Abstract
Objective
Oxidized LDL (oxLDL) and oxLDL antibodies form immune complexes (IC) that reflect essential components in the development of atherosclerosis: dyslipidemia, oxidative stress and induction of a pro-inflammatory humoral immune response. We measured oxLDL in IC (oxLDL-IC) isolated from patients with type 1 diabetes to assess the relationship between oxLDL-IC and coronary artery calcification (CAC).
Methods
OxLDL was measured in IC isolated from baseline samples from a subgroup of 476 patients of the Diabetes Control and Complications Trial (DCCT). CAC was determined by computed tomography (CT) 11–20 years later. Multivariable log-binomial regression models were used to estimate the risk ratios associated with having a high CAC score with an increase of 1 standard deviation (SD) of the natural logarithm of oxLDL-IC.
Results
Multivariable regression models indicate that a 1 SD increase in the levels of oxLDL-IC was associated with a 37% increase in the risk of having high CAC score (RR = 1.36; 95 % CI: 1.12–1.67) at follow up after adjustment for DCCT treatment group, retinopathy/AER groups, gender and CT scanning site as well as baseline age, diabetes duration and HbA1C %. Further adjustment for smoking status, blood pressure and LDL resulted in a risk ratio of 1.23 (95% CI: 1.01–1.50) which remained statistically significant indicating that baseline oxLDL-IC is independently associated with the development of CAC.
Discussion
Increased levels of oxLDL-IC are associated with the development of coronary calcification. This observation reinforces previously published clinical and experimental data demonstrating that oxLD-IC have pro-inflammatory and proatherogenic properties.
INTRODUCTION
As the inflammatory nature of atherosclerosis has gained acceptance, research into the triggers initiating and/or perpetuating inflammatory reactions in the wall of large and medium vessels has expanded. Insults of many different kinds may be involved, but recent research efforts has focused on investigating pro-inflammatory immune mechanisms. These may involve innate immune signals, mediated by pattern-recognition receptors such as scavenger receptors for modified LDL, and Toll-like receptors[1] as well as adaptive immunity signals, both cell-mediated [2] and antibody-mediated.[3] Establishing a hierarchy for these mechanisms is challenging, in part because of their multiplicity and diversity and in part because the data on which such ranking can be based was obtained in animal models or in vitro experimental systems.[1–4]
Based on in vitro [5, 6], ex-vivo studies,[7] as well as in clinical studies,[8–11] evidence has been accumulating over the years, supporting the pathogenic role of the humoral response to modified lipoproteins. This is mainly due to the fact that modified LDL and the corresponding antibodies form immune complexes (mLDL-IC), which are able to activate phagocytic cells through engagement of Fc receptors.[12, 13] Engagement of Fc receptors by mLDL-IC is particularly significant because it delivers stronger activating signals to phagocytic cells than engagement of scavenger receptors by modified LDL.[5]
Definitive evaluation of the role that the humoral immune response plays in human atherosclerosis cannot be established in animal model studies for reasons mentioned above and summarized in previous publications.[3] We have shown that the concentrations of circulating LDL-IC, measured as surrogates of IC formed in the vessel wall,[14, 15] correlate with accepted end-points for atherosclerotic and renal disease in patients with type 1 diabetes.[8–10, 16] However, several of these studies have limitations due to their small sample size and/or the lack of definition of the nature of the modified lipoprotein(s) involved in LDL-IC formation.
In recent years we have developed capture assays for different forms of modified lipoproteins.[17] This has allowed us to modify our assay for LDL-IC by measuring the concentration of specific types of modified LDL in IC isolated from the sera of patients with type 1 diabetes and study the correlation of these concentrations with objective measures of atherosclerotic disease, such as arterial calcium scores. Furthermore, the availability of samples collected two decades ago has allowed us to evaluate the potential prognostic significance of modified LDL-IC on the development of arterial calcification over time.
RESEARCH DESIGN AND METHODS
This study was performed on a non-random subgroup of 476 subjects from the DCCT/EDIC cohort who had oxLDL-IC levels measured on samples obtained at entry into the DCCT as well as coronary artery calcification (CAC) scores performed during the EDIC phase of the study (11–20 years after enrollment in the DCCT), as a marker for coronary artery disease (CAD). [18] The original DCCT cohort included 1,441 patients who were 13–39 years of age and had type 1 diabetes for 1–15 years at study entry.[19] The DCCT cohort was randomized to intensive or conventional insulin therapy and followed for an average of 6.5 years. In 1993, the interventional phase of the study was stopped and in 1994, the observational phase of the DCCT/EDIC study (EDIC phase) was initiated [20], aimed at assessing the development of macrovascular disease in type 1 diabetes. During the EDIC phase, all of the patients were under the care of their personal health care provider and were encouraged to practice intensive insulin therapy. At DCCT Baseline (1983–1989), none of the patients had hypertension (defined as ≥ 140 and/or ≥ 90 mmHg) or dyslipidemia (defined as total cholesterol > 200 and/or LDL > 160 mg/dl).
