Abstract
Docosahexaenoic acid (DHA), the most abundant essential n-3 polyunsaturated fatty acid in the CNS, emerged recently together with eicosapentaenoic acid (EPA) and DHA/EPA metabolic derivatives as a major player in the resolution of inflammation. Protective antiinflammatory effects of DHA were reported in clinical studies and animal models of colitis, sepsis, and stroke. Here we report for the first time a beneficial effect of dietary n-3 fatty acids in experimental autoimmune encephalomyelitis (EAE), a model for human multiple sclerosis. In the present study we investigated the effects of DHA on the function of bone marrow-derived dendritic cells (DC) in CD4+ T cell stimulation and differentiation. Pretreatment of DC with DHA prevented LPS-induced DC maturation, maintaining an immature phenotype characterized by low expression of costimulatory molecules and lack of proinflammatory cytokine production (IL-12p70, IL-6 and IL-23). DHA-treated DC were poor stimulators of antigen-specific T cells in terms of proliferation and Th1/Th17 differentiation. This was associated with an increase in p27(kip1), a cell cycle arresting agent, and with decreases in Tbet, GATA-3 and RORγt, master transcription factors for Th1, Th2, and Th17. In contrast, T cells co-cultured with DC-DHA express higher levels of TGFβ and Foxp3, without exhibiting a functional Treg phenotype. Similar to the in vitro results, the beneficial effect of DHA in EAE was associated with reduced numbers of IFNγ- and IL-17-producing CD4+ T cells in both spleen and CNS.
Keywords: Docosahexaenoic acid, Experimental autoimmune encephalomyelitis, IL-12, IL-23, Th1, Th17, Foxp3, Tbet, RORγt
INTRODUCTION
In contrast to n-6 polyunsaturated fatty acids (PUFA) such as arachidonic acid (AA) which mediate predominantly proinflammatory effects, the n-3 docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) are mostly anti-inflammatory. The n-3 PUFAs were reported to be protective in animal models of Alzheimer’s and Parkinson’s disease, in the ischemia-reperfusion model of stroke, and more recently in spinal cord and traumatic brain injury models (Bailes and Mills, 2010; Bousquet et al., 2008; Calon et al., 2004; Lopez-Vales et al., 2010; Marcheselli et al., 2003). In human subjects, high intake of dietary DHA and EPA affected the expression of more than 1,000 genes, reducing proinflammatory and atherogenic related gene expression (Bouwens et al., 2009). In patients with multiple sclerosis (MS), several studies reported lower levels of inflammatory cytokines and MMP9 and significant improvement in quality of life following 3–6 months of n-3 fatty acids supplementation (Gallai et al., 1995; Shinto et al., 2009; Weinstock-Guttman et al., 2005).
Although MS etiology remains elusive, the pathology relies mainly on autoimmune mechanisms that involve activated antigen-specific T cells as major players in addition to macrophages, dendritic cells (DC) and B cells (Frohman et al., 2006; Slavin et al., 2010) Experimental autoimmune encephalomyelitis (EAE) is a widely used animal model for MS. Multiple T cell subsets including CD4+ Th1 and Th17, γδT cells, CD8+ and Treg have been shown to be involved in EAE, with Th1 and Th17 as major pathogenic cells (Slavin et al., 2010). DC play an essential role in antigen presentation and T cell activation in EAE, with CNS perivascular conventional/myeloid DC (cDC/mDC) activating myelin-specific T cells and inducing differentiation into encephalitogenic Th1/Th17 (Bailey et al., 2007; Slavin et al., 2010).
DC represent an essential cellular link between innate and adaptive immunity. DC are professional antigen presenting cells whose major role is the uptake, processing and presentation of antigens to naïve CD4+ and CD8+ T cells. Following antigen uptake, cDC mature, upregulating MHCII and co-stimulatory molecules, secreting various cytokines and chemokines, and acquiring a new chemokine receptor pattern which enables them to migrate to neighboring lymph nodes. CD4+ T cell activation and differentiation in vivo is mediated through cognate interactions of naïve T cells with antigen-presenting cells, primarily cDC, which provide signaling through MHCII/antigen complexes and costimulatory molecules. Cytokines secreted by mature cDC play an important role in CD4+ T cell differentiation, with IL-12 supporting Th1, and IL-6, TGFβ and IL-23 supporting Th17 differentiation. We and others reported that exposure to DHA maintains cDC stimulated with TLR ligands in an immature state characterized by low MHCII, CD40, CD80, CD86 and CCR7 expression, and lack of inflammatory cytokine production (Kong et al., 2010; Wang et al., 2007; Zapata-Gonzalez et al., 2008; Zeyda et al., 2005). In our previous study (Kong et al., 2010) we did not address the effects of DHA-treated cDC on T cell activation and differentiation. Here we report for the first time that exposure to DHA prevents cDC to induce antigen-specific naïve CD4+ T cells to differentiate into Th17 cells, and that DHA-treated DC arrest T cells in G0/G1 phase through increased expression of p27(kip1). We also report for the first time that dietary DHA reduces EAE disease severity and that this correlates with a significant reduction in both Th1 and Th17 cells in spleen and the CNS.
MATERIALS AND METHODS
Mice
B10.A mice (I-Ek), C57BL/6 mice (H-2b), TCR-Cyt-5CC7-I/Rag1−/− transgenic (PCCF-specific TCR Tg; I-Ek) and C57BL/6-Tg (Tcra2D2,Tcrb2D2)1Kuch/J (MOG35–55 specific TCR) were purchased from Jackson Laboratory (Bar Harbor, ME) and Taconic (Hudson, NY). Transgenic mice were bred and maintained in the Temple University School of Medicine animal facility (Philadelphia, PA) under pathogen-free conditions. All mice used were between 6 and 10 wk of age. Mice were handled and housed in accordance with the guidelines of the Temple University Animal Care and Use Committee.
Reagents
Recombinant murine GM-CSF, recombinant murine CCL19, IL-12, IL-6, were purchased from Peprotech Inc (Rocky Hill, NJ). Docosahexaenoic Acid was purchased from Cayman Chemical (Ann Arbor, MI). Lipopolysaccharide (LPS) (Escherichia coli O26:B6), pertussis toxin (PTX), streptavidin-peroxidase, phorbol myristate acetate (PMA) and ionomycin were purchased from Sigma-Aldrich (St. Louis, MO). CD4 and CD11c MicroBeads were purchased from Miltenyi Biotec (Bergish-Gladbach, Germany). Recombinant IL-23, capture and biotinylated anti-mouse IL-23 antibody, PE-conjugated anti-mouse PD-L1 and CD25, FITC-conjugated anti-mouse PD-L2, mouse regulatory T cell staining kit, APC-conjugated anti-mouse INFγ were purchased from eBioscience (San Diego, CA). FITC-conjugated anti-mouse CD80, CD86, CD40, MHCII, CD4, CD44; PerCP-Cy™5.5 conjugated anti-mouse CD69, PE-conjugated anti-mouse IL-17, recombinant mouse IL-10, IFNγ and capture and biotinylated anti-mouse IL-2, IL-12p70, IL-6, IL-10, IFNγ; GolgiPlug, annexin V-FITC apoptosis detection kit I, Cytofix/Cytoperm, Perm/Wash buffer, TMB Substrate Reagent Set, and the Cycle TEST PLUS DNA Reagent Kit were purchased from BD PharMingen (San Diego, CA). Pigeon cytochrome c fragment (PCCF), myelin oligodendrocyte glycoprotein (MOG)35–55, proteolipid protein (PLP)139–151, CFSE Cell Proliferation Kit, 1X HBSS and 10X HBSS were purchased from Invitrogen Corporation (Carlsbad, CA). Capture and biotinylated anti-mouse IL-17, recombinant mouse IL-17, recombinant TGFβ, and recombinant mouse IL-2 were purchased from R&D Systems (Minneapolis, MN). DNase I grade II and Liberase TL were purchased from Roche (Indianapolis, IN). Ketamine HCl was purchased from Fort Dodge Animal Health (Fort Dodge, IA). Xylazine was purchased from Butler Animal Health Supply (Dublin, OH). 0.5 M EDTA was purchased from Promega Corporation (Madison, WI). Percoll was purchased from GE Healthcare (Piscataway, NJ). Mycobacterium tuberculosis H37 RA was purchased from Difco (Detroit, MI).
Generation and purification of DC from bone marrow
DC were generated from bone marrow as described previously (Kong et al., 2010). On day 7, the non-adherent cells were harvested and purified by immunomagnetic sorting with anti-CD11c–coated magnetic beads using the autoMACS system according to the manufacturer’s instructions (Miltenyi Biotec). The purity of the sorted cells was determined by FACS analysis (>96% CD11c+ cells).
Isolation of CD4+ T cells
Purified CD4+ T cells were isolated from the spleen of PCCF-specific TCR-Tg mice or MOG35–55 specific TCR-Tg mice by positive immunomagnetic selection using anti-CD4 mAb magnetic beads (Miltenyi Biotec). The purified T cells were 98% CD4+ as determined by FACS analysis.
