Summary
Objectives
There is a resurgence of interest in lung-sparing extirpative surgery for malignant pleural mesothelioma with recent reports of better survival and fewer adverse consequences than with extrapleural pneumonectomy. However, these operations are not well-characterized and to offer evidence-based clinical recommendations and to plan future trials a summary of what is already known is required.
Design
A formal literature search was performed and all recovered titles were sequentially sifted by title, abstract and full-text reading according to prespecified criteria. Papers were selected if they contained data relevant to the area of enquiry. Quantitative synthesis and textual analysis, appropriate to the material, were performed.
Setting
Follow-up studies of patients undergoing surgery for malignant pleural mesothelioma in specialist thoracic or cardiothoracic units.
Participants
Among the operated patients described in these papers, a total of 1270 patients had undergone lung-sparing surgery for mesothelioma.
Results
There were no randomized trials or other forms of controlled studies. From 464 titles, 26 papers contained sufficient data on 1270 patients to be included in the systematic review. Operative descriptions for all series were extracted and tabulated and variation was found in the nature of surgery within and between series, and the degree of detail with which it was described. There was more operative detail in recent papers. All available numerical data were extracted, tabulated and summarized using quantitative methods. The average survival at 1, 2, 3, 4 and 5 years was 51%, 26%, 16%, 11% and 9%, respectively. There were no data on patients’ performance status, symptomatic change, or other patient reported outcomes.
Conclusions
In the absence of any form of control data, no conclusions can be drawn concerning survival differences or symptomatic benefits attributable to surgery. As mesothelioma surgery is restricted to a selected minority of patients who often have multiple therapies, future research will require controlled studies with explicit definitions of the clinical and surgical intent.
Introduction
The incidence of malignant pleural mesothelioma (MPM) in Europe is still rising and Britain has the highest mesothelioma death rate in the world. Asbestos exposure was at its greatest between 1950 and 1970 and death rates are expected to peak in the next few years mirroring the pattern of asbestos imports.1–3 The number of cases of the disease faced in Europe as a whole is high and there is an incalculable burden of disease expected in the less developed world.
No part of this practice was subjected to randomized trial prior to the Mesothelioma and Radical Surgery (MARS) trial. Extrapleural pneumonectomy (EPP) accompanied by adjuvant therapy was regarded as offering the best prospect of prolonged survival following Sugarbaker's report about 10 years ago4 but a large multivariate analysis comparing the results of EPP with pleurectomy/decorticiaton (PD) has since shown better survival for the lung sparing form of surgery.5 It was stressed by the authors that this difference in favour of PD was despite the observation that the group undergoing PD were considered to have a relatively worse prognosis and to be less favourable cases for surgery.5 At the same time there has been an increased interest in PD6–8 and it is given prominence in recent European guidelines.9
In the literature a range of terms appear but pleurectomy/decortication appears in both European9 and American guidance.10 Pleurectomy may be termed partial, total or radical while the relative incompleteness of the extirpation of the cancer is signified by terms such as debulking, occasionally cytoreduction, and increasingly by the term macroscopic complete resection. What these operations have in common is that they are all extirpative surgery for mesothelioma, which does not include pneumonectomy. It is this surgery that is the subject of this systematic review.
Materials and methods
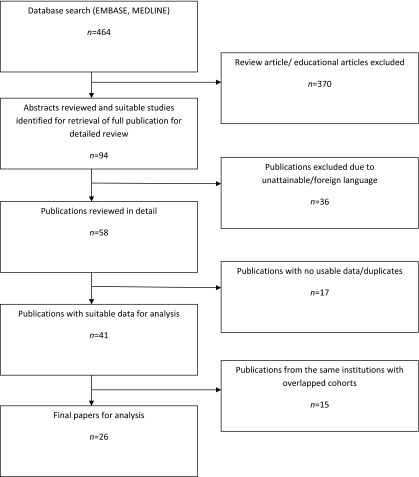
The literature was searched using a formal strategy (Appendix 1 – see http://jrsm.rsmjournals.com/cgi/content/full/jrsm.2010.100345/DC1 ). Manuscript titles were searched to identify papers that might include data concerning survival following non-EPP surgery for mesothelioma, with or without multimodality treatment. Papers excluded at the first selection (ET,CT) were teaching and review articles, technical reports, and those containing no relevant data concerning surgical treatment. A further selection was made to remove any registries that overlapped with institutional reports and single institutional studies that were superseded by later reports from the same institution (ET, TT). Obvious exclusions were made on the basis of abstracts, and further exclusions after reading 58 full articles (Figure 1). To exclude any selection bias, decisions about inclusions and exclusions were made in discussion between all four authors before any analysis or data synthesis, purely on the basis of whether usable data could be extracted.
Figure 1.