Of the 1,441 DCCT participants, 90–95% entered the EDIC study and 905 of these individuals had blood collected longitudinally as part of a sub-study on biomarkers of macrovascular disease. From these 905 subjects, 518 patients were selected for measurement of oxLDL-IC in a case-control study for which cases of albuminuria, elevated carotid artery intima-media thickness (≥ 25% Stenosis at a lesion) and severe retinopathy (EDTRS ≥ 10) were oversampled (i.e. all available cases were sampled) resulting in 157 of the 518 patients having one of these three endpoints and 361 of the 518 patients having none of these endpoints. Of the 518 with oxLDL-IC measured 476 also had CAC measured during EDIC.[21]
Serum samples were obtained after an overnight fast at entry into the DCCT study (between 1983–89) and assayed at the time for HbA1c, creatinine and lipids or stored at −70°C. The frozen serum was used to assay the modified LDL-IC (see below). Lipid levels were repeated in the frozen serum samples and there was no significant difference between the levels measured in 1983–89 and those measured at the time the modified LDL-IC were measured. The DCCT and EDIC studies were approved by the Institutional Review Board of all participating DCCT/EDIC centers and all participants provided written informed consent.
Assessment of coronary artery calcification
CAC was determined by computed tomography (CT), performed 7–9 years after the end of the DCCT in 1,205 (86%) of the surviving 1,404 participants. CT was performed using a C-150 cardiac-gated electron beam CT scanner (n = 9; Imatron, San Francisco, CA), a Lightspeed (n = 7; General Electric Medical Systems, Waukesha, WI) or a Volume Zoom (Siemens, Erlanger, Germany) multidetector CT system, a Lightspeed Marconi MX-8000 (GE), or a Somatom 4+ (Siemens) (n = 3). All participants were scanned twice over calibration phantoms of known physical calcium concentration. Scans were read centrally at the Harbor-UCLA (University of California, Los Angeles) Research and Education Institute (Torrance, CA) to identify and quantify CAC. The average score from the two scans was used in the analysis. Readers were masked to subject identity and prior treatment assignment. Details concerning standardization and reliability of the CAC measurement have been previously published. [18]
OxLDL-IC Measurement
We measured oxLDL by first precipitating circulating immune complexes from serum and then fractionating these IC by protein G affinity chromatography, separating the predominant IgG antibody from modified LDL, as previously described.[16, 22] The concentration of OxLDL in the IC was then assayed with a capture assay developed in our laboratory using a specific oxLDL antibody.[17] The development of standards for calibration of the oxLDL assay, as well as sensitivity, reproducibility, and recovery data for the capture assay have been reported elsewhere[17]. The effect of long term freezing at −&0°C was carefully assessed and found to have no effect in the measurements performed. The levels of oxLDL in human circulating IC were expressed in function of the amount of apolipoprotein B contained in the IC and the final values were given as the concentration per mL of serum
Other procedures
At the baseline DCCT examination, each participant completed a physical examination, medical history, electrocardiogram and laboratory testing including serum creatinine, and hemoglobin A1c [20, 23]. Lipid profiles and 4-hour urine collections for measurement of AER and creatinine clearance were also obtained. Covariates for the current analyses were obtained from DCCT baseline history, physical examination and laboratory data (fasting lipids, renal function, and hemoglobin A1c). The methodology used to perform the routine measurements used as conventional risk factors in this study were guided by the DCCT/EDIC study protocols and have been described elsewhere as mentioned above [20, 23]. Retinopathy was assessed by obtaining stereo fundus photographs in all participants [24].
Statistical Analysis
In the analyses performed oxidized LDL-IC at DCCT baseline was used to determine a person’s exposure status and CAC levels 11–20 years later were the outcomes of interest. Values of oxLDL-IC were log transformed due to their skewed, non-normal distribution. The study population was divided into those with minimal to moderate CAC scores (Low Group: < 100 Agatston units) and those with increased to extensive CAC scores (High Group: ≥100 Agatstons units).[25, 26] Standard descriptive and clinical characteristics at DCCT baseline were summarized for the entire study population as well as stratified by CAC outcome. A two-sided Wilcoxon Ranks Sum test was used to compare continuous descriptive and clinical measures between CAC groups. All categorical measures were compared using Pearson’s Chi-Square test statistic or Fishers Exact Test when appropriate. The Cochran-Armitage test for trend was used to assess the overall linear trend in the proportion of participants with high CAC scores across the tertiles of oxLDL-IC (chosen for ease of representation).