FACS Analysis
Cells were subjected to FACS analysis in a 3-color FACS Calibur (BD Biosciences, Mountain View, CA). Data were collected for 10,000 cells and analyzed using Cellquest software from BD Biosciences (San Jose, CA). DC or T cells washed with ice cold PBS and incubated for 30 minutes at 4°C with various FITC/PE/APC/PerCP conjugated antibodies and were analyzed by flow cytometry. For the detection of Foxp3, cells were first stained with anti-CD4 and anti-CD25, fixed with Cytofix/Cytoperm buffer, incubated with anti-Foxp3, and analyzed by FACS. The specificity of the primary Abs was established with appropriate isotype-matched controls.
T cell proliferation assay
DC-CD4+ T cell co-cultures or splenocytes were cultured in 96-well flat bottom plates. On day 3 of co-culture, [3H]-thymidine (1µCi per well) was added and incorporation was measured after 16h. Cells were harvested on fiberglass filters, and [3H]-thymidine incorporation was measured in a liquid scintillation counter.
Proliferation suppressive assays were performed as follows: 1×105 MOG35–55 specific CD4+ T cells were activated with MOG-pulsed DC or DC-DHA in the presence or absence of 2 ng/ml TGFβ and 50 U/ml IL-2 for 3 days, rested for 2 days in the presence of IL-2 and re-cultured with CFSE-labeled (5 µM, according to the manufacturer’s Iprotocol) naïve MOG Tg-CD4+ T cells (0.1× 105) and 0.1× 104 MOG-pulsed DC, in 200 µl medium in 96-well plates. Three days later, proliferation was assessed by CFSE dilution using FACS. The proliferation of naïve CD4+ T cells was based on gated CFSE labeled cells.
Apoptosis assay with annexin V and propidium iodide (PI) staining
After treatment, DC or T cells were washed and adjusted to 1 × 106 cells/ml in staining buffer. Annexin V and PI staining was performed according to the protocol provided by BD Pharmingen, and the cells were analyzed immediately by flow cytometry.
Cell cycle analysis
DC or DC treated with DHA (DC-DHA) were pulsed with 50µg/ml MOG35–55 and stimulated with 0.1 µg/ml LPS for 24 h. After extensive washing, DC or DC-DHA were co-cultured with MOG35–55 specific CD4+ T cells at a 1/20 ratio. Three days later, cell cycle analysis was performed on activated T cells using the Cycle TEST PLUS DNA Reagent Kit according to the manufacturer's instructions. Samples were analyzed by FACS.
Cytokine ELISA
Supernatants from DC, DC-CD4+ T cell co-cultures or splenocyte cultures were harvested and subjected to sandwich ELISA for IL-12p70, IL-23, IL-6, IL-10, IL-2, IFNγ and IL-17. The detection limits were: 15 pg/ml for IL-6, IL-17, IL-2 and IL-10, 30 pg/ml for IL-23, IFNγ and IL-12p70.
Chemotaxis assay
Purified DC were preincubated with 50µM DHA for 24h, followed by an additional 24h treatment with 0.1 µg/ml LPS and assayed for migration in response to the chemokine CCL19 (100 ng/ml). The lower chambers of Transwell plates (8.0µm pore size; Corning, Acton, MA) were filled with 600µl serum-free medium with or without CCL19. DC (1×105 cells in 0.1 ml) resuspended in serum-free medium were deposited in the upper chambers of the Transwell plates and allowed to migrate for 4h at 37°C in 5% CO2. The numbers of migrated DC harvested from the lower chambers were counted by FACS (60-second counts).
Real-time RT-PCR
The expression of p27(kip1), Tbet, GATA3, RORC, Foxp3, CCR5, CCR7, IL-10 and TGFβ were detected by SYBR Green-based real-time RT-PCR. RNA was prepared from 4×106 T cells activated by DC or DC-DHA using an Ultraspec RNA isolation system according to the manufacturer’s instructions (Biotecx Laboratories, Houston, TX). RNA (1 µg) was reversed transcribed to cDNA and subjected to real time PCR. The PCR mixture (20 µl), consists of 4 µl diluted cDNA, 16 µl of SYBR Green containing the PCR master mix and 150 nM of each primer. Real-time PCR was performed using the Stratagene Mx3005P. The following primers were used: p27(kip1) sense, 5'-CGGCGGCAAGGTTTGGAGAGG-3' and antisense, 5'- GGAGGAGGCAGGAGGAGGTGG-3'; Tbet sense, 5'-CGGTA CCAGAGCGGCAAGT-3', and antisense, 5'-CATGCTGCCTTCTGCCTTTC-3'; GATA3 sense, 5'-TACTTGCGTTTTTCGCAGGA-3', and antisense, 5'-GATCTGTCGCTTTCGGGCCT-3'; RORC sense, 5'-GCGGAGCAGACACACTTACA-3', and antisense, 5'-TCCACCACCACAGCTGAGAGG-3'; Foxp3 sense, 5'- CAGCTGCCTACAGTGCCCCTA-3', and antisense 5'-CATTTGCCAGCAGTGGGTAG-3'; CCR5 sense, 5′-CATCGATTATGGTATGTCAGC ACC-3′ and antisense, 5′-CAGAATGGTAGTGTGAGCAGGAA-3′; CCR7 sense 5′- CCAGGAAAAACGTGCTGGTG-3′ and antisense 5′-GGCCAGGTTGAGCAGGTAG G-3′; IL-10 sense, 5'-ACCTGCTCCACTGCCTTGCT-3', and antisense 5'-GGTTGCCAAGCCTTATCGGA-3'; TGFβ sense, 5'-GACCTGGGTTGGAAGTGGATC-3', and antisense 5'-GAAGTT GGCATGGTAGCCCTT-3'; β-actin sense, 5’-TCCACCA CCACAGCTGAGAGG-3’ and antisense, 5’-CAGCTTCTCTTTGATGTCACG-3’. The cycling conditions were 95°C for 15 s, 75°C for 1 min, 57°C for 30 sec, for 40 cycles, followed by a melting point determination or dissociation curves. The expression level of each gene is indicated by the number of cycles needed for the cDNA amplification to reach a threshold. The amount of DNA is calculated from the number of cycles by using standard curves and the results are normalized to β-actin.
EAE induction
C57BL/6 mice (3 wks old) were fed with control or DHA diet for 5 weeks as described before (Kong et al. 2010). Mice were injected with 200µg MOG33–55 peptide emulsified in complete Freund’s adjuvant containing Mycobacterium tuberculosis H37 RA (final concentration 2mg/ml) s.c. on day 0 and 100 ng pertussis toxin (PTX) i.p. on day 0 and on day 2. Clinical scores were as follows: 0, normal mouse, no overt signs of disease; 1, limp tail or hind limb weakness; 2, limp tail and hind limb weakness; 3, partial hind limb paralysis; 4, complete hind limb paralysis; 5, moribund state. At stage 5, animals were euthanized and removed from the calculation for the clinical score. Both clinical scores and weight were followed for 60 days.
Isolation of inflammatory CD4+ T cells from central nervous system (CNS)
C57BL/6 mice were immunized as described before. Isolation of mononuclear cells was performed at peak of clinical disease (day 18). Mice were anesthetized with 20 µl of mix of ketamine HCl and xylazine and perfused through the left cardiac ventricle with 30 ml of HBSS containing 2mM EDTA. The brain was dissected and spinal cord was flushed out with HBSS. CNS tissue was digested with 10 ml HBSS containing DNAse I (0.1 mg/ml for brain and 0.05 mg/ml for spinal cord) and Liberase (0.05 mg/ml for brain and 0.025 mg/ml for spinal cord) for 45 min at 37°C with shaking, followed by blocking solution (10% FCS, 10 mM EDTA in HBSS). The tissue was pelleted and resuspended in 10 ml of 30% isotonic Percoll (diluted with 10x HBSS and distilled water), underlaid with 5 ml of 70% isotonic Percoll. Mononuclear cells were isolated from the 30/70 interphase after gradient centrifugation. Cells were washed with RPMI 1640 medium. Mononuclear cells were cultured in the presence of PMA (50 ng/ml), ionomycin (500ng/ml) and GolgiPlug (1µl/ml) for 4h. Cells were stained with FITC anti-CD4 for 30 min, fixed and permeabilized using Cytofix/Cytoperm and Perm/Wash buffer according to the manufacturer’s instructions. Cells were stained with APC anti-IFNγ and PE anti-IL-17 for 30 min. FACS analysis was performed. T cells were identified by gating on CD4+ cells. The number of cytokine producing cells was calculated from the percentage of cytokine-positive CD4+ T cells. We determined the number of CD4+ T cells in the CNS by multiplying the percentage of positive cells by the total number of mononuclear cells isolated from the CNS.
Statistical analysis
Results are described as mean +/− SD. Comparisons between two groups were done using Student t test, whereas comparisons among multiple groups were done by one way ANOVA. Bonferroni test was used for post hoc comparisons among multiple groups where appropriate. Statistical significance was determined as p values less than 0.05. All statistical analyses were performed using SPSS 12.0 software.