Flow diagram to show the stepwise process of sifting and selecting papers
Papers were individually searched and data extracted (ET) and all data included in analyses were confirmed in working sessions with two or more of the authors working together (ET, FF, TT). We looked for the start and end dates of the reported series, the number of patients reported, sex, age, tumour histology, stage, asbestos exposure, laterality, type of surgery performed, any adjuvant therapy, all survival data available, respiratory outcomes, and patterns of recurrence. We performed a textual analysis of the descriptions of operations performed. We constructed evidence tables and created graphical displays of aggregated and discrete data as appropriate.
Results
The search resulted in 464 titles which by various inclusion/exclusion processes resulted in 26 papers containing data on lung-sparing surgery (Figure 1).5,7,8,11–33 There were no randomized trials or studies comparing the outcomes of surgery with a non-operated control group. The reports were heterogeneous, some reporting various forms of surgery within the same report and others reporting surgical patients within an overall experience including non-operated patients. This made analysis difficult so we have been careful not to over interpret any findings but to present them with the stated limitations.
A total of 1270 patients are included in the reports used in the analysis although the number incorporated varies from one data summary to another because data were not available for every analysis in all papers. The (weighted) mean age in the whole series was 62 years. The average age shows a small upward trend over the 30 years of reported data but the age range of patients included is essentially unchanging over 25 years of reporting (Figure 2). Male patients are in the majority in all reports with an average of 80% men.
Figure 2.
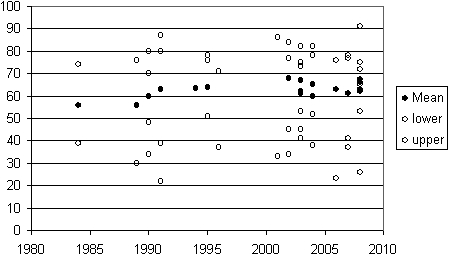
Patient age over time. For publications where the age in years (vertical axis) of operated patients is provided (mean age for 16 studies and the range in 23). The data are plotted against the year of publication
Asbestos exposure was recorded in 13 papers7,8,13,16,17,21,22,24,25,27,31–33 and was positive in 64% of patients (682/1061 patients) with a range of 17–95%. The rate in some reports is given for all mesothelioma patients, not just those operated. The variation in the asbestos exposure rate is more likely to be attributable to the assiduity of recording rather than a real epidemiological variation.
In four reports5,11–13 including 564 patients (individual series included 15, 26, 245 and 278 patients) 60% had right-sided disease with the proportion ranging from 52–62% for the four series included. This is consistent and the difference between right and left may simply reflect the relative surface area of pleura at risk.
In 14 reports8,12,13,17,22,24,26 the breakdown of operated patients by histology is provided ( Figure 3). The dominance of epithelioid type is evident. The report of Clarke et al.14 is an outlier by virtue of the large number of mixed tumours reported. It is relatively recent and from a group with a large experience and may illustrate a recognition that the dichotomy for epithelioid versus sarcomatoid histology is often not possible.
Figure 3.
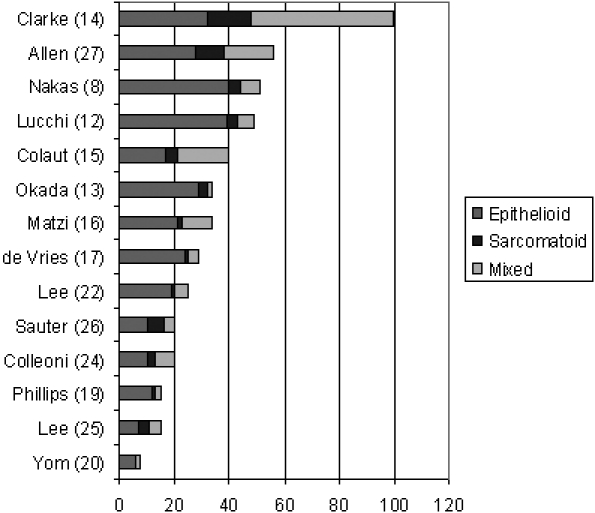
For the 14 papers in which the histology of operated patients was found the absolute number of cases of each classification is illustrated, ranked by total cases to aid the eye
In seven reports8,20,21,24,25,31,32 there is a breakdown by stage of operated patients (Table 1). The staging systems have varied over time and are more comprehensively reported in some recent papers, for example in 2008 by Nakas.8 The authors state ‘The patients that had radical P/D were staged according to the WHO and IMIG staging systems but this was not appropriate for group NR [non-radical]’. Use of a different staging system for different operations clearly represented a warning to us as we considered how to aggregate data. It is also likely from the table that in later series8,21,23 higher stage patients are planned for PD (lower stage being offered EPP) while earlier more of the lower (more selected) stage patients had surgery in the form of PD. There is likely to be deliberate selection of different types of patients, depending on whether the group offered either or both operations at the time, making further analysis unlikely to be informative. As an illustration of the potential for over interpretation of data to mislead, consider Table 1. There is an impression of relatively longer survival with increasing proportion of advanced stage. Further analysis along these lines would be unlikely to add useful knowledge about the role of PD in the management of mesothelioma patients and whether it is more appropriate in one pathological stage than in another.