Due to the skewed nature of the CAC scores, unadjusted and multivariable log-binomial (Relative Risk) regression models with robust error variance estimates [27] were used to estimate the risk ratios associated with the prevalence of high CAC scores with an increase of one standard deviation of the natural logarithm of oxLDL-IC (1 SD = 0.9137 mg/L). Risk Models were analyzed both unadjusted and adjusted for DCCT randomized treatment group, baseline age, gender, duration of type 1 diabetes, HbA1C %, CT scanning location, DCCT baseline retinopathy cohort (primary vs. secondary), and AER (mg/24hr). Further multivariable models looked at the addition of baseline systolic blood pressure, baseline smoking status and baseline LDL-Cholesterol.
Multivariable Tobit regression models were used to examine the association of the observed quantitative CAC scores with oxLDL-IC. Calcification scores below the lower limit of quantification (0.935 Agaston Units) were not observable and were assumed immeasurable (but present) and censored. All measurable CAC scores were natural log transformed and decreased by subtracting the natural logarithm of the lowest detectable CAC score prior to model fitting. Tobit models were implemented using maximum likelihood methods and adjusted for this apparent left censoring of the CAC distribution.[28] This method provided a single association measure between oxLDL-IC measures and CAC scores.[29] Tobit regression models used in the analysis were fit using the QLIM Procedure in SAS 9.2 and were adjusted for the same covariates as in the risk ratio models.
All statistical analyses were performed using the SAS System version 9.2 (SAS Institute, Cary, NC, USA). A type I error rate was controlled for significance at 0.05 for all analysis, and p-values have not been adjusted for multiple comparisons.
RESULTS
For descriptive purposes, oxLDL-IC was broken into tertiles and Table 1 shows the baseline demographics and clinical data of the patients in each tertile. Increases in oxLDL-IC were associated with increases in the duration of type 1 diabetes, length of DCCT follow-up, cholesterol (s), and baseline AER. Those with higher oxLDL-IC were more likely to have been in the standard treatment group and to be male. The correlation of oxLDL-IC with LDL-cholesterol level, while statistically significant, was of moderate magnitude (Rho = 0.22, p<0.001). Comparison of DCCT baseline characteristics of the 476 participants included in the current study and those of the 965 participants excluded revealed longer duration of diabetes, higher body mass index and higher AER in the participants included into the study compared to those excluded. Those included were also less likely to be in the primary retinopathy cohort. Included and excluded participants were of similar age and sex, were similarly likely to smoke and drink alcohol and had similar lipid, blood pressure and hemoglobin A1c measures at baseline.
Table 1.
Demographic and clinical characteristics measured at DCCT Baseline by OxLDL-IC Tertile Grouping. Continuous characteristics are denoted as mean ± standard deviation and categorical characteristics are denoted as n (%).
| OxLDL-IC Tertiles |
P-Value | |||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| n=158 | n=159 | n=159 | ||
| Age (yrs) | 26.9 ± 6.9 | 27.5 ± 6.6 | 27.3 ± 7.4 | 0.751 |
| BMI (kg/m2) | 23.0 ± 2.8 | 23.5 ± 2.5 | 23.8 ± 3.2 | 0.067 |
| Duration of T1D (yrs) | 5.5 ± 4.1 | 5.6 ± 4.2 | 6.5 ± 4.2 | 0.031 |
| DCCT Follow up (yrs) | 6.1 ± 1.6 | 6.4 ± 1.8 | 6.9 ± 1.8 | 0.001 |
| EDIC Follow up (yrs) | 9.0 ± 0.5 | 9.1 ± 0.5 | 9.1 ± 0.5 | 0.196 |
| SBP (mmHg) | 113.2 ± 11.5 | 115.0 ± 11.0 | 115.6 ± 11.0 | 0.123 |
| DBP (mmHg) | 72.7 ± 9.0 | 72.5 ± 9.2 | 74.3 ± 8.7 | 0.054 |
| MAP (mmHg) | 86.2 ± 9.0 | 86.7 ± 8.6 | 88.1 ± 8.5 | 0.051 |
| Cholesterol (mg/dl) | 169.5 ± 29.7 | 171.5 ± 35.1 | 183.3 ± 32.7 | <0.001 |