RESULTS
DHA prevents LPS-induced DC maturation and cytokine production
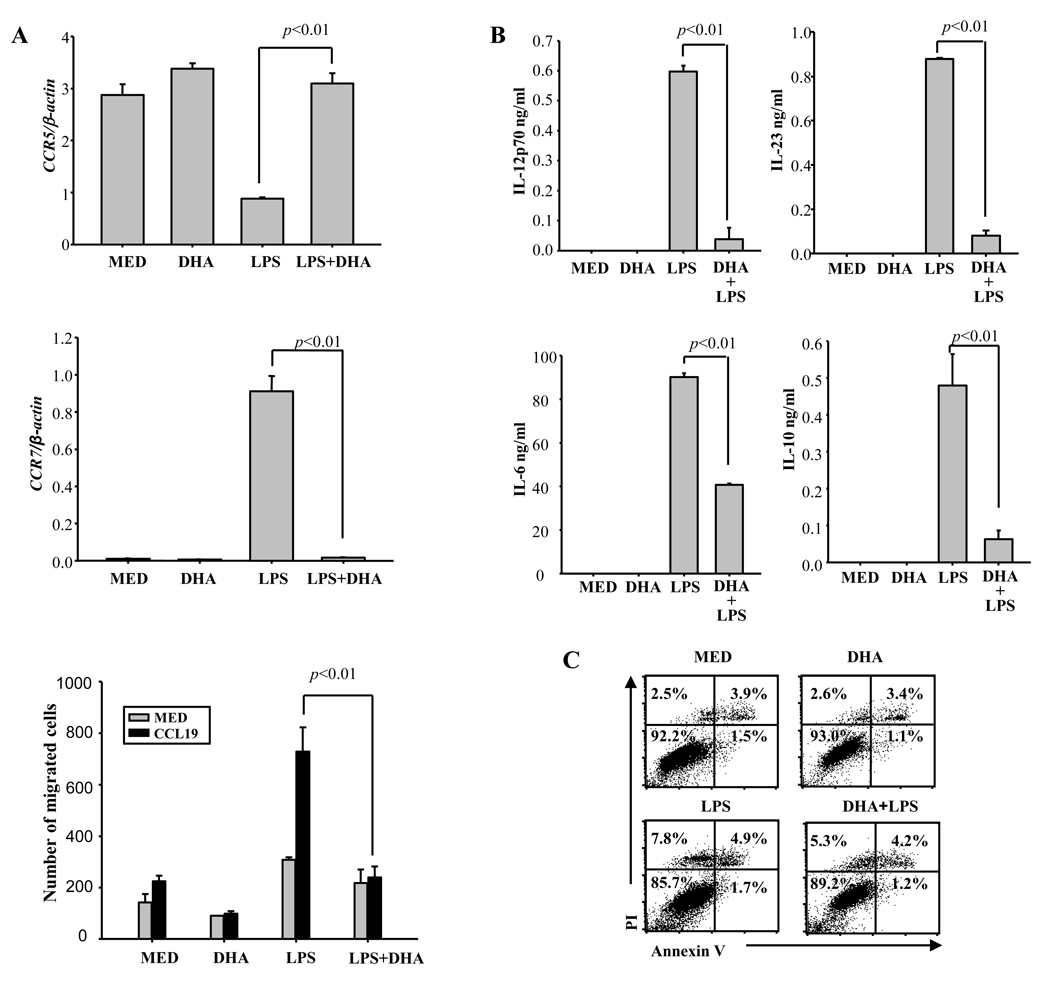
Purified CD11c+ bone marrow-derived DC were exposed to DHA for 24h followed by LPS stimulation. In previous experiments we established the optimal dose of DHA as 50 µM (Kong et al., 2010). 24h later we analyzed the levels of CD40, CD80, CD86, PD-L1 and PD-L2 by flow cytometry. In immature DC, DHA treatment inhibited the expression of MHCII and increased slightly the expression of CD40 and CD86 (Table 1). As reported previously, we found that DHA prevented the upregulation of costimulatory molecules (CD40/80/86) both in terms of percentage positive cells and mean fluorescence intensity (MFI) (Table 1). In contrast, DHA did not significantly affect the percentage of PD-L1 and PD-L2 positive cells, although it reduced the levels of PD-L1 MFI (Table 1). In contrast to immature DC which express high levels of CCR5 and migrate to inflammatory sites, mature DC upregulate CCR7 and migrate in response to CCL19/21, chemokines constitutively expressed in secondary lymphoid organs. Immature DC treated with or without DHA expressed high levels of CCR5. LPS treatment reduced CCR5 expression in control DC, but not in DHA-treated DC (Fig. 1A – upper panel). As expected, LPS induced CCR7 in control DC. In contrast, there was no CCR7 induction in DHA-treated DC (Fig. 1A – middle panel). In agreement with observed CCR7 expression, LPS-treated control DC migrated in response to CCL19, whereas DHA-treated DC did not (Fig. 1A – lower panel).
TABLE 1.
DHA pretreatment maintains the immature phenotype in LPS treated DC
| MED | DHA | LPS | LPS+DHA | ||
|---|---|---|---|---|---|
| MHCII | % of cells | 17.6±0.6 | 4.2±1.2 # | 31.6±2.2 | 7.24±1.5* |
| MFI | 192.1± 9.3 | 97.4±4.2 # | 236.1±28.7 | 123.8±16.4* | |
| CD40 | % of cells | 4.1±2.5 | 14.4±2.7 # | 73.9±0.8 | 31.6±7.3 * |
| MFI | 163.2±9.7 | 105.4±7.8# | 162.9±4.8 | 103.6±5.1* | |
| CD80 | % of cells | 7.1±0.9 | 7.1±1.7 | 37±5.8 | 8.1±2.9 * |
| MFI | 72.8±0.4 | 103.2±2.1 | 128.8±7.1 | 79.6±4.4* | |
| CD86 | % of cells | 3.8±1.1 | 8.5±2.7# | 37.7±1.1 | 7.7±0.3 * |
| MFI | 82.2±0.2 | 64.5±4.0 | 88.3±1.3 | 60.4±2.9* | |
| PD-L1 | % of cells | 94±0.6 | 95±0.3 | 98±0.3 | 94±0.1 |
| MFI | 277.4±44.6 | 294.0±28.2 | 722.4±51.5 | 494.2±25.4* | |
| PD-L2 | % of cells | 52±0.6 | 48±1.1 | 40±0.7 | 41±0.4 |
| MFI | 48.1±0.9 | 39.2±3.4 | 56.5±1.7 | 43.8±8.8 | |
Data are means ± SD, n=3. # indicates difference from medium control: p<0.05;
indicates difference from LPS treatment: p <0.01.
MED: medium control. Percentage of positive cells was based on isotype control.
CD11c+ DC (1×106/ml) were cultured in the presence or absence of 50 µM of DHA for 24h, followed by treatment with 0.1µg of LPS for another 24h. Cells were incubated with various Abs for 30 min, and the expression of MHCII, CD40, CD80, CD86, PD-L1, PD-L2 was detected by flow cytometry.
Fig. 1. DHA prevents CCR7 expression and cytokine production in LPS-treated DC.
(A) CD11c+ DC were treated with 50 µM DHA for 24h, followed by LPS (0.1 µg/ml) for 24h and CCR5 (upper panel) and CCR7 (middle panel) expression was determined by real time RT-PCR. Migration of 1×105 DC toward 100 ng/ml CCL 19 was determined in a 4h Transwell chemotaxis assay as described in Methods (lower panel). (B) CD11c+ DC (1×106/ml for IL-12p70 and IL-6, and 2×106/ml for IL-23 and IL-10) were cultured in the presence or absence of 50 µM of DHA for 24h, followed by 0.1µg of LPS treatment for 12h (for IL-23) or 24h (for IL-12p70, IL-6 and IL-10). Supernatants were subjected to ELISA. Data represent the mean +/− SD of three experiments performed in triplicate. (C) CD11c+ DC were preincubated with 50 µM DHA for 24h, followed by treatment with 0.1 µg LPS for 24h. Cells were stained with Propidium Iodide (PI) and Annexin V and apoptosis was analyzed by flow cytometry (quadrant values represent percentage of cells). One representative experiment of three is shown. Med- medium control.
We reported previously that DHA also prevented cytokine production by LPS-stimulated DC (Kong et al., 2010). Here, we confirmed that DHA abolished production of IL-12p70, IL-23 and IL-10, and significantly inhibited IL-6 release (Fig. 1B). The reduction in cytokine release was not due to DC apoptosis, since exposure to DHA of immature or LPS-stimulated DC did not increase the percentage of apoptotic/necrotic cells (Fig. 1C). These results indicate that pretreatment with DHA maintains an immature phenotype in LPS-stimulated DC, in terms of surface markers, directional chemotactic migration, and cytokine production.