Table 1.
Staging and median survival in months as given in seven reports
A textual analysis of the nature of surgery described in these 26 reports is summarized in Table 2 where the papers are presented in chronological order of publication. Where authors give discrete descriptions of more than one form of lung-sparing surgery, they appear in separate rows. It is evident that there is considerable variation in the surgery performed. Most authors use the word ‘pleurectomy’ but it is often qualified as total, radical, non-radical, sub-total or partial. ‘Decortication’ is less often used but descriptions of surgery become more detailed over time and the abbreviation P/D for pleurectomy and decortication appears more recently. In many of these publications a mixture of operations are reported and we have only extracted data on PD in this paper. Sometimes there was a gradation in the surgery performed: in some reports if EPP was the intention but intrapoperatively that surgery could not be performed, these patients had some other, presumably lesser, but not always well characterized, form of surgery. For example, Allen gives a long and very detailed description of extrapleural pneumonectomy but if, for any of a number of patient or pathological factors, EPP was precluded in their view, the surgery performed was categorized by the one word, pleurectomy.27
Table 2.
A textual analysis of the operative descriptions for lung sparing extirpative surgery in its various forms. Where a study included more than one form of surgery (as determined by the authors, for example the Mayo Clinic report of Schipper et al.) these are described separately. The studies are in date order of reporting
| Author | From | Until | Series* | Stated surgical extent and intent† | Pericardium resected | Diaphragm resected |
|---|---|---|---|---|---|---|
| Law 198433 | 1971 | 1980 | 28/150 | Non-radical parietal pleurectomy and decortication of the lung, removing the bulk of the tumour as discussed by Butchart | ||
| Achatzy 198932 | 1969 | 1985 | 48/245 | Total pleurectomy‡ | 1 | |
| Achatzy 198932 | 1969 | 1985 | 72/245 | Partial pleurectomy | ||
| Ball 199030 | 1981 | 1985 | 13/35 | … radical surgery, i.e. pleurectomy with attempted removal of all macroscopic disease | ||
| Harvey 199031 | 1965 | 1988 | 9/94 | … palliative surgery consisting of decortication and pleurectomy | 1 | |
| Brancatisano 199128 | 1984 | 1989 | 45/45 | … subtotal parietal pleurectomy … costal parietal pleura was resected sparing the mediastinal and diaphragmatic pleurae | ||
| Branscheid 199129 | 1978 | 1989 | 82/301 | … palliative decortication … | ||
| Allen 199427 | 1958 | 1993 | 56/96 | Pleurectomy§ | ||
| Lee 199525 | 1986 | 1993 | 15/15 | At thoracotomy, most gross disease was resected in areas involving the pericardium, parietal, visceral, and mediastinal pleura. Disease involving the surface of the diaphragm underwent resection, but frank invasion of the diaphragm or chest wall precluded a complete resection | ||
| Sauter 199526 | 1988 | 1992 | 20/20 | An extrapleural stripping of the tumor was performed from the apex of the lung to the diaphragm and along the pericardium in an attempt to remove all gross disease. The phrenic nerve was preserved if necessary on an isthmus of pericardium. While clearing of the chest wall and much of the lung was usually possible, in many cases gross disease was left behind on the diaphragm and/or the mediastinum | ||
| Colleoni 199624 | 1990 | 1994 | 20/20 | A resection of all gross tumor with minimal residual disease (defined as no nodules >1 cm in diameter and 0.5 cm in thickness was possible in only seven patients; 13 cases had gross residual disease | ||
| Ceresoli 200123 | 1986 | 1989 | 54/121 | Surgical intervention was palliative pleurectomy with extensive debulking of tumor in all cases | ||
| Aziz 200221 | 1989 | 1999 | 47/302 | A lesser procedure such as decortication/pleurectomy was considered for locally extensive disease … | ||
| Lee 200222 | 1986 | 1993 | 15/15 | A complete extrapleural dissection was performed on all patients. Occasionally, patients with locally extensive disease through the endothoracic fascia received limited en bloc chest wall resection. All efforts were made to avoid entry into the peritoneum. Patients underwent complete visceral pleurectomy, including clean dissection of the pulmonary artery and hilar structures | 8 | |
| De Vries 200317 | 1976 | 2001 | 29/46 | Although an attempt was made to remove all macroscopic visible tumor, the pleurectomies were usually incomplete, especially over the diaphragmatic and mediastinal surfaces | ||
| Monneuse 200318 | 1990 | 2000 | 16/24 | The extent of surgery was tailored according to the disease and the patient's condition. Maximal attempts were made to remove all macroscopic tumour | ||
| Phillips 200319 | 1989 | 1999 | 15/70 | Pleurectomy confined to the parietal pleura | ||
| Yom 200320 | 2000 | 2001 | 8/9 | Pleurectomy and decortication | ||