| HDL (mg/dl) | 53.4 ± 12.9 | 49.9 ± 11.7 | 49.0 ± 11.9 | 0.004 |
| LDL (mg/dl) | 101.8 ± 26.6 | 106.1 ± 30.8 | 116.5 ± 28.2 | <0.001 |
| Trig (mg/dl) | 72.2 ± 31.1 | 77.6 ± 38.9 | 89.2 ± 44.7 | <0.001 |
| HbA1C % | 8.8 ± 1.5 | 8.7 ± 1.6 | 9.1 ± 1.7 | 0.088 |
| AER (mg/24 Hrs) | 14.9 ± 13.5 | 15.8 ± 21.6 | 18.0 ± 17.8 | 0.022 |
| Serum Creatinine | 0.81 ± 0.16 | 0.80 ± 0.14 | 0.80 ± 0.15 | 0.536 |
| Intensive Treatment, n (%) | 82 (51.9) | 77 (48.4) | 58 (36.5) | 0.015 |
| Primary Ret Cohort | 75 (47.5) | 83 (52.2) | 55 (34.6) | 0.005 |
| Male | 70 (44.3) | 86 (54.1) | 93 (58.5) | 0.035 |
| Smoker (current at BL) | 29 (18.4) | 36 (22.6) | 35 (22.0) | 0.600 |
| Drinker (current at BL) | 35 (22.2) | 24 (15.1) | 34 (21.4) | 0.220 |
BMI = Body Mass Index, SBP = Systolic Blood Pressure, DBP = Diastolic Blood Pressure, MAP = Mean Arterial Pressure, HDL = High-Density Lipoprotein Cholesterol, LDL = Low-Density Lipoprotein, Trig = triglycerides, AER = Albumin Excretion Rate
Continuous measures are compared with Wilcoxon Ranks Sum statistics. Pearson Chi Square statistics are used for Categorical measures.
Demographic and clinical differences between CAC groups (0–100 and >100) are summarized in table 2. At DCCT baseline, the mean age of the study population (n=476) was 27.2 ± 7.0 years, the mean duration of diabetes was 5.8 ± 4.1 years, 52.3 % (n=249) were males, 97.3 % (n=463) were Caucasian and 45.6% (n=217) were assigned to the DCCT intensive treatment group. In the high CAC group, duration of type 1 diabetes was higher (5.7 ± 4.1 vs. 6.9 ± 4.5, p < 0.001) and the proportion of smokers was higher than in the low CAC group (17.9 % vs. 39.1 %, p < 0.001). LDL-cholesterol, total cholesterol and systolic blood pressure levels as well as the proportion of men were also higher in the high CAC group than the low CAC group.
Table 2.
Demographic and clinical characteristics measured at DCCT Baseline by CAC Grouping. Continuous characteristics are denoted as mean ± standard deviation and categorical characteristics are denoted as n (%).
| DCCT Baseline Characteristic (Mean±SE) | Overall N=476 |
CAC Groupings | P-Value | |
|---|---|---|---|---|
| 0 – 100 n=407 |
> 100 n=69 |
|||
| Age (yrs) | 27.2 ± 7.0 | 26.5 ± 6.8 | 31.7 ± 5.9 | <0.001 |
| BMI(kg/m2) | 23.5 ± 2.8 | 23.3 ± 2.8 | 24.4 ± 3.1 | 0.009 |
| Duration of T1D (yrs) | 5.8 ± 4.1 | 5.7 ± 4.1 | 6.9 ± 4.5 | 0.036 |
| SBP (mmHg) | 114.6 ± 11.2 | 114.0 ± 11.3 | 117.9 ± 10.3 | 0.009 |
| DBP (mmHg) | 73.2 ± 9.0 | 73.1 ± 9.1 | 73.9 ± 8.1 | 0.431 |
| MAP (mmHg) | 87.0 ± 8.7 | 86.7 ± 8.8 | 88.5 ± 7.9 | 0.090 |
| Cholesterol (mg/dl) | 174.8 ± 33.1 | 172.1 ± 32.2 | 190.7 ± 34.0 | <0.001 |
| HDL (mg/dl) | 50.7 ± 12.3 | 51.2 ± 12.4 | 47.9 ± 11.3 | 0.056 |
| LDL (mg/dl) | 108.1 ± 29.2 | 105.2 ± 28.1 | 125.7 ± 29.6 | <0.001 |
| Trig (mg/dl) | 79.7 ± 39.2 | 78.6 ± 39.4 | 85.9 ± 37.8 | 0.067 |
| HbA1C % | 8.9 ± 1.6 | 8.8 ± 1.6 | 9.0 ± 1.7 | 0.463 |
| AER (mg/24 Hrs) | 16.3 ± 17.9 | 15.7 ± 15.6 | 19.8 ± 28.1 | 0.180 |
| Serum Creatinine | 0.80 ± 0.15 | 0.80 ± 0.15 | 0.81 ± 0.15 | 0.507 |
| OxLDL-IC at DCCT Entry (mg/L) | 5.03 ± 0.91 | 4.98 ± 0.92 | 5.38 ± 0.81 | <0.001 |
| Intensive Treatment, n (%) | 217 (45.6) | 189 (46.4) | 28 (40.6) | 0.366 |
| Primary Ret Cohort | 213 (44.8) | 189 (46.4) | 24 (34.8) | 0.072 |
| Male | 249 (52.3) | 200 (49.1) | 49 (71.0) | <0.001 |
| Smoker (current at BL) | 100 (21.0) | 73 (17.9) | 27 (39.1) | <0.001 |
| Drinker (current at BL) | 93 (19.5) | 77 (18.9) | 16 (23.2) | 0.406 |
BMI = Body Mass Index, SBP = Systolic Blood Pressure, DBP = Diastolic Blood Pressure, MAP = Mean Arterial Pressure, HDL = High-Density Lipoprotein Cholesterol, LDL = Low-Density Lipoprotein, Trig = triglycerides, AER = Albumin Excretion Rate
Continuous measures are compared with Wilcoxon Ranks Sum statistics. Pearson Chi Square statistics are used for Categorical measures.