DHA treatment impairs the capacity of DC to activate naïve CD4+ T cells
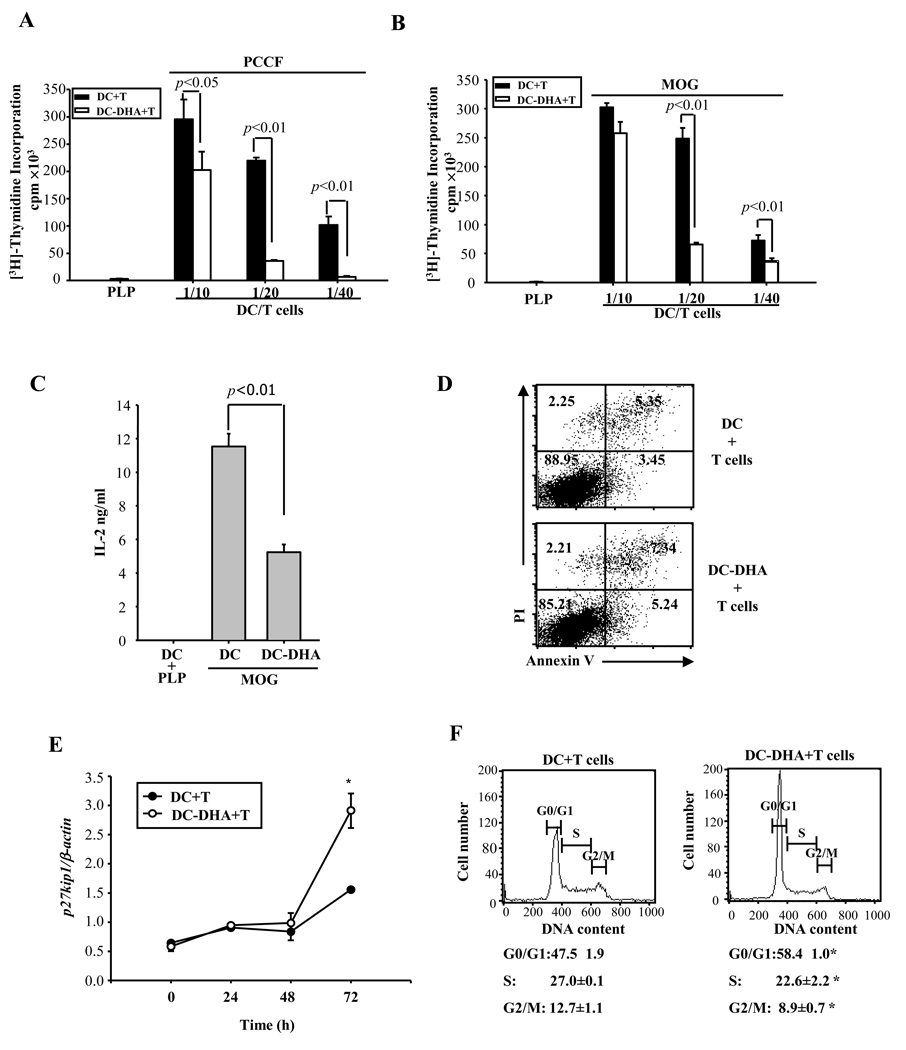
Next, we assessed the capacity of DHA-treated DC to activate naïve T cells in two TCR transgenic systems. PCCF- or MOG-specific CD4+ T cells were purified from the spleen of TCR transgenic mice and co-cultured with various numbers of DC pretreated with DHA for 24h (DC-DHA) or with control DC (DC). DC-DHA were washed extensively to remove DHA, followed by treatment with LPS and pulsing with either PCCF or MOG (or PLP as negative control) for 24h prior to co-culture with CD4+ T cells. Control DC were treated the same way with the exception of DHA pretreatment. DC-DHA induced much less T cell proliferation compared to control DC (Fig. 2A–B). In agreement with reduced T cell activation by DC-DHA, we observed decreased expression of the T cell activation markers CD25, CD44 and CD69 (Table 2). T cells co-cultured with DC-DHA also produced significantly lower levels of IL-2 (Fig. 2C). Since reduced proliferation could be due to T cell apoptosis, we assessed apoptosis by flow cytometry. There was no significant change in apoptosis in co-cultures with DC-DHA compared to control DC (Fig. 2D).
Fig. 2. DC-DHA are poor stimulators of antigen specific CD4+ T cells.
(A and B) CD11c+ DC from B10.A mice (A) or C57BL/6 (B) were treated with (DC-DHA) or without (DC) 50 µM DHA for 24 h, stimulated with LPS and pulsed with 5 µM PCCF (pigeon cytochrome c fragment), 50 µg/ml MOG35–55 (myelin oligodendrocyte glycoprotein), or PLP (proteolipid protein) (20 µg/ml; nonspecific Ag) for an additional 24h, followed by extensive washing. Various numbers of DC-DHA or DC were cultured with TCR Tg CD4+ T cells (2×105 cells/well) (PCCF-specific in A and MOG-specific in B) in 96-well plates for 3 days. [3H]-thymidine (1µCi per well) was added and incorporation was measured after 16h. (C) DC from C57BL/6 mice were cultured with MOG35–55 specific CD4+ T cells at 1/20 ratio. Three days later, supernatant was collected and IL-2 production was determined by ELISA. (D) The apoptotic status of T cells was assessed using flow cytometry (annexin V/PI staining) after 3 days of co-culture and quadrant values represent percentage of cells. One representative experiment of three is shown. (E) DC and DC-DHA were cultured with MOG-specific CD4+ T cells at 1/20 ratio. T cells were collected after 0 h, 24 h, 48h, and 72h and p27(kip1) expression was detected by real time RT-PCR. * P<0.05, compared with DC-T cells co-cultures. (F) Cell cycle analysis was performed on activated CD4+T cells after 3 days of co-culture with DC or DC-DHA pulsed with MOG. The changes in cell cycle were quantified by flow cytometry after PI staining as described in Methods. The experiment was performed three times, and the ratios of cells in G0/G1, S, and G2/M phase were expressed as mean ± SD. * P<0.05, compared with DC-T cells co-cultures.
Table 2.
DC-DHA activated T cells express lower levels of CD25, CD44 and CD69.
| DC + T cells | DC-DHA + T cells | |||
|---|---|---|---|---|
| % of cells | MFI | % of cells | MFI | |
| CD 25 | 86.9±0.5 | 426.3±4.5 | 74.9±1.3* | 196.7±9.7* |
| CD44 | 64.2±0.4 | 162.7±3.7 | 54.7±1.3* | 135.9±0.1* |
| CD69 | 64.0±1.3 | 143.8±3.5 | 48.3±1.4* | 118.6±0.5* |
Data are means ± SD, n=3. Asterisk indicates difference from LPS treatment:
P<0.01.
MED: medium control. Percentage of positive cells was based on isotype control.
CD11c+ DC from C57BL/6 mice were treated with or without 50 µM DHA (DC-DHA and DC) for 24h, stimulated with 0.1 µg/ml LPS and pulsed with 50 µg/ml of MOG 35–55.. DC (1×104 cells) were cultured with MOG-specific Tg CD4+ T cells (2×105 cells) for 3 days. The expression of CD25, CD44, CD69 on gated CD4+ T cells was detected by flow cytometry.
Cell cycle exit can be also responsible for reduced proliferation. DHA has been reported to promote neuronal and retinal phosphoreceptor differentiation by inducing cell cycle arrest in the G1 phase through prolonged and increased expression of p27(kip1) (Insua et al., 2003; Katakura et al., 2009). p27(kip1) expression was measured by real time RT-PCR at different time points in co-cultures with DC or DC-DHA. A significant increase was observed at 72h in T cells co-cultured with DC-DHA (Fig. 2E). To determine whether the increase in p27(kip1) is indeed associated with changes in T cell cycle, we performed cell cycle analysis by FACS using propidium iodide labeled T cells. Indeed, in agreement with the observed increase in p27(kip1), a higher percentage of T cells remained in G0/G1 phase when co-cultured with DC-DHA (Fig. 2F).