| Colaut 200415 | 1985 | 2002 | 40 | Surgical pleurectomy was preformed to remove all gross tumour or to obtain significant debulking. Partial or total pleurectomy of the visceral pleura depended on the extent of the tumour | ||
| Matzi 200416 | 1993 | 2003 | 34 | … debulking and decortication … All gross disease was removed, leaving as little residual tumour as possible | 2 | |
| Clarke 200614 | 1989 | 1999 | 100 | Decortication of the tumor was carried out as completely as possible, removing the parietal pleura and any tumor on the surface of the lung, and taking care to open all the fissures | ||
| Lucchi 200712 | 1999 | 2004 | 49 | … P/D consisting of the removal of the parietal and mediastinal pleura of the involved areas of the visceral pleura, with minimal resection of the lung if necessary. In case of minimal involvement of the pericardium and diaphragm, they were resected and sutured; however, they were never replaced with a mesh as for the radical P/D … | ||
| Okada 200813 | 1986 | 2006 | 34/87 | P/D was defined as total removal of the parietal pleura, visceral pleural, mediastinal pleura, pericardium and diaphragm | ≤34 | ≤34** |
| Flores 20085 | 1990 | 2006 | 278/663 | P/D removed tumor with the parietal and visceral pleurae and pericardium and/or diaphragm when necessary without removing the entire underlying lung | ≤278 | ≤278 |
| Schipper 200811 | 1985 | 2003 | 31/285 | … subtotal pleurectomy was defined as removal of up to 70% of the parietal pleura with debulking of as much of the mesothelioma as possible | ||
| Schipper 200811 | 1985 | 2003 | 10/285 | Total pleurectomy consisted of a complete extrapleural stripping of the parietal and mediastinal pleura and involved visceral pleura from the ipsilateral hemithorax without performing pulmonary resection. Total pleurectomy also included resection of the diaphragm and pericardium when necessary | ≤10 | ≤10 |
| Nakas 20087 | 51/102 | Non-radical: the operative objective was to remove the bulk of the tumour including both visceral and parietal pleura, to re-expand the trapped lung … The diaphragmatic and mediastinal surfaces were spared | ||||
| Nakas 20087 | 51/102 | Radical: with radical P/D the surgical objective was to achieve complete macroscopic clearance of the tumour with removal of the pericardium and diaphragm if required. Pericardium and diaphragm were reconstructed using prosthetic patches | ≤51 | ≤51 |
Where a report includes a mixture of operations and/or operated and non-operated patients the number of patients having lung sparing extirpative surgery is given as the numerator and the total number of patients in the report as the denominator
Some of these operations included planned multimodality therapies but for the purposes of this table we confine ourselves to the technical details of the extirpative surgery
The authors state ‘Decortication and pleurectomy, when possible, is the treatment of choice’ so the total versus partial presumably applies to either or both components of the surgery
The brevity of this operative detail is in marked contrast to the very full description of EPP performed in the other 40 patients in the same series
Resection of pericardium and diaphragm was sometimes not removed, or partially removed, in an unstated number of patients. ‘Partial or no removal of pericardium or diaphragm was sometimes done for a parietal pleural tumor separable from the pericardium or diaphragm.’
There is a consistent theme of modifying the operation according to the intra-operative findings. In earlier papers this tended towards conservativism, non-radical, sparing the diaphragm, sparing the mediastinal pleura and with a textual reminder that the surgery has a palliative intent. For example ‘A lesser procedure such as decortication/pleurectomy was considered for locally extensive disease but only for relief of pain or shortness of breath’. ‘The aim of this procedure was to attempt palliation of troublesome symptoms particularly chest pain and pleural effusion.’21 In some, particularly more recent series, PD is performed with radical intent.8
We judge that resection of the diaphragm or pericardium was infrequently performed in early series because it is specifically mentioned if done16,31,32 whereas it is included in the general operative description in more recent reports.5,8,11,13 It appears that it was optional with a degree of selection for appropriateness but it is often unclear in how many patients these steps were taken (Table 2).
The operative mortality, 1, 2, 3, 4 and 5-year survival, and median survival are given in Table 3. Some data were as supplied by the authors in text or tables and some were read from their figures. The survival data at each year from first to fifth are illustrated graphically with symbols weighted by the number of patients in the series (<25, 25–49, 50–99, >99) ( Figure 4). To explore any trend in outcome over time, for 22 reports where the median survival and the start and end dates of the clinical series are provided, the data are presented graphically in Figure 5.
Table 3.
