTTCCTGTAACAATGCA-3′. The following temperature-time profile was used: initial denaturation at 95°C for 5 min, 25 cycles of 95°C for 1 min, 55°C for 45 sec and 72°C for 45 sec, and a final elongation step at 72°C for 2 min. PCR products were electrophoresed on an 1% agarose gel containing ethidium bromide and visualized on an UV transilluminator.
For quantitative Real-Time PCR cDNA samples were diluted 1∶10 in RNase-free water. 2 µl of each sample, 0,2 µM of primers, c-myc for and c-myc rev (see above) or GAPDH for: 5′-TGCACCACCAACTGCTTAGC-3′ and GAPDH rev: 5′-GGCATGGACTGTGGTCATGAG-3′, and SsoFastTM EvaGreen® supermix with Low ROX (BIO-RAD) in a total volume of 20 µl were used. PCR was performed in StepONE Real-Time PCR-system: 95°C for 2 min, 40 cycles of 95°C for 5 sec and 62°C for 20 sec, and analyzed with StepONE software v2.0 (Applied Biosystems).
Yeast two-hybrid assay
The full-length IncA of Cp. psittaci DC15 was PCR amplified using chromosomal DNA of this species as template and the following primer sets: IncAPsi1: 5′-GCGAATTCATGACATCACCAGTAGAATCT-3′ and IncAPsi2: 5′-GCGGATCCTTACTGTTCATATATTGGGAA-3′ (aa 1-383). The cytoplasmic domain of IncA protein was PCR amplified in an analogous manner using the following primer set: IncAPsi4: 5′-GCGAATTCACTCCCTCTCAAGTGGCACGC-3′ (aa 121-383) and IncAPsi2. PCR fragments were digested with EcoRI-BamHI and transferred into the GAL4 BD destination vector pGBKT7 (CLONTECH). The C-terminal deletion fragment of G3BP1 was PCR amplified using the following primer set: G3BP1d: 5′-GCGAATTCGATGATTCTGAGCCTGTTCAG-3′ and G3BP1b: 5′-GCCGTCGACTCACTGCCGTGGCGCAAGCC-3′and DsRed-G3BP1 vector (kindly provided by Zhi-Min Yuan, Harvard School of Public Health, Boston, USA) as a template. PCR fragment was digested with EcoRI-SalI and transferred into the GAL4 AD destination vector pGADT7 (CLONTECH). S. cerevisiae Y190 (CLONTECH) expressing pGBKT7-IncA (aa 121-383) was mated for 6 hours with S. cerevisiae Y187 expressing the HeLa cDNA MATCHMAKER 3 library in the activation domain (AD) vector pGADT7-Rec (CLONTECH). Interacting clones were first selected by growth on vector-selective medium also lacking histidine but containing 5 mM 3-amino-1,2,4-triazole (3-AT). Emerging colonies were checked for activity of the second reporter gene lacZ by performing the colony-lift filter assay using X-Gal (5-Bromo-4-chloro-3-indolyl-α-D-galactopyranoside) as a substrate. HeLa cDNA-expressing plasmids were recovered from these strains, propagated in E. coli and sequenced to identify coding sequences. For quantitative two-hybrid assays S. cerevisiae Y190 was transformed with corresponding pGBKT7- and pGADT7-constructs and β-galactosidase liquid culture assays, using o-nitrophenyl β-D-galactopyranoside (ONPG) as substrate were performed according to the manufacturer's instructions (CLONTECH) [41]. The β-galactosidase values represent the mean numbers and standard deviations of three independent experiments.
Mutagenesis screen procedure
Construction of a mutated cytoplasmic domain of Cp. psittaci IncA disabled to interact with G3BP1 was performed in S. cerevisiae using error-prone PCR as already described [42]. Briefly, codon adapted cytoplasmic domain of IncA (aa 121-383) from Cp. psittaci was amplified by PCR analogous to the mammalian construct (see above) and cloned as a NdeI-EcoRI-fragment into pGBKT7 in frame with the enhanced green flourescence protein (EGFP) from pEGFP-C1 vector (CLONTECH) resulting in a GAL4 DB-IncA-EGFP fusion protein. The EGFP protein allowed to exclude frame shift mutations in the mutated IncA domain constructs. PCR primers (T7SEQ: 5′-GTAATACGACTCACTATAGGGCGA-3′ and GFPrev: 5′-GCTGAACTTGTGGCCGTTTAC-3′) flanking the IncA domain were used to amplify this region using error-prone PCR conditions as described [42] but utilizing SAWADY Taq-DNA-Polymerase (PEQLAB). The liniarized GAL4 DB-EGFP plasmid and the randomized PCR fragment were then co-transformed into yeast strain Y190 expressing the GAL4 AD-G3BP1 library plasmid (Fig. 1B). The high efficiency of homologous recombination in yeast, between the linearized plasmid and the PCR fragment, enabled expression of randomized cytoplasmic IncA domain chimeric proteins within yeast cells, permitting detection of those not binding to G3BP1 but still expressing EGFP via flourescence microscopy and colony-lift filter assay as described above.