DHA modulates CD4+ T cell differentiation
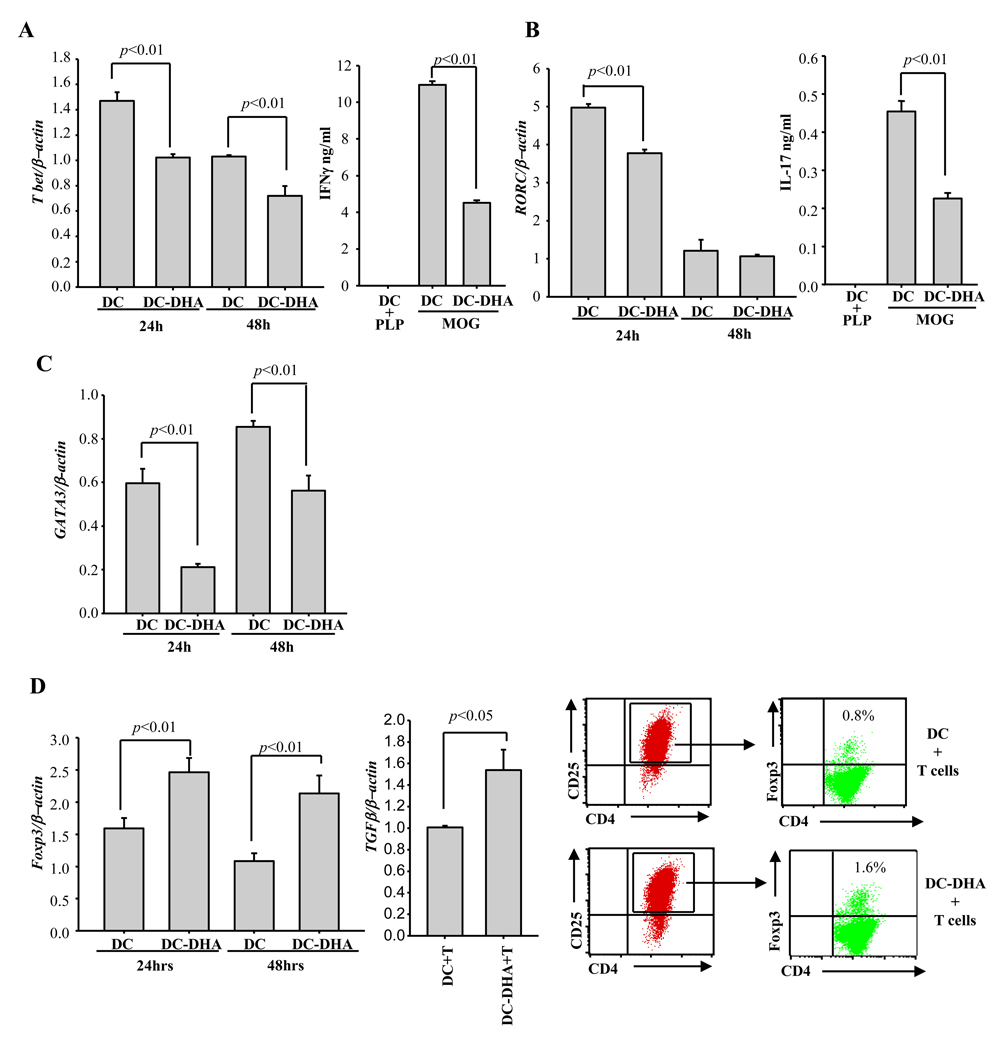
To assess the effect of DC-DHA on CD4+ T cell differentiation, we co-cultured naïve T cells with LPS-activated, MOG-pulsed DC or DC-DHA for 24 and 48h and measured T cell expression of the master transcription factors Tbet (Th1), RORγt (Th17), GATA-3 (Th2) and Foxp3 (Treg) by real time RT-PCR. Maximum expression of Tbet and RORγt occurred at 24h, and expression of both transcription factors was reduced in T cells co-cultured with in DC-DHA (Fig. 3A–B). GATA-3 expression was also reduced by DHA at 24 and 48h (Fig. 3C). Supernatants collected after 72h were subjected to ELISA. As expected, IFNγ and IL-17 were decreased in co-cultures containing DC-DHA (Fig. 3A–B). IL-4 secretion was undetectable in T cells co-cultured with control DC, as well as DC-DHA. Although IL-10 production could not be detected by ELISA, similar levels of IL-10 mRNA were observed in T cells co-cultured with control or DC-DHA (data not shown). In contrast to Tbet, GATA-3 and RORγt, Foxp3 and TGFβ expression were increased in T cells co-cultured with DC-DHA (Fig. 3D). Increased numbers of CD4+CD25+Foxp3+ T cells were also observed by flow cytometry in co-cultures with DC-DHA (Fig. 3D), suggesting that DC-DHA might contribute to the generation of CD4+Foxp3+ iTreg.
Fig. 3. DC-DHA affects CD4+ T cell differentiation.
DC from C57BL/6 mice were treated with or without 50 µM DHA for 24h, followed by 0.1 µg LPS and pulsing with MOG35–55 for another 24h and extensive washing. DC and DC treated with DHA (DC-DHA) were cultured with MOG35–55-specific CD4+ T cells at 1/20 ratio. 24 and 48h later, T cells were collected, the expression of Tbet (A), RORC (B), GATA-3 (C) and Foxp3 (D, left panel) were assessed by real time RT-PCR. Three days later, supernatants were collected and the production of IFNγ and IL-17 was determined by ELISA (A and B). T cells were collected and subjected to real time RT-PCR to detect expression of TGFβ (D, middle panel). Foxp3+ expression was analyzed by flow cytometry in gated CD4+CD25+ T cells. (D, right panels). One representative experiment of three is shown.
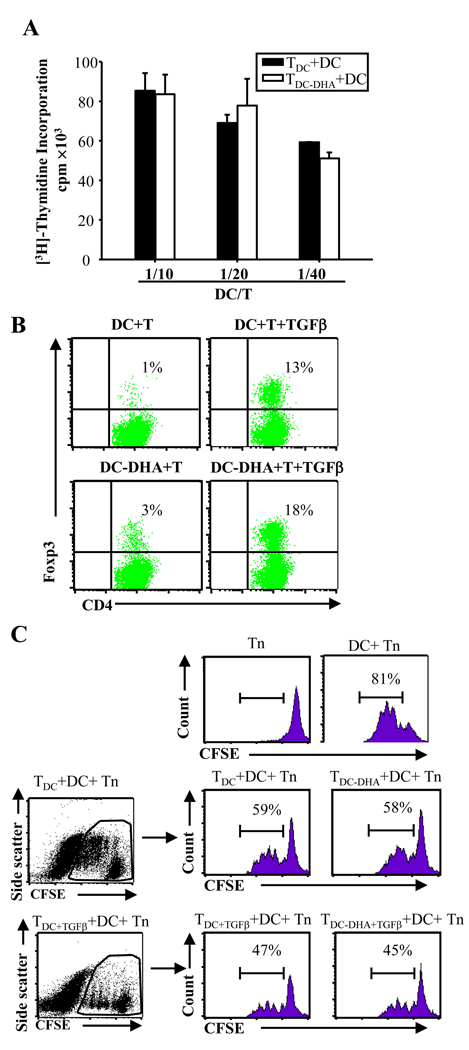
To test this possibility, CD4+ T cells were isolated following co-culture with DC-DHA, rested in the presence of exogenous IL-2, and restimulated with LPS-treated, MOG-pulsed DC. The T cells previously exposed to DC-DHA were not anergic, since they proliferated at the same rate as T cells initially stimulated with control DC (Fig. 4A). To determine whether TGFβ is required for the induction of functional Treg by DC-DHA, TDC-DHA and TDC were generated in the presence of TGFβ, followed by co-culture with naïve T cells and regular DC. The percentages of CD4+CD25+Foxp3+ T cells increased in both TDC-DHA and TDC (Fig. 4B). To test the possible suppressive activity of T cells exposed to DC-DHA (TDC-DHA), the cells were co-cultured with CFSE-labeled MOG-specific naïve T cells in the presence of LPS-stimulated, MOG-pulsed DC. Proliferation of naïve T cells was similar in the presence of TDC-DHA or TDC (Fig. 4C). Also, we did not observe any difference between proliferation of naïve T cells in the presence of TDC+DGFβ or TDC-DHA+TGFβ (Fig. 4C). These results suggest that although DC-DHA induce a higher percentage of CD4+CD25+Foxp3+ T cells, these cells apparently lack the ability to suppress the proliferation of effector T cells.
Fig. 4. DC-DHA activated CD4+T cells are not anergic or suppressive.
(A) Activated T cells (TDC or TDC-DHA) were generated from MOG35–55-specific CD4+ T cells cultured with DC or DC treated with docosahexaenoic acid (DC-DHA) pulsed with MOG35–55 and activated with LPS. TDC or TDC-DHA (2×105 cells/well) were cultured with different number of regular DC stimulated with LPS and pulsed with MOG35–55. Three days later, proliferation was measured. (B) Activated T cells were generated from MOG-specific CD4+ T cells cultured with DC or DC-DHA in the presence or absence of 2 ng/ml TGFβ plus 50U IL-2. The Foxp3+ expression was analyzed in the same way as in Fig 3 (D). (C) 1×105 cells/well activated T cells (TDC, TDC-DHA, TDC+TGFβ and TDC-DHA+TGFβ generated from naïve MOG-specific CD4+ T cells cultured with DC, DC-DHA, DC+TGFβ and DC-DHA+TGFβ, respectively) were co-cultured with CFSE-labeled syngeneic MOG-specific naïve CD4+ T cells (Tn) (1×105 cells/well) in the presence of DC (1×104 cells/well) pulsed with 50 µg/ml MOG35–55. T cell proliferation was determined by CFSE dilution using FACS. One representative experiment of three is shown.