All survival data that could be extracted from the reports. Where data are stated in the paper, in text or tables, these have been used. Where the data were displayed graphically the proportion alive at 1, 2, 3, 4 and 5 years has been read from the graphs to supplement the data reported in text and tables
| Author | Start year | End year | n | Op mort (%) | 1 year survival (%) | 2 year survival (%) | 3 year survival (%) | 4 year survival (%) | 5 year survival (%) | Median (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| Achatzy32 | 1969 | 1985 | 120 | 2 | 10.1 | |||||
| Alberts40 | 1965 | 1985 | 26 | 10.9 | ||||||
| Allen27 | 1958 | 1993 | 56 | 5 | 30 | 9 | 5 | 9.0 | ||
| Aziz21 | 1989 | 1999 | 47 | 0 | 50 | 0 | 0 | 0 | 14.0 | |
| Ball30 | 1981 | 1985 | 13 | 55 | 20 | 18 | 8 | 17.0 | ||
| Brancatisano28 | 1984 | 1989 | 45 | 2 | 58 | 21 | 16.0 | |||
| Branscheid29 | 1978 | 1989 | 82 | 2 | 35 | 20 | 17 | 10.5 | ||
| Ceresoli23 | 1986 | 1999 | 54 | 50 | 12.5 | |||||
| Clarke14 | 1989 | 1999 | 100 | 2 | 14.6 | |||||
| Colaut15 | 1985 | 2002 | 40 | 28 | 17 | |||||
| Colleoni24 | 1990 | 1994 | 20 | 44 | 32 | 0 | 0 | 11.5 | ||
| de Vries17 | 1976 | 2001 | 29 | 4 | 14 | 10 | 8 | 5 | 9.0 | |
| Flores5 | 1990 | 2006 | 278 | 4 | 62 | 30 | 20 | 15 | 16.0 | |
| Harvey31 | 1965 | 1988 | 9 | 43 | 0 | 0 | 0 | 12.0 | ||
| Law33 | 1971 | 1980 | 28 | 82 | 32 | 11 | 20.0 | |||
| Lee J25 | 1986 | 1993 | 15 | 37 | 15 | 7 | 0 | 0 | 11.5 | |
| Lee T22 | 1995 | 2000 | 26 | 64 | 32 | 18 | 12 | 18.1 | ||
| Lucchi12 | 1999 | 2004 | 49 | 0 | 60 | 23 | 26.0 | |||
| Matzi16 | 1993 | 2003 | 34 | |||||||
| Monneuse18 | 1990 | 2000 | 16 | 6 | 69 | 50 | 42 | 8 | 41.3 | |
| Nakas (Radical)7 | 51 | 6 | 53 | 41 | 25 | 13 | 15.3 | |||
| Nakas (Non-radical)7 | 51 | 10 | 32 | 10 | 2 | 0 | 0 | 7.1 | ||
| Okada13 | 1986 | 2006 | 34 | 0 | 60 | 40 | 24 | 19 | 10 | 17.0 |
| Phillips19 | 1989 | 1999 | 15 | 54 | 40 | 33 | 28 | 28 | 14.0 | |
| Sauter26 | 1988 | 1992 | 20 | 50 | 25 | 12.0 | ||||
| Schipper SP11 | 31 | 3 | 30 | 15 | 4 | 4 | 0 | 8.1 | ||
| Schipper TP11 | 10 | 0 | 80 | 35 | 35 | 35 | 35 | 17.2 | ||
| Yom20 | 2000 | 2001 | 8 | 25 | 13 | 6.5 | ||||
| Weighted average | 4 | 51 | 26 | 16 | 11 | 9 | ||||
Figure 4.
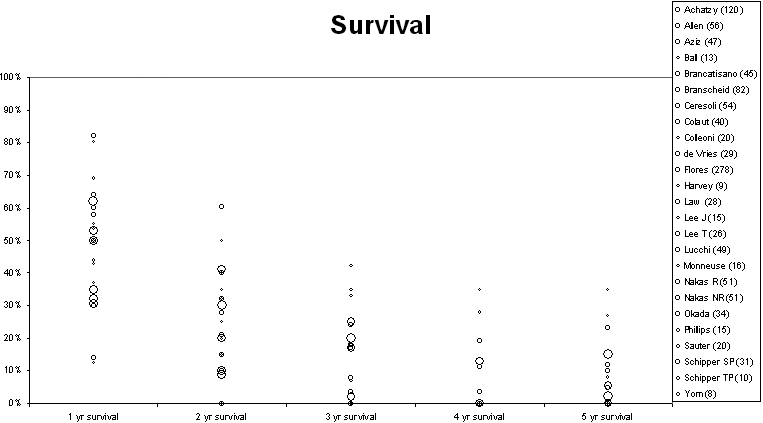
Survival at 1–5 years from 23 papers providing data either in the text or graphs. The circle size is proportional to the number of patients reported in the series as in the key
Figure 5.
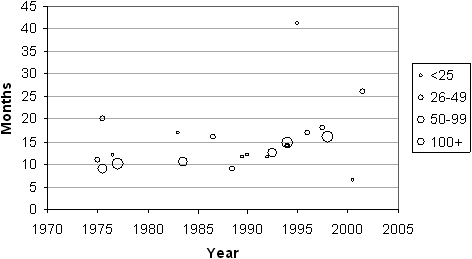
For 22 reports where the median survival (months) and the start and end dates of the clinical series are provided, they have been plotted graphically against the mid-point of the series. The size of circles is proportional to the number of patients reported in the series (see key)
Adjuvant therapies were used (probably) in all later series but it was very variable, and when reported, the stage of disease and the interventions used were not well enough characterized to attempt analysis. There were no data on performance status, quality of life, symptomatic change, or other patient reported outcomes.