GST pull-down
To produce GST-tagged IncA protein fragments, full-length and C-terminal cytoplasmic fragments from IncA of Cp. psittaci DC15 were transferred from corresponding pGBKT7-constructs (see Yeast two-hybrid assay section) into expression vector pGEX-4T-1 (GE Healthcare) encoding GST. To obtain GST-tagged full-length G3BP1 protein (aa 1-466) the corresponding DNA fragment was amplified by PCR using the following primers: forward-5′-GCGAATTCATGGTGATGGAGAAGCCTTAGT-3′, reverse-5′-GCCGTCGACTCACTGCCGTGGCGCAAGCC and DsRED-G3BP1 vector as template. EcoRI-SalI-digested PCR fragment was transferred into pGEX-4T-1. Plasmids containing sequences encoding GST, Cp. psittaci DC15 GST-IncA (aa 1-383), GST-IncA (aa 120-383) and GST-G3BP1(aa 1-466) were transformed into E. coli strain BL21-CodonPlus-RIL (Stratagene). 13 ml portions of overnight cultures were filled up to 20 ml with LB medium containing 100 µg of ampicillin/ml and fusion derivates were induced with 1 mM isopropyl β-D-thiogalactoside (IPTG) for induction of protein expression. After 1,5 h incubation cells were collected by centrifugation, resuspended in 1 ml PBS containing 1 mM PMSF and 10 mM DTT, and then sonicated. After sonification 1% Triton X-100 was added and samples were incubated for 30 min at 4°C. Then the lysates were cleared by centrifugation and the supernatants were subjected to Glutathione Sepharose affinity chromatography for purification of the GST fusion proteins as described by the manufacturer (GE Healthcare). [35S]-methionine-labeled G3BP1 full-length and IncA cytoplasmic domain proteins were synthesized in vitro by coupled T7 RNA polymerase-mediated transcription and translation (IVT) in a reticulocyte lysate system as described by the manufacturer (Promega). Glutathione Sepharose beads (50 µl) liganded by either GST-tagged protein fragments or GST alone were washed with HB-buffer (20 mM HEPES, 100 mM KCl, 5 mM MgCl2, 0,5% Igepal-CA630, 0.5 mM dithiothreitol, pH 7.4) and then resuspended in 100 µl HB-buffer containing 0.5% Igepal CA630 and 10 µl [35S]-methionine-labeled protein. After incubation at 4°C by constantly rotating for 2 hours, the beads were washed with HB-buffer extensively. Proteins were eluted from the beads using SDS sample buffer. After separation by 10% SDS-PAGE, G3BP1 and IncA cytoplasmic domain were revealed by exposure on KODAK X-OMAT AR film (Kodak). Analogous GST pull-down experiments were performed with Hep-2 cell lysates instead of [35S]-methionine-labeled G3BP1. The associated proteins were pelleted by centrifugation, washed and analyzed for full-length G3BP1 by Western blot analysis as described in the co-immunoprecipitation and Immunoblotting sections.
Acknowledgments
We are indebted to Zhi-Min Yuan for providing us with the DsRed-G3BP1 vector. The skillful technical assistance of Annemarie Carlstedt, Grit Mrotzek and Andrea Hartmann is gratefully acknowledged.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by the German Federal Ministry of Education and Research (BMBF), grant 01 KI 0724 to H.P.S. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Harkinezhad T, Geens T, Vanrompay D. Chlamydophila psittaci infections in birds: A review with emphasis on zoonotic consequences. Vet Microbiol. 2009;135:68–77. doi: 10.1016/j.vetmic.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 2.Szeredi L, Hotzel H, Sachse K. High prevalence of chlamydial (Chlamydophila psittaci) infection in fetal membranes of aborted equine fetuses. Vet Res Commun. 2005;29:37–49. doi: 10.1007/s11259-005-0835-1. [DOI] [PubMed] [Google Scholar]
- 3.Pantchev A, Sting R, Bauerfeind R, Tyczka J, Sachse K. Detection of all Chlamydophila and Chlamydia spp. of veterinary interest using species-specific real-time PCR assays. Comp Immunol Microbiol Infect Dis. 2009 doi: 10.1016/j.cimid.2009.08.002. In press. [DOI] [PubMed] [Google Scholar]
- 4.Heinzen RA, Scidmore MA, Rockey DD, Hackstadt T. Differential interaction with endocytic and exocytic pathways distinguish parasitophorous vacuoles of Coxiella burnetii and Chlamydia trachomatis. Infect Immun. 1996;64:796–809. doi: 10.1128/iai.64.3.796-809.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delevoye C, Nilges M, Dautry-Varsat A, Subtil A. Conservation of the biochemical properties of IncA from Chlamydia trachomatis and Chlamydia caviae. J Biol Chem. 2004;279:46896–46906. doi: 10.1074/jbc.M407227200. [DOI] [PubMed] [Google Scholar]