Dietary DHA has a beneficial effect in EAE
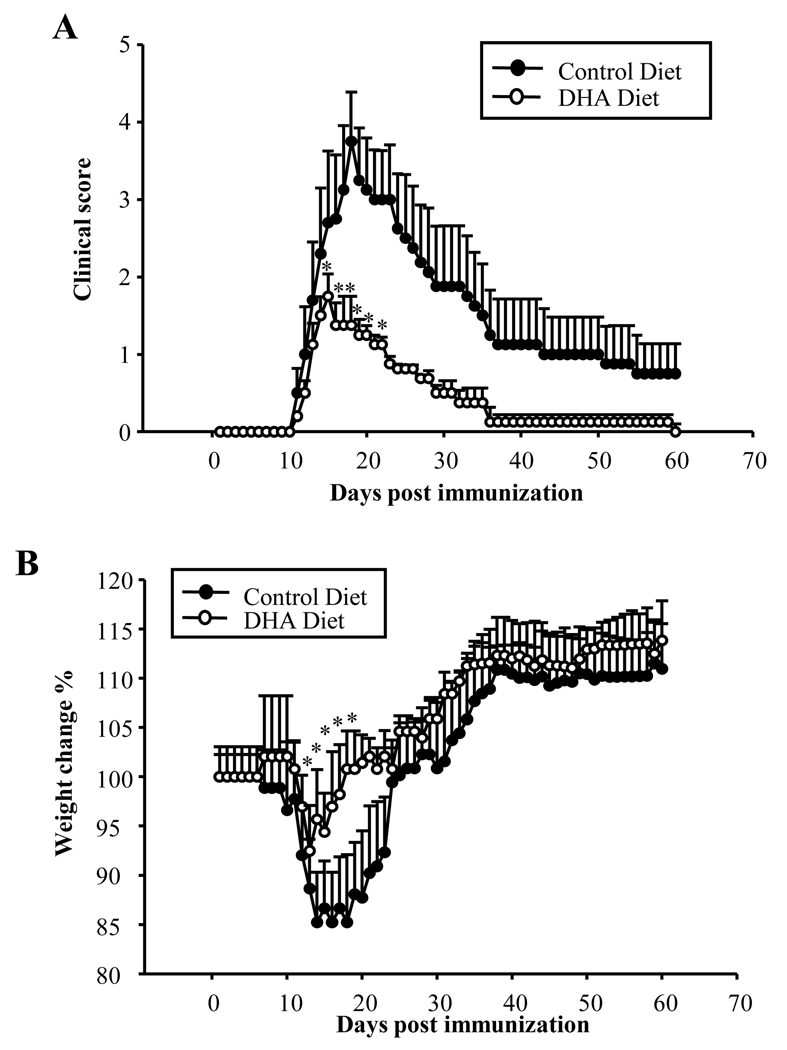
Both Th1 and Th17 cells play an important role in the development of autoimmune diseases, including EAE. Since exposure of DC to DHA inhibits T cell differentiation into Th1/Th17 subsets, we assessed the effect of dietary DHA in an active EAE model. Mice were fed a DHA-enriched diet for 5 wks, followed by EAE induction as described in Methods. Dietary DHA did not delay EAE onset, but significantly reduced disease severity and decreased mortality (Fig. 5A; Table 3). In terms of weight loss, the mice on the enriched DHA diet lost significantly less weight and recovered faster than those on the control diet (Fig. 5B).
Fig. 5. Dietary DHA suppresses experimental autoimmune encephalomyelitis.
C57BL/6 mice were fed a control (n=13) or DHA diet (n=13) for 5 wks. Mice were immunized with MOG 35–55 as described in Methods. Clinical scores and weight were followed daily for 60 days. At the end of the observation period three mice had died in the control group and one in the DHA group. (A) Kinetics of mean clinical score; (B) Percentage change in weight. * p<0.05, compared with control group.
Table 3.
Incidence and mortality in EAE mice fed control or DHA diet.
| Diet | No. of mice | Incidence | Mortality |
|---|---|---|---|
| Control diet | 13 | 12/13 | 3/13 |
| DHA diet | 13 | 11/13 | 1/13 |
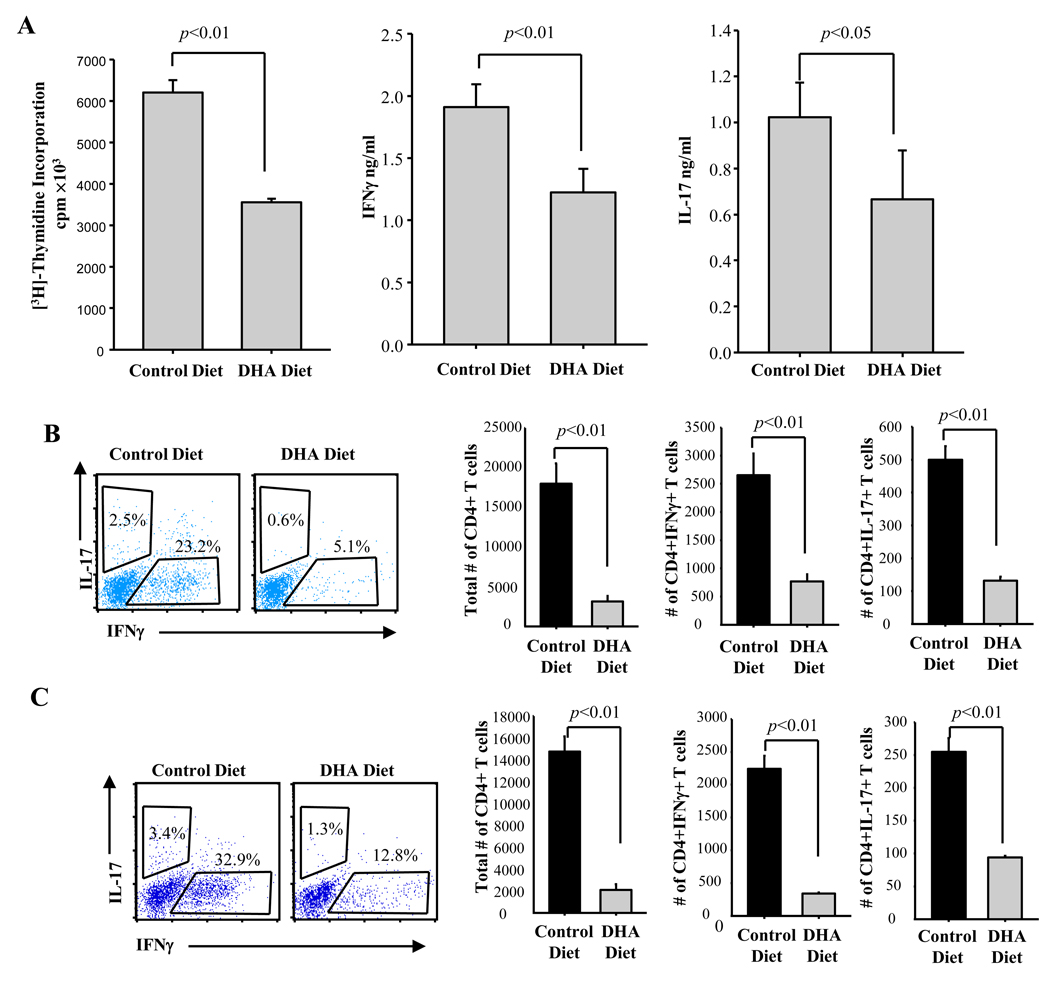
To investigate whether the protective effect of dietary DHA is due to the inhibition of T cell proliferation and differentiation into Th1/Th17 effectors, splenocytes obtained on day 11 were restimulated ex vivo with MOG35–55, and tested for proliferation and for IFNγ and IL-17 production. There were significant decreases in proliferation and IFNγ/IL-17 secretion in splenocytes from mice on the DHA enriched diet (Fig. 6A). We also investigated the numbers of brain and spinal cord infiltrating CD4+ T cells, and their functional phenotype through intracellular staining for IFNγ and IL-17 on day 18 (peak of clinical disease). Both the percentage and numbers of CD4+IFNγ+ (Th1) and CD4+IL-17+ (Th17) cells are much lower in the CNS of mice on the DHA enriched diet (Fig. 6B–C).
Fig. 6. Dietary DHA inhibits Th1 and Th17 differentiation and reduces the numbers of CNS infiltrating Th1, Th17 and total CD4+ T cells.
C57BL/6 mice fed a control or DHA diet were immunized with MOG35–55 as described in Methods. (A) Splenocytes (2×106/ml) isolated from spleen on day 11 post immunization (before disease onset) were cultured ex vivo in the presence of 50 µg MOG35–55 for 3 days. Proliferation was determined by [3H]-thymidine incorporation. Supernatants were subjected to ELISA for IL-17 and IFNγ. One representative experiment out of three is shown. (B–C) Intracellular cytokine staining of FITC-gated CD4+ T cells isolated from brain (B) or spinal cord (C) of mice at peak of disease (day 18) (cells pooled from 3 animals) (left panels show flow cytometric analysis; right panels show calculated numbers of cytokine-producing cells). Data are representative of two independent experiments.
DISCUSSION
Initially the anti-inflammatory effects of DHA and EPA have been attributed to the inhibition of cycloxygenase-mediated AA metabolism. However, recent studies established that DHA and EPA also alter the composition of lipid rafts in immune cells, act as ligands for nuclear receptors such as peroxisome proliferator-activated receptors (PPARs) and retinoid X receptors (RXR), and generate endogenous metabolites involved in the resolution of inflammation such as resolvins, docosatrienes and neuroprotectins (Bannenberg, 2009; Calder, 2008; Chapkin et al., 2009; Das, 2006; Kohli and Levy, 2009; Serhan et al., 2008a).
In agreement with the anti-inflammatory properties of n-3 fatty acids, fat-1 transgenic mice which synthesize n-3 PUFA from n-6 PUFA exhibit reduced systemic inflammation in acute pancreatitis, and are more susceptible to pulmonary TB due to reduced inflammatory responses in macrophages (Bonilla et al., 2010; Weylandt et al., 2008). Dietary n-3 DHA and EPA were shown to be protective in models of inflammatory/autoimmune diseases such as polymicrobial sepsis, periodontitis, peritonitis and colitis (Arita et al., 2005; Camuesco et al., 2005; Hasturk et al., 2007; Hudert et al., 2006; Tsou et al., 2008). The effect of DHA on host susceptibility to infectious diseases is controversial. Dietary n-3 fatty acids did not have a negative impact on resistance to Pseudomonas aeruginosa in mice (Tiesset et al., 2009), but guinea pigs were reported to be more susceptible to Mycobacterium tuberculosis (McFarland et al., 2008). Dietary DHA also delayed reovirus clearance in a murine enteric reovirus infection model (Beli et al., 2008) and induced high morbidity and mortality in influenza infections (Schwerbrock et al., 2009).