The overall prevalence of high CAC scores was 14.5 % (69/476). The prevalence of having high CAC across oxLDL-IC tertiles is shown in figure 1. Prevalence of high CAC scores increases as the levels of oxLDL-IC increase. (Trend P value < 0.001).
Figure 1.
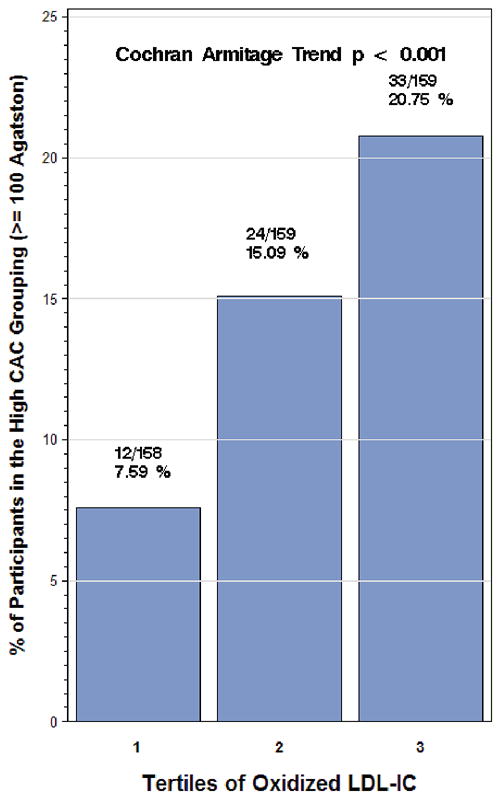
Incidence of CAC scores (≥ 100 Agatstons) by tertile of oxidized LDL-Immune Complex. The tertiles of oxLDL-IC are 1: 4–106 (mg/L) 2: 107–246, and 3: 247–1382
Table 3 presents the unadjusted and multivariable risk ratios for significant CAC associated with a one standard deviation increase in the natural logarithm of oxLDL-IC. In the unadjusted analysis, those with a one SD change in oxLDL-IC at DCCT baseline had a 51 percent increase in the risk of having a high CAC score (RR = 1.51; 95 % CI: 1.20–1.88: p < 0.001) at follow-up. Once adjusted for common CAC, risk factors and DCCT design variables (DCCT treatment group, baseline retinopathy cohort, baseline diabetes duration, baseline Ln AER, baseline HbA1C %, gender, CT scanning site, and age), the increased risk of a high CAC score associated with the change in oxLDL-IC remained significant (RR = 1.36; 95 % CI: 1.12–1.67: p = 0.003). The addition of LDL, SBP, and smoking status into the model, attenuated the risk ratio slightly, but it remained significant (RR = 1.26; 95 % CI: 1.03–1.54: p = 0.024) and of magnitude only slightly lower than the relative risk associated with a one standard deviation increase in baseline LDL (RR = 1.41; 95 % CI: 1.17–1.70: p < 0.001).
Table 3.
Unadjusted and Multivariate Relative Risk Regression Results for oxLDL-IC association with High CAC status.