- 6.Ying S, Pettengill M, Ojcius DM, Häcker G. Host-cell survival and death during Chlamydia infection. Curr Immunol Rev. 2007;3:31–40. doi: 10.2174/157339507779802179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valdivia RH. Chlamydia effector proteins and new insights into chlamydial cellular microbiology. Curr Opin Microbiol. 2008;11:53–59. doi: 10.1016/j.mib.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Peters J, Wilson DP, Myers G, Timms P, Bavoil PM. Type III secretion a la Chlamydia. Trends Microbiol. 2007;15:241–251. doi: 10.1016/j.tim.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Bannantine JP, Griffiths RS, Viratyosin W, Brown WJ, Rockey DD. A secondary structure motif predictive of protein localization to the chlamydial inclusion membrane. Cell Microbiol. 2000;2:35–47. doi: 10.1046/j.1462-5822.2000.00029.x. [DOI] [PubMed] [Google Scholar]
- 10.Betts HJ, Wolf K, Fields KA. Effector protein modulation of host cells: examples in the Chlamydia spp. arsenal. Curr Opin Microbiol. 2008;11:1–7. doi: 10.1016/j.mib.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Delevoye C, Nilges M, Dehoux P, Paumet F, Perrinet S, et al. SNARE protein mimicry by an intracellular bacterium. PLoS Pathog. 2008;4:e1000022. doi: 10.1371/journal.ppat.1000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paumet F, Wesolowski J, Garcia-Diaz A, Delevoye C, Aulner N, et al. Intracellular bacteria encode inhibitory SNARE-like proteins. PLoS ONE. 2009;4:e7375. doi: 10.1371/journal.pone.0007375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rzomp KA, Moorhead AR, Scidmore MA. The GTPase Rab4 interacts with Chlamydia trachomatis inclusion membrane protein CT229. Infect Immun. 2006;74:5362–5373. doi: 10.1128/IAI.00539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortes C, Rzomp KA, Tvinnereim A, Scidmore MA, Wizel B. Chlamydia pneumoniae inclusion membrane protein Cpn0585 interacts with multiple Rab GTPases. Infect Immun. 2007;75:5586–5596. doi: 10.1128/IAI.01020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scidmore MA, Hackstadt T. Mammalian 14-3-3β associates with the Chlamydia trachomatis inclusion membrane via its interaction with IncG. Mol Microbiol. 2001;39:1638–1650. doi: 10.1046/j.1365-2958.2001.02355.x. [DOI] [PubMed] [Google Scholar]
- 16.Wolf K, Plano GV, Fields KA. A protein secreted by the respiratory pathogen Chlamydia pneumoniae impairs IL-17 signalling via interaction with human Act1. Cell Microbiol. 2009 doi: 10.1111/j.1462-5822.2009.01290.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parker F, Maurier F, Delumeau I, Duchesne M, Faucher D, et al. A Ras-GTPase-activating protein SH3-domain-binding protein. Mol Cell Biol. 1996;16:2561–2569. doi: 10.1128/mcb.16.6.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallouzi IE, Parker F, Chebli K, Maurier F, Labourier E, et al. A novel phosphorylation-dependent Rnase activity of GAP-SH3 binding protein: a potential link between signal transduction and RNA stability. Mol Cell Biol. 1998;18:3956–3965. doi: 10.1128/mcb.18.7.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irvine K, Stirling R, Hume D, Kennedy D. Rasputin, more promiscous than ever: a review of G3BP. Int J Dev Biol. 2004;48:1065–1077. doi: 10.1387/ijdb.041893ki. [DOI] [PubMed] [Google Scholar]
- 20.Beeckman DSA, Geens T, Timmermans JP, Van Oostveldt P, Vanrompay DCG. Identification and characterization of a type III secretion system in Chlamydophila psittaci. Vet Res. 2008;39:27. doi: 10.1051/vetres:2008002. [DOI] [PubMed] [Google Scholar]
- 21.Tourriere H, Chebli K, Zekri L, Courselaud B, Blanchard JM, et al. The RasGAP-associated endoribonuclease G3BP assembles stress granules. J Cell Biol. 2003;160:823–831. doi: 10.1083/jcb.200212128. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Buchan JR, Parker R. Eukaryotic stress granules: the ins and outs of translation. Mol Cell. 2009;36:932–941. doi: 10.1016/j.molcel.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soncini C, Berdo I, Draetta G. Ras-GAP SH3 domain binding protein (G3BP) is a modulator of USP10, a novel human ubiquitin specific protease. Oncogene. 2001;20:3869–3879. doi: 10.1038/sj.onc.1204553. [DOI] [PubMed] [Google Scholar]