Recently, DHA also emerged as a major player in neuroinflammation. DHA fully crosses the brain blood barrier through passive diffusion (Ouellet et al., 2009), and is by far the most abundant n-3 fatty acid in the phospholipids of brain and retina (Bazan, 2009). DHA and its derivative neuroprotectin D1 (NPD1) promote neuronal and retinal pigment epithelial cell survival by inducing antiapoptotic and neuroprotective gene-expression programs in models of ischemia-reperfusion, experimental brain damage, Alzheimer disease, and oxidative stress (Bazan, 2009; Jicha and Markesbery, 2010; Lukiw and Bazan, 2010; Palacios-Pelaez et al., 2010). Although encouraging data for n-3 fatty acid supplementation in MS emerged from a number of studies (Gallai et al., 1995; Shinto et al., 2009; Weinstock-Guttman et al., 2005), there are no published data on the effects of DHA/EPA or their derivatives in EAE. The present study is the first to report a significant beneficial effect on clinical EAE scores and a significant decrease in the numbers of encephalitogenic Th1/Th17 cells in spleen and CNS of EAE mice that received dietary n-3 fatty acids. In a previous study we compared LPS-inoculated mice fed control- or DHA-enriched diets and concluded that splenic DC from mice on the DHA diet expressed less IL-12 and IL-23 (Kong et al., 2010). The present study shows fewer Th1 and Th17 cells in the spleen and CNS of EAE mice fed a DHA-enriched diet. Together these observations support the idea that dietary DHA inhibits T cell proliferation and Th1/Th17 differentiation, at least partially, through effects on myeloid DC. Whether CD4+ T cells from EAE mice fed the DHA diet are less encephalitogenic will have to be further tested in the EAE transfer model.
The ability of n-3 fatty acids and of their metabolic derivatives to reduce and resolve inflammation focused attention on immune cells. In addition to well documented inhibitory effects on transendothelial neutrophil migration and enhanced macrophage-mediated clearance of apoptotic PMNs by resolvins and protectins (Serhan, 2007; Serhan et al., 2008a; Serhan et al., 2008b), several recent studies reported n-3 fatty acids effects on dendritic cells and T lymphocytes.
In vitro experiments using human monocyte-derived DC showed that DHA, and to a lesser degree EPA, prevents LPS-induced upregulation of MHCII and costimulatory molecules (CD80, CD86, CD40) and cytokine secretion (Wang et al., 2007; Zapata-Gonzalez et al., 2008; Zeyda et al., 2005). We reported similar findings in murine bone marrow-derived DC and concluded that DHA maintains DC in an immature stage characterized by low expression of costimulatory molecules, lack of cytokine/chemokine production, high endocytic activity, and maintenance of the CCR5hiCCR7lo chemokine receptor pattern which prevents DC migration in response to lymph node-derived chemokines (Kong et al., 2010). Cytokines released by activated DC are essential in T cell differentiation, with IL-12p70 promoting Th1, while IL-6 and TGFβ promote Th17 differentiation, and IL-23 maintains the Th17 functional phenotype. The combined effects of DHA on costimulatory molecules, IL-12, IL-6 and IL-23 production, and on DC migration are strong indicators that DHA might also affect T cell activation and differentiation.
Several studies using mitogenic stimulation of purified T cells showed a reduction in T cell proliferation and IL-2 production following direct treatment with DHA or dietary n-3 fatty acids (Fan et al., 2004; Jolly et al., 1997; Wallace et al., 2001). The present study extends these observations to a physiologically relevant situation, using antigen-specific stimulation of T cells through antigen presentation by DC, and shows that pre-exposure of DC to DHA leads to a significant decrease in their capacity to stimulate T cells and affects CD4+ T cell differentiation. The reduction in T cell proliferation is not due to apoptosis, but mostly related to cell cycle arrest, due to increased levels of p27(kip1) in T cells co-cultured with DHA-treated DC. P27 is a member of the Cip/Kip family of universal cyclin-dependent kinase inhibitors which arrest cells in the G1 phase (Lloyd et al., 1999). TGFβ is the major activator of p27 through increased transcription, decreased proteosomal degradation, and increased nuclear translocation (Kamesaki et al., 1998; Lecanda et al., 2009). Based on increased expression of both TGFβ and p27 in T cells co-cultured with DHA-treated DC we propose that T cell proliferation is inhibited through the TGFβ ➔ p27(kip1) axis.
In terms of CD4+ T cell differentiation, we observed that DC-DHA have profound effects. Expression of the master transcription factors Tbet and RORγt was reduced, and as a result, differentiation into Th1 and Th17 cells was inhibited. A similar decrease in Tbet and Th1 differentiation was reported with dietary DHA for T cells restimulated ex vivo in the absence of DC (Attakpa et al., 2009). However, we could not substantiate the increase in Th2 cells reported by Attapka and colleagues, since in our experimental system GATA-3 expression was also downregulated and IL-4 levels were undetectable. In contrast to Tbet, GATA-3 and RORγt, DC-DHA increased Foxp3 expression (mRNA and protein) in CD4+ T cells both in the absence and presence of exogenous TGFβ. However, surprisingly, T cells did not become anergic and did not exhibit suppressive activity, suggesting that DC-DHA were not tolerogenic. Although the existence of human Foxp3+ T cells that are not anergic or suppressive has been reported (Morgan et al., 2005; Tran et al., 2007), traditionally, all mouse Foxp3+ T cells have been considered to be functional Treg. However, a recent study reported on the disconnect between induction of Foxp3 and suppressive activity in murine T cells treated with DHA (Yessoufou et al., 2009). Similar to Yessoufou and colleagues, we saw increases in Foxp3, CTLA-4, GITR (results not shown) and TGFβ in CD4+ T cells. However, in contrast to Yessoufou et al, in our experimental system this occurred with T cells co-cultured with DHA-treated DC, which excludes a direct effect of DHA on T cells. One possible explanation for expression of Foxp3 in the absence of T cell anergy or suppressive activity is the need for additional factor(s) for inducing regulatory functions. Recently, both NFAT and RUNX1 have been shown to be critical for Foxp3 induction of CTLA-4 and CD25, repression of IL-2, and for maintaining the Treg/Th17 balance (Hermann-Kleiter and Baier, 2010; Zhang et al., 2008). The effect of DHA-treated DC on the expression of NFAT and RUNX1 in CD4+ T cells remains to be established.
In conclusion, our studies established that pre-exposure of DC to DHA (DC-DHA) prior to stimulation through TLRs maintains the immature phenotype in terms of low expression of co-stimulatory molecules and lack of proinflammatory cytokine expression (IL-12p70, IL-23, and IL-6). As a result, DC-DHA exhibit low stimulatory activity for CD4+ T cells, leading to reduced proliferation of naïve antigen-specific T cells (TDC-DHA) and increased expression of the cell cycle arresting agent p27(kip1). In contrast to T cells co-cultured with regular DC, TDC-DHA differentiation into Th1, Th2 and Th17 is drastically inhibited, in agreement with reductions in Tbet, GATA-3 and RORγt. On the other hand, TDC-DHA express higher levels of TGFβ and Foxp3. We propose that TGFβ is the major factor responsible for the inhibition of T cell proliferation through the induction of p27(kip1). The significance of increased expression of Foxp3 in TDC-DHA cells remains unclear at this point, since TDC-DHA are neither anergic nor suppressive for other T cells. A possible explanation is that DC-DHA lack the capacity to induce additional factors such as NFAT and/or RUNX1 shown previously to interact with Foxp3 in controlling Treg/Th17 differentiation and maintenance of specific functional phenotypes.
ACKNOWLEDGMENTS
This study was supported by the following grants: NIH/NIAID 2RO1AI052306 and Pennsylvania CURE Tobacco Settlement Formula (DG)
Abbreviations
- AA
arachidonic acid
- cDC
conventional dendritic cells
- CNS
central nervous system
- DC
dendritic cells
- DHA
docosahexaenoic acid
- EAE
experimental autoimmune encephalomyelitis
- EPA
eicosapentaenoic acid
- LPS
lipopolysaccharide
- MFI
mean fluorescence intensity
- MOG
myelin oligodendrocyte glycoprotein
- MS
multiple sclerosis
- PCCF
Pigeon cytochrome c fragment
- PTX
pertussis toxin
- PMA
phorbol myristate acetate
- PI
propidium iodide
- PLP
proteolipid protein
- PUFA
polyunsaturated fatty acids.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: All authors declare that there are no conflicts of interest.