| Relative risks associated with risk factors of CAC | Model 1 (Unadjusted) | Model 2 | Model 3 | Model 4* |
|---|---|---|---|---|
| oxLDL-IC (1 SD Increase) | 1.51 (1.20–1.88) § | 1.36 (1.12–1.67) § | 1.35 (1.10–1.65) § | 1.26 (1.03–1.54) † |
| Baseline LN AER | 1.17 (0.89–1.54) | 1.07 (0.83–1.37) | 1.03 (0.80–1.33) | |
| Experimental Treatment Group | 0.95 (0.61–1.47) | 0.97 (0.63–1.48) | 0.98 (0.64–1.52) | |
| Secondary Retinopathy Cohort | 0.85 (0.45–1.61) | 0.88 (0.47–1.65) | 0.82 (0.43–1.56) | |
| HbA1C (1 unit increase, %) | 1.08 (0.93–1.25) | 1.07 (0.91–1.24) | 1.05 (0.91–1.21) | |
| Diabetes Duration at BL (1 yr increase) | 1.07 (1.01–1.15) † | 1.07 (1.01–1.14)† | 1.08 (1.01–1.15)† | |
| Age at BL (1 year increase) | 1.12 (1.08–1.16) § | 1.11 (1.06–1.15) § | 1.09 (1.05–1.14) § | |
| Male (men versus women) | 1.92 (1.20–3.09) § | 1.68 (1.02–2.78) † | 1.70 (1.04–2.76) § | |
| SBP (10 unit increase, mmHg) | 1.18 (0.98–1.42) | 1.20 (0.99–1.44) | ||
| Baseline Smoking (yes vs. no) | 1.71 (1.11–2.64) † | 1.56 (1.01–2.41) † | ||
| LDL (1 SD increase) | 1.41 (1.17–1.70) § |
Risk Models:
1: Ox LDL IC only
2: Adjusted for DCCT Treatment Group, Retinopathy Cohort, age, gender, baseline HbA1C %, CT Scanning location, Baseline AER, and Baseline Diabetes Duration.
3: Additionally Adjusted for Smoking Status and Systolic Blood Pressure
4: Additionally adjusted for baseline LDL
p < 0.05
p < 0.01
LDL was chosen for model inclusion based on significance within the model. HDL, Cholesterol, Triglycerides, and BMI were highly non-significant when included in the model, and were therefore removed from model 4.
The results of the Tobit regression models were stronger than those from the risk models. In the unadjusted analysis, a one standard deviation increase in oxLDL-IC level resulted in a nearly 3-fold increase in CAC scores (2.98: 95 % CI: 1.59–5.59: χ2 = 11.68, p < 0.001). Adjustment for common CAC risk factors and DCCT design variables attenuated the association somewhat, but a greater than 2-fold increase in mean CAC scores remained (2.15: 95% CI: 1.24–3.72: χ2 = 7.43, p = 0.006). Further adjustment for LDL levels, SBP, and smoking status, further attenuated the effect of baseline oxLDL-IC on CAC score (1.63: 95% CI: 0.95–2.79: χ2 = 3.13, p = 0.076). In all fully adjusted models, the possible effect modification of DCCT treatment group, baseline retinopathy cohort, and gender on OxLDL-IC were tested. None were found to be significant and were removed from the model.
DISCUSSION
Increased levels of oxLDL-IC at DCCT baseline are associated with an increased risk of having clinically significant coronary artery calcification 11 to 20 years later in patients with type 1 diabetes. When adjustments for different variables were introduced, particularly LDL level, the effect of an increase in oxLDL-IC level was attenuated, the relative risk decreasing from 1.51 to 1.23 but remained statistically significant. As expected, the degree of dyslipidemia, and in particular the levels of LDL-cholesterol did correlate with the levels of oxLDL-IC, because higher levels of LDL will inevitably result in higher levels of oxLDL, the necessary antigen for the formation of oxLDL-IC. In a previous study we have demonstrated that the total LDL particle number as well as the concentrations of both small LDL and large LDL particles (assessed by nuclear magnetic resonance spectroscopy) were responsible for LDL-IC formation in the DCCT/EDIC cohort.[30] In the full model, LDL itself remained significant, suggesting, not surprisingly, that it also has an effect on coronary calcification independent of oxLDL-IC.
A limitation of the study is the lack of a random sampling. To increase the statistical power available participants were oversampled (i.e. all available cases were sampled) for one of three endpoints none of which are the endpoint for the current report: albuminuria, elevated carotid artery intima-media thickness and retinopathy. To overcome this selection bias throughout all analyses we have controlled for baseline markers of diabetes severity (i.e., DCCT retinopathy status, diabetes duration, and hemoglobin A1C) and albuminuria (i.e., the case-control selection criteria). Additionally, we determined that neither markers of diabetes severity, albuminuria nor DCCT treatment group were acting as effect modifiers of associations of interest, thus indicating that the predictive ability of IC was similar across different levels of these variables. However, some residual confounding variable that we were unable to account for could still be present in our analysis.