Discussion
The evidence tables and figures provide an historical record of the outcomes of 50 years of lung-sparing surgery for MPM. There are no randomized studies and no direct or indirect comparison with the survival of comparable unoperated patients. The limitations on the inferences that can be drawn from retrospective case series have been previously enumerated.34 It is of course negotiable which papers to include and which to exclude. These decisions were made in the interests of finding data rather than to fit any prior belief and we hope that we have not overlooked any publication that would cast new or further light on the research question – which is the effect of lung sparing extirpative surgery for malignant pleural mesothelioma.
As a result of applying the test that the included publications should provide data that enabled some level of analysis, exclusion of some important papers concerning PD were made. An example is the report by Grossebner et al.35 It describes a videothoracoscopic approach with the emphasis on diagnosis, pleural fluid control and lung mobilization, all with palliative intent. This is the subject of an ongoing randomized controlled trial.36 Also excluded was a study where patients having ‘maximum debulking surgery’ either EPP or PD and complex adjuvant therapy were randomized, half having photodynamic therapy.37 As the purpose of that study was evaluation of photodynamic therapy and the surgery was mixed it was excluded from our analysis. However, other studies with primary intent of reporting one or more adjuvant therapies were included if they also included data in lung sparing surgery which could be analysed. The judgements were made on the basis of utility of the data for the present analysis. It should be emphasized that we had no patient level data and that the analyses are illustrative rather than definitive.
Figure 4 shows the survival for first to fifth year after surgery and Figure 5 shows the median survival with the size of symbols proportional to the number of patients in the series. We have not attempted to produce an aggregated statistic because of the heterogeneous nature of surgery but the eye can see that there is a consistency in results among the larger series with smaller series tending to be outliers. The illustration shows that six larger series, with more than 50 and more than 100 cases included, had median survivals of 10–15 months. Does this represent longer survival than these patients would have had without resection? In a report of 945 patients with malignant pleural mesothelioma38 those who had no surgery (n=387) had a mean survival of 16.8 months and those who had an exploratory thoracotomy but no resection (147/558; 31%) survived on average 17.8 months.39 It is understood that these were patients with less favorable clinical and pathological characteristics than those selected for surgery, but they fared as well as the operated patients.
The work of those deriving better staging systems is of great importance. It is noteworthy that in a series of 262 patients undergoing a range of treatments in South Africa, performance status and Butchart staging were correlated with survival across all treatment groups.40 Clearly standardization of a form of staging generally applicable to preoperative patients (rather than relying on intra-operative or pathological evaluation) will be essential in any future trials.
Between the 1960s and the present, the time span of the 1270 operations summarized, there will have been changes in practice and case selection, and in the use of effective chemotherapy. The variety of textual accounts in Table 1, with surgeons making intra-operative decisions on how much can be removed, illustrates the imperative to standardize the operation for any future studies if they are to have meaning and allow surgeons to compare their own with reported results, and the work of others. This is evident in the work of Waller's group in Leicester, UK.6–8 It is also likely that any impression of longer survival in more recent series may be due to other factors such as lead time bias due to earlier detection in the modern era, stage migration with contemporary imaging, and the likely effect of reporting bias. Therefore any discernable change cannot reliably be ascribed to newer rather than earlier techniques. More sophisticated analyses with newer statistical techniques would not clarify whether there is a difference in survival attributable to any particular factor. The question remains whether any of the operations described in Table 2 make a difference to outcome.
In 1988 Alberts wrote ‘Despite enthusiastic reports of response to treatment, untreated patients with malignant pleural mesothelioma may survive just as long as treated patients’.40 The authors cited the Brompton group's analysis of surgical results in support of this contention.33 Achatzy et al. observed ‘It is interesting to note that the long-term prognosis of patients treated non-operatively was better than that of surgically treated patients. In the non-operative group, the 5-year survival was 11.4% (5 of 44 patients) whereas in the operative group, the comparable figure was 2.2% (4 of 178 patients)’ and yet they concluded ‘Decortication and pleurectomy, when possible, is the treatment of choice’.32 Since lung-sparing surgery is being promoted as a clinical option for MPM patients in the modern era, knowledge of these results should be available to inform patients. If we are to move the emphasis from making an impact on survival to improving the quality of that survival, then more information is required concerning patient reported outcomes, their ability to breathe, and their quality of life.