- 24.Cohen M, Stutz F, Belgareh N, Haguenauer-Tsapis R, Dargemont C. Ubp3 requires a cofactor, Bre5, to specifically de-ubiquitinate the COPII protein, Sec23. Nature Cell Biol. 2003;5:661–667. doi: 10.1038/ncb1003. [DOI] [PubMed] [Google Scholar]
- 25.Sears RC. The life cycle of c-Myc. From synthesis to degradation. Cell Cycle. 2004;3:1133–1137. [PubMed] [Google Scholar]
- 26.Eilers M, Eisenmann RN. Myc's broad reach. Genes Dev. 2008;22:2755–2766. doi: 10.1101/gad.1712408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer N, Penn LZ. Reflecting on 25 years with MYC. Nature Rev Cancer. 2008;8:976–990. doi: 10.1038/nrc2231. [DOI] [PubMed] [Google Scholar]
- 28.Hermeking H, Eick D. Mediation of c-Myc-induced apoptosis by p53. Science. 1994;265:2091–2093. doi: 10.1126/science.8091232. [DOI] [PubMed] [Google Scholar]
- 29.Wagner AJ, Kokontis JM, Hay N. Myc-mediated apoptosis requires wild-type p53 in a manner independent of cell cycle arrest and the ability of p53 to induce p21. Genes Dev. 1994;8:2817–2830. doi: 10.1101/gad.8.23.2817. [DOI] [PubMed] [Google Scholar]
- 30.Zindy F, Eischen CM, Randle DH, Kamijo T, Cleveland JL, et al. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 1998;12:2424–2433. doi: 10.1101/gad.12.15.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nilsson JA, Cleveland JL. Myc pathways provoking cell suicide and cancer. Oncogene. 2003;22:9007–9021. doi: 10.1038/sj.onc.1207261. [DOI] [PubMed] [Google Scholar]
- 32.Böhme L, Rudel T. Host cell death machinery as a target for bacterial pathogens. Microbes Infect. 2009;11:1063–1070. doi: 10.1016/j.micinf.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 33.Fischer SF, Vier J, Kirschnek S, Klos A, Hess S, et al. Chlamydia inhibit host cell apoptosis by degradation of proapoptotic BH3-only proteins. J Exp Med. 2004;200:905–916. doi: 10.1084/jem.20040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajalingam K, Sharma M, Lohmann C, Oswald M, Thieck O, et al. Mcl-1 is a key regulator of apoptosis resistance in Chlamydia trachomatis-infected cells. PLoS ONE. 2008;3:e3102. doi: 10.1371/journal.pone.0003102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moulder JW. Interaction of chlamydiae and host cells in vitro. Microbiol Rev. 1991;55:143–190. doi: 10.1128/mr.55.1.143-190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horoschak KD, Moulder JW. Devision of single host cells after infection with chlamydiae. Infect Immun. 1978;19:281–286. doi: 10.1128/iai.19.1.281-286.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greene W, Zhong G. Inhibition of host cell cytokinesis by Chlamydia trachomatis infection. J Infect. 2003;47:45–51. doi: 10.1016/s0163-4453(03)00039-2. [DOI] [PubMed] [Google Scholar]
- 38.Balsara ZR, Misaghi S, Lafave JN, Starnbach MN. Chlamydia trachomatis infection induces cleavage of the mitotic cyclin B1. Infect Immun. 2006;74:5602–5608. doi: 10.1128/IAI.00266-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goellner S, Schubert E, Liebler-Tenorio E, Hotzel H, Saluz HP, et al. Transcriptional response patterns of Chlamydophila psittaci in different in vitro models of persistent infection. Infect Immun. 2006;74:4801–4808. doi: 10.1128/IAI.01487-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wollmann Y, Schmidt U, Wieland GD, Zipfel PF, Saluz HP, et al. The DNA topoisomerase IIβ binding protein I (TopBP1) interacts with poly (ADP-ribose) polymerase (PARP-1). J Cell Biochem. 2007;102:171–182. doi: 10.1002/jcb.21292. [DOI] [PubMed] [Google Scholar]
- 41.Breeden L, Nasmyth K. Cell cycle control of the yeast HO gene: cis- and trans-acting regulators. Cell. 1987;48:389–397. doi: 10.1016/0092-8674(87)90190-5. [DOI] [PubMed] [Google Scholar]
- 42.Schneider S, Buchert M, Georgiev O, Catimel B, Halford M, et al. Mutagenesis and selection of PDZ domains that bind new protein targets. Nature Biotechnol. 1999;17:170–175. doi: 10.1038/6172. [DOI] [PubMed] [Google Scholar]