Previous publications from our group and others had pointed to the potential pathogenic role of oxLDL-IC. Orchard et al. reported that levels of LDL-containing IC measured at baseline were directly related to subsequent CAD.[10] In a nested case-control study including 49 incident cases of myocardial infarction, angina, or death attributed to CAD and 49 control subjects, matched for age, gender, and duration of diabetes, using multivariate analysis, modified LDL-IC, assessed by measuring the cholesterol content in isolated immune complexes were shown to be independent predictors of CAD. In addition, a complementary study from our group using the same patient cohort showed that the IC from the patients that developed CAD, contained higher LDL concentrations and higher concentrations of IgG than those measured in IC isolated from control cases.[8] A later study carried out in 1050 patients from the DCCT/EDIC cohort showed that LDL-IC (measured by the concentration of cholesterol and ApoB in precipitated IC) were present in higher levels in patients that showed progression of the intima-media thickening (IMT) over a follow-up period of 4 to 6 years.[9]
Using capture assays for modified LDL we have also revisited the correlation between modified LDL IC levels and IMT in a DCCT/EDIC cohort of 479 patients who had levels of oxLDL and AGE-LDL IC at DCCT Baseline, and for whom IMT was measured 8 – 14 years later. After adjusting for treatment group, retinopathy status, age, sex, diabetes duration, hemoglobin A1c and ultrasonography equipment oxLDL-IC and AGE-LDL-IC each significantly predicted internal and common carotid IMT at EDIC year 1 and 6.[31]
In conclusion, the humoral immune response to modified LDL in humans appears to be a prime factor in the progression of atherosclerosis in humans. The demonstration that increased levels of oxLDL-IC predict the development of coronary artery calcification is a very significant finding supporting previous evidence and pointing to a pathogenic role of modified LDL immune complexes.
Acknowledgments
This work was supported by a Program Project funded by the National Institutes of Health/NHLBI (PO1 HL 55782), by two RO1 Grant funded by NIH/NIDDK (R01 DK081352 and R01 DK088778) and by a Juvenile Diabetes Foundation Grant (2006-49). The work was also supported by the Research Service of the Ralph H. Johnson Department of Veterans Affairs Medical Center.
The DCCT/EDIC was sponsored through research contracts from the Division of Diabetes, Endocrinology and Metabolic Diseases (NIDDK) of the NIH. Additional support was provided by the National Center for Research Resources through the GCRC program and by Genentech Inc through a Cooperative Research and Development Agreement with the NIDDK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lundberg AM, Hansson GK. Innate immune signals in atherosclerosis. Clin Immunol. 2010;134:5–24. doi: 10.1016/j.clim.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 2.Andersson J, Libby P, Hansson GK. Adaptive immunity and atherosclerosis. Clin Immunol. 2010;134:33–46. doi: 10.1016/j.clim.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Lopes-Virella MF, Virella G. Clinical significance of the humoral immune response to modified LDL. Clin Immunol. 2010;134:55–65. doi: 10.1016/j.clim.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amir S, Binder CJ. Experimental immunotherapeutic approaches for atherosclerosis. Clin Immunol. 2010;134:66–79. doi: 10.1016/j.clim.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saad AF, Virella G, Chassereau C, et al. OxLDL immune complexes activate complement and induce cytokine production by MonoMac 6 cells and human macrophages. J Lipid Res. 2006;47:1975–83. doi: 10.1194/jlr.M600064-JLR200. Epub 2006 Jun 27. [DOI] [PubMed] [Google Scholar]
- 6.Virella G, Atchley DH, Koskinen S, et al. Pro-atherogenic and pro-inflammatory properties of immune complexes prepared with purified human oxLDL antibodies and human oxLDL. Clin Immunol. 2002;105:81–92. doi: 10.1006/clim.2002.5269. [DOI] [PubMed] [Google Scholar]
- 7.Mironova M, Virella G, Virella-Lowell I, et al. Anti-modified LDL antibodies and LDL-containing immune complexes in IDDM patients and healthy controls. Clin Immunol Immunopath. 1997;85:73–82. doi: 10.1006/clin.1997.4404. [DOI] [PubMed] [Google Scholar]
- 8.Lopes-Virella MF, Virella G, Orchard TJ, et al. Antibodies to oxidized LDL and LDL-containing immune complexes as risk factors for coronary artery disease in diabetes mellitus. Clin Immunol. 1999;90:165–72. doi: 10.1006/clim.1998.4631. [DOI] [PubMed] [Google Scholar]