Footnotes
DECLARATIONS —
Competing interests None declared
Funding The Clinical Operational Research Unit receives funding from the UK Department of Health Policy Research Programme
Ethical approval Not applicable
Guarantor TT
Contributorship All authors contributed equally
Supplementary Material
Appendix 1
Acknowledgements
None
References
- 1.Peto J, Hodgson JT, Matthews FE, Jones JR. Continuing increase in mesothelioma mortality in Britain. Lancet 1995;345:535–9 [DOI] [PubMed] [Google Scholar]
- 2.Peto J, Decarli A, La Vecchia C, Levi F, Negri E. The European mesothelioma epidemic. Br J Cancer 1999;79:666–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rake C, Gilham C, Hatch J, Danton J, Peto J. Occupational, domestic and environmental mesothelioma risks in the British population: a case control study. Br J Cancer 2009;100:1175–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugarbaker DJ, Flores RM, Jaklitsch MT, et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg 1999;117:54–63 [DOI] [PubMed] [Google Scholar]
- 5.Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg 2008;135:620–6 [DOI] [PubMed] [Google Scholar]
- 6.Martin-Ucar AE, Nakas A, Edwards JG, Waller DA. Case-control study between extrapleural pneumonectomy and radical pleurectomy/decortication for pathological N2 malignant pleural mesothelioma. Eur J Cardiothorac Surg 2007;31:765–70 [DOI] [PubMed] [Google Scholar]
- 7.Nakas A, Martin Ucar AE, Edwards JG, Waller DA. The role of video assisted thoracoscopic pleurectomy/decortication in the therapeutic management of malignant pleural mesothelioma. Eur J Cardiothorac Surg 2008;33:83–8 [DOI] [PubMed] [Google Scholar]
- 8.Nakas A, Trousse DS, Martin-Ucar AE, Waller DA. Open lung-sparing surgery for malignant pleural mesothelioma: the benefits of a radical approach within multimodality therapy. Eur J Cardiothorac Surg 2008;34:886–91 [DOI] [PubMed] [Google Scholar]
- 9.Scherpereel A, Astoul P, Baas P, et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J 2010;35:479–95 [DOI] [PubMed] [Google Scholar]
- 10.Ettinger DS. Malignant Pleural Mesothelioma. Fort Washington, PA: National Comprehensive Cancer Network; 2010. See www.nccn.org. [DOI] [PubMed] [Google Scholar]
- 11.Schipper PH, Nichols FC, Thomse KM, et al. Malignant pleural mesothelioma: surgical management in 285 patients. Ann Thorac Surg 2008;85:257–64 [DOI] [PubMed] [Google Scholar]
- 12.Lucchi M, Chella A, Melfi F, et al. Four-modality therapy in malignant pleural mesothelioma: a phase II study. J Thorac Oncol 2007;2:237–42 [DOI] [PubMed] [Google Scholar]
- 13.Okada M, Mimura T, Ohbayashi C, Sakuma T, Soejima T, Tsubota N. Radical surgery for malignant pleural mesothelioma: results and prognosis. Interact Cardiovasc Thorac Surg 2008;7:102–6 [DOI] [PubMed] [Google Scholar]
- 14.Clarke CP, Knight SR, Daniel FJ, Seevanayagam S. Management of malignant mesothelioma by decortication and adjunct phototherapy. Asian Cardiovasc.Thorac Ann 2006;14:206–9 [DOI] [PubMed] [Google Scholar]
- 15.Colaut F, Toniolo L, Vicario G, et al. Pleurectomy/decortication plus chemotherapy: outcomes of 40 cases of malignant pleural mesothelioma. Chir Ital 2004;56:781–6 [PubMed] [Google Scholar]
- 16.Matzi V, Maier A, Woltsche M, Smolle-Juttner FM. Polyhematoporphyrin-mediated photodynamic therapy and decortication in palliation of malignant pleural mesothelioma: a clinical pilot study. Interact Cardiovasc Thorac Surg 2004;3:52–6 [DOI] [PubMed] [Google Scholar]
- 17.de Vries WJ, Long MA. Treatment of mesothelioma in Bloemfontein, South Africa. Eur J Cardiothorac Surg 2003;24:434–40 [DOI] [PubMed] [Google Scholar]
- 18.Monneuse O, Beaujard AC, Guibert B, et al. Long-term results of intrathoracic chemohyperthermia (ITCH) for the treatment of pleural malignancies. Br J Cancer 2003;88:1839–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phillips PG, Asimakopoulos G, Maiwand MO. Malignant pleural mesothelioma: outcome of limited surgical management. Interact Cardiovasc Thorac Surg 2003;2:30–4 [DOI] [PubMed] [Google Scholar]
- 20.Yom SS, Busch TM, Friedberg JS, et al. Elevated serum cytokine levels in mesothelioma patients who have undergone pleurectomy or extrapleural pneumonectomy and adjuvant intraoperative photodynamic therapy. Photochem Photobiol 2003;78:75–81 [DOI] [PubMed] [Google Scholar]