REFERENCES
- Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, Blumberg RS, Serhan CN. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci U S A. 2005;102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attakpa E, Hichami A, Simonin AM, Sanson EG, Dramane KL, Khan NA. Docosahexaenoic acid modulates the expression of T-bet and GATA-3 transcription factors, independently of PPARalpha, through suppression of MAP kinase activation. Biochimie. 2009;91:1359–1365. doi: 10.1016/j.biochi.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Bailes JE, Mills JD. Docosahexaenoic acid (DHA) Reduces Traumatic Axonal Injury in a Rodent Head Injury Model. J Neurotrauma. 2010 doi: 10.1089/neu.2009.1239. [DOI] [PubMed] [Google Scholar]
- Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4+ T(H)−17 cells in relapsing EAE. Nat Immunol. 2007;8:172–180. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- Bannenberg GL. Resolvins: Current understanding and future potential in the control of inflammation. Curr Opin Drug Discov Devel. 2009;12:644–658. [PubMed] [Google Scholar]
- Bazan NG. Cellular and molecular events mediated by docosahexaenoic acid-derived neuroprotectin D1 signaling in photoreceptor cell survival and brain protection. Prostaglandins Leukot Essent Fatty Acids. 2009;81:205–211. doi: 10.1016/j.plefa.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beli E, Li M, Cuff C, Pestka JJ. Docosahexaenoic acid-enriched fish oil consumption modulates immunoglobulin responses to and clearance of enteric reovirus infection in mice. J Nutr. 2008;138:813–819. doi: 10.1093/jn/138.4.813. [DOI] [PubMed] [Google Scholar]
- Bonilla DL, Fan YY, Chapkin RS, McMurray DN. Transgenic mice enriched in omega-3 fatty acids are more susceptible to pulmonary tuberculosis: impaired resistance to tuberculosis in fat-1 mice. J Infect Dis. 201:399–408. doi: 10.1086/650344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla DL, Fan YY, Chapkin RS, McMurray DN. Transgenic mice enriched in omega-3 fatty acids are more susceptible to pulmonary tuberculosis: impaired resistance to tuberculosis in fat-1 mice. J Infect Dis. 2010;201:399–408. doi: 10.1086/650344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet M, Saint-Pierre M, Julien C, Salem N, Jr, Cicchetti F, Calon F. Beneficial effects of dietary omega-3 polyunsaturated fatty acid on toxin-induced neuronal degeneration in an animal model of Parkinson’s disease. Faseb J. 2008;22:1213–1225. doi: 10.1096/fj.07-9677com. [DOI] [PubMed] [Google Scholar]
- Bouwens M, van de Rest O, Dellschaft N, Bromhaar MG, de Groot LC, Geleijnse JM, Muller M, Afman LA. Fish-oil supplementation induces antiinflammatory gene expression profiles in human blood mononuclear cells. Am J Clin Nutr. 2009;90:415–424. doi: 10.3945/ajcn.2009.27680. [DOI] [PubMed] [Google Scholar]
- Calder PC. The relationship between the fatty acid composition of immune cells and their function. Prostaglandins Leukot Essent Fatty Acids. 2008;79:101–108. doi: 10.1016/j.plefa.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Calon F, Lim GP, Yang F, Morihara T, Teter B, Ubeda O, Rostaing P, Triller A, Salem N, Jr, Ashe KH, Frautschy SA, Cole GM. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer’s disease mouse model. Neuron. 2004;43:633–645. doi: 10.1016/j.neuron.2004.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camuesco D, Galvez J, Nieto A, Comalada M, Rodriguez-Cabezas ME, Concha A, Xaus J, Zarzuelo A. Dietary olive oil supplemented with fish oil, rich in EPA and DHA (n-3) polyunsaturated fatty acids, attenuates colonic inflammation in rats with DSS-induced colitis. J Nutr. 2005;135:687–694. doi: 10.1093/jn/135.4.687. [DOI] [PubMed] [Google Scholar]
- Chapkin RS, Kim W, Lupton JR, McMurray DN. Dietary docosahexaenoic and eicosapentaenoic acid: emerging mediators of inflammation. Prostaglandins Leukot Essent Fatty Acids. 2009;81:187–191. doi: 10.1016/j.plefa.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das UN. Essential fatty acids: biochemistry, physiology and pathology. Biotechnol J. 2006;1:420–439. doi: 10.1002/biot.200600012. [DOI] [PubMed] [Google Scholar]
- Fan YY, Ly LH, Barhoumi R, McMurray DN, Chapkin RS. Dietary docosahexaenoic acid suppresses T cell protein kinase C theta lipid raft recruitment and IL-2 production. J Immunol. 2004;173:6151–6160. doi: 10.4049/jimmunol.173.10.6151. [DOI] [PubMed] [Google Scholar]
- Frohman EM, Racke MK, Raine CS. Multiple sclerosis--the plaque and its pathogenesis. N Engl J Med. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- Gallai V, Sarchielli P, Trequattrini A, Franceschini M, Floridi A, Firenze C, Alberti A, Di Benedetto D, Stragliotto E. Cytokine secretion and eicosanoid production in the peripheral blood mononuclear cells of MS patients undergoing dietary supplementation with n-3 polyunsaturated fatty acids. J Neuroimmunol. 1995;56:143–153. doi: 10.1016/0165-5728(94)00140-j. [DOI] [PubMed] [Google Scholar]
- Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, Van Dyke TE. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 2007;179:7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- Hermann-Kleiter N, Baier G. NFAT pulls the strings during CD4+ T helper cell effector functions. Blood. 2010;115:2989–2997. doi: 10.1182/blood-2009-10-233585. [DOI] [PubMed] [Google Scholar]
- Hudert CA, Weylandt KH, Lu Y, Wang J, Hong S, Dignass A, Serhan CN, Kang JX. Transgenic mice rich in endogenous omega-3 fatty acids are protected from colitis. Proc Natl Acad Sci U S A. 2006;103:11276–11281. doi: 10.1073/pnas.0601280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insua MF, Garelli A, Rotstein NP, German OL, Arias A, Politi LE. Cell cycle regulation in retinal progenitors by glia-derived neurotrophic factor and docosahexaenoic acid. Invest Ophthalmol Vis Sci. 2003;44:2235–2244. doi: 10.1167/iovs.02-0952. [DOI] [PubMed] [Google Scholar]
- Jicha GA, Markesbery WR. Omega-3 fatty acids: potential role in the management of early Alzheimer’s disease. Clin Interv Aging. 2010;5:45–61. doi: 10.2147/cia.s5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly CA, Jiang YH, Chapkin RS, McMurray DN. Dietary (n-3) polyunsaturated fatty acids suppress murine lymphoproliferation, interleukin-2 secretion, and the formation of diacylglycerol and ceramide. J Nutr. 1997;127:37–43. doi: 10.1093/jn/127.1.37. [DOI] [PubMed] [Google Scholar]
- Kamesaki H, Nishizawa K, Michaud GY, Cossman J, Kiyono T. TGF-beta 1 induces the cyclin-dependent kinase inhibitor p27Kip1 mRNA and protein in murine B cells. J Immunol. 1998;160:770–777. [PubMed] [Google Scholar]
- Katakura M, Hashimoto M, Shahdat HM, Gamoh S, Okui T, Matsuzaki K, Shido O. Docosahexaenoic acid promotes neuronal differentiation by regulating basic helix-loop-helix transcription factors and cell cycle in neural stem cells. Neuroscience. 2009;160:651–660. doi: 10.1016/j.neuroscience.2009.02.057. [DOI] [PubMed] [Google Scholar]
- Kohli P, Levy BD. Resolvins and protectins: mediating solutions to inflammation. Br J Pharmacol. 2009;158:960–971. doi: 10.1111/j.1476-5381.2009.00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong W, Yen JH, Vassiliou E, Adhikary S, Toscano MG, Ganea D. Docosahexaenoic acid prevents dendritic cell maturation and in vitro and in vivo expression of the IL-12 cytokine family. Lipids Health Dis. 9:12. doi: 10.1186/1476-511X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong W, Yen JH, Vassiliou E, Adhikary S, Toscano MG, Ganea D. Docosahexaenoic acid prevents dendritic cell maturation and in vitro and in vivo expression of the IL-12 cytokine family. Lipids Health Dis. 2010;9:12. doi: 10.1186/1476-511X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecanda J, Ganapathy V, D’Aquino-Ardalan C, Evans B, Cadacio C, Ayala A, Gold I. TGFbeta prevents proteasomal degradation of the cyclin-dependent kinase inhibitor p27kip1 for cell cycle arrest. Cell Cycle. 2009;8:742–756. doi: 10.4161/cc.8.5.7871. [DOI] [PubMed] [Google Scholar]
- Lloyd RV, Erickson LA, Jin L, Kulig E, Qian X, Cheville JC, Scheithauer BW. p27kip1: a multifunctional cyclin-dependent kinase inhibitor with prognostic significance in human cancers. Am J Pathol. 1999;154:313–323. doi: 10.1016/S0002-9440(10)65277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Vales R, Redensek A, Skinner TA, Rathore KI, Ghasemlou N, Wojewodka G, DeSanctis J, Radzioch D, David S. Fenretinide promotes functional recovery and tissue protection after spinal cord contusion injury in mice. J Neurosci. 2010;30:3220–3226. doi: 10.1523/JNEUROSCI.5770-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukiw WJ, Bazan NG. Inflammatory, apoptotic, and survival gene signaling in Alzheimer’s disease. A review on the bioactivity of neuroprotectin D1 and apoptosis. Mol Neurobiol. 2