- 9.Lopes-Virella MF, McHenry MB, Lipsitz S, et al. Immune complexes containing modified lipoproteins are related to the progression of internal carotid intima-media thickness in patients with type 1 diabetes. Atherosclerosis. 2007;190:359–69. doi: 10.1016/j.atherosclerosis.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Orchard TJ, Virella G, Forrest KY, et al. Antibodies to oxidized LDL predict coronary artery disease in type 1 diabetes: a nested case-control study from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes. 1999;48:1454–8. doi: 10.2337/diabetes.48.7.1454. [DOI] [PubMed] [Google Scholar]
- 11.Yishak AA, Costacou T, Virella G, et al. Novel predictors of overt nephropathy in subjects with type 1 diabetes. A nested case control study from the Pittsburgh Epidemiology of Diabetes Complications cohort. Nephrol Dial Transplant. 2006;21:93–100. doi: 10.1093/ndt/gfi103. Epub 2005 Sep 6. [DOI] [PubMed] [Google Scholar]
- 12.Griffith RL, Virella GT, Stevenson HC, et al. Low density lipoprotein metabolism by human macrophages activated with low density lipoprotein immune complexes. A possible mechanism of foam cell formation. J Exp Med. 1988;168:1041–59. doi: 10.1084/jem.168.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopes-Virella MF, Binzafar N, Rackley S, et al. The uptake of LDL-IC by human macrophages: predominant involvement of the Fc gamma RI receptor. Atherosclerosis. 1997;135:161–70. doi: 10.1016/s0021-9150(97)00157-3. [DOI] [PubMed] [Google Scholar]
- 14.Yla-Herttuala S, Palinski W, Rosenfeld ME, et al. Lipoproteins in normal and atherosclerotic aorta. European Heart J. 1990;11:88–9. doi: 10.1093/eurheartj/11.suppl_e.88. [DOI] [PubMed] [Google Scholar]
- 15.Yla-Herttuala S, Palinski W, Butler S, et al. Rabbit and human atherosclerotic lesions contain IgG that recognizes epitopes of oxidized LDL. Arterioscler Thromb. 1994;14:32–40. doi: 10.1161/01.atv.14.1.32. [DOI] [PubMed] [Google Scholar]
- 16.Atchley DH, Lopes-Virella MF, Zheng D, et al. Oxidized LDL-Anti-Oxidized LDL immune complexes and diabetic nephropathy. Diabetologia. 2002;45:1562–71. doi: 10.1007/s00125-002-0962-y. [DOI] [PubMed] [Google Scholar]
- 17.Virella G, Derrick MB, Pate V, et al. Development of capture assays for different modifications of human low-density lipoprotein. Clin Diagn Lab Immunol. 2005;12:68–75. doi: 10.1128/CDLI.12.1.68-75.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cleary PA, Orchard TJ, Genuth S, et al. The effect of intensive glycemic treatment on coronary artery calcification in type 1 diabetic participants of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes. 2006;55:3556–65. doi: 10.2337/db06-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New England J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 20.Epidemiology of Diabetes Interventions and Complications (EDIC) Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22:99–111. doi: 10.2337/diacare.22.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodis HN, Mack WJ, LaBree L, et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128:262–9. doi: 10.7326/0003-4819-128-4-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 22.Virella G, Thorpe S, Alderson NL, et al. Definition of the immunogenic forms of modified human LDL recognized by human autoantibodies and by rabbit hyperimmune antibodies. J Lipid Res. 2004;45:1859–67. doi: 10.1194/jlr.M400095-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Feasibility of centralized measurements of glycated hemoglobin in the Diabetes Control and Complications Trial: a multicenter study. The DCCT Research Group. Clin Chem. 1987;33:2267–71. [PubMed] [Google Scholar]
- 24.Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy: ETDRS report number 12. Ophthalmology. 1991;98 (Suppl):823–33. [PubMed] [Google Scholar]
- 25.Greenland P, Bonow RO, Brundage BH, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2007;49:378–402. doi: 10.1016/j.jacc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann U, Brady TJ, Muller J. Cardiology patient page. Use of new imaging techniques to screen for coronary artery disease. Circulation. 2003;108:e50–3. doi: 10.1161/01.CIR.0000085363.88377.F2. [DOI] [PubMed] [Google Scholar]
- 27.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 28.Tobin J. Estimation of Relationships for limited Dependent Variables. Econometrica. 1958;26:24–36. [Google Scholar]
- 29.Reilly MP, Wolfe ML, Localio AR, et al. Coronary artery calcification and cardiovascular risk factors: impact of the analytic approach. Atherosclerosis. 2004;173:69–78. doi: 10.1016/j.atherosclerosis.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 30.Klein RL, Carter RE, Jenkins AJ, et al. LDL-containing immune complexes in the DCCT/EDIC cohort: associations with lipoprotein subclasses. J Diabetes Complications. doi: 10.1016/j.jdiacomp.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopes-Virella MF, Hunt KJ, Baker NL, et al. The Levels of Oxidized LDL and AGE- LDL in Circulating Immune Complexes are strongly associated with increased levels of Carotid Intima-Media Thickness and its progression in Type 1 Diabetes Diabetes. In print. [DOI] [PMC free article] [PubMed] [Google Scholar]