- 21.Aziz T, Jilaihawi A, Prakash D. The management of malignant pleural mesothelioma; single centre experience in 10 years. Eur J Cardiothorac Surg 2002;22:298–305 [DOI] [PubMed] [Google Scholar]
- 22.Lee TT, Everett DL, Shu HK, et al. Radical pleurectomy/decortication and intraoperative radiotherapy followed by conformal radiation with or without chemotherapy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2002;124:1183–9 [DOI] [PubMed] [Google Scholar]
- 23.Ceresoli GL, Locati LD, Ferreri AJ, et al. Therapeutic outcome according to histologic subtype in 121 patients with malignant pleural mesothelioma. Lung Cancer 2001;34:279–87 [DOI] [PubMed] [Google Scholar]
- 24.Colleoni M, Sartori F, Calabro F, et al. Surgery followed by intracavitary plus systemic chemotherapy in malignant pleural mesothelioma. Tumori 1996;82:53–6 [DOI] [PubMed] [Google Scholar]
- 25.Lee JD, Perez S, Wang HJ, Figlin RA, Holmes EC. Intrapleural chemotherapy for patients with incompletely resected malignant mesothelioma: the UCLA experience. J Surg Oncol 1995;60:262–7 [DOI] [PubMed] [Google Scholar]
- 26.Sauter ER, Langer C, Coia LR, Goldberg M, Keller SM. Optimal management of malignant mesothelioma after subtotal pleurectomy: revisiting the role of intrapleural chemotherapy and postoperative radiation. J Surg Oncol 1995;60:100–5 [DOI] [PubMed] [Google Scholar]
- 27.Allen KB, Faber LP, Warren WH. Malignant pleural mesothelioma. Extrapleural pneumonectomy and pleurectomy. Chest Surg Clin N Am 1994;4:113–26 [PubMed] [Google Scholar]
- 28.Brancatisano RP, Joseph MG, McCaughan BC. Pleurectomy for mesothelioma. Med J Aust 1991;154:455–7, 460 [PubMed] [Google Scholar]
- 29.Branscheid D, Krysa S, Bauer E, Bulzebruck H, Schirren J. Diagnostic and therapeutic strategy in malignant pleural mesothelioma. Eur J Cardiothorac Surg 1991;5:466–72 [DOI] [PubMed] [Google Scholar]
- 30.Ball DL, Cruickshank DG. The treatment of malignant mesothelioma of the pleura: review of a 5-year experience, with special reference to radiotherapy. Am J Clin Oncol 1990;13:4–9 [DOI] [PubMed] [Google Scholar]
- 31.Harvey JC, Fleischman EH, Kagan AR, Streeter OE. Malignant pleural mesothelioma: a survival study. J Surg Oncol 1990;45:40–2 [DOI] [PubMed] [Google Scholar]
- 32.Achatzy R, Beba W, Ritschler R, et al. The diagnosis, therapy and prognosis of diffuse malignant mesothelioma. Eur J Cardiothorac Surg 1989;3:445–7 [DOI] [PubMed] [Google Scholar]
- 33.Law MR, Gregor A, Hodson ME, Bloom HJ, Turner-Warwick M. Malignant mesothelioma of the pleura: a study of 52 treated and 64 untreated patients. Thorax 1984;39:255–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Treasure T, Utley M. Ten traps for the unwary in surgical series: a case study in mesothelioma reports. J Thorac Cardiovasc Surg 2007;133:1414–18 [DOI] [PubMed] [Google Scholar]
- 35.Grossebner MW, Arifi AA, Goddard M, Ritchie AJ. Mesothelioma–VATS biopsy and lung mobilization improves diagnosis and palliation. Eur J Cardiothorac Surg 1999;16:619–23 [DOI] [PubMed] [Google Scholar]
- 36.Rintoul R, Winter R. MESOVATS. London: UK Clinical Research Network; 2010. See http://public.ukcrn.org.uk/search/StudyDetail.aspx?StudyID=1352 [Google Scholar]
- 37.Pass HI, Temeck BK, Kranda K, et al. Phase III randomized trial of surgery with or without intraoperative photodynamic therapy and postoperative immunochemotherapy for malignant pleural mesothelioma. Ann Surg Oncol 1997;4:628–33 [DOI] [PubMed] [Google Scholar]
- 38.Flores RM, Zakowski M, Venkatraman E, et al. Prognostic factors in the treatment of malignant pleural mesothelioma at a large tertiary referral center. J Thorac Oncol 2007;2:957–65 [DOI] [PubMed] [Google Scholar]
- 39.Utley M, Fiorentino F, Treasure T. Obtaining an upper estimate of the survival benefit associated with surgery for mesothelioma. Eur J Cardiothorac Surg 2010;38:241–4 [DOI] [PubMed] [Google Scholar]
- 40.Alberts AS, Falkson G, Goedhals L, Vorobiof DA, Van der Merwe CA. Malignant pleural mesothelioma: a disease unaffected by current therapeutic maneuvers. J Clin Oncol 1988;6:527–35 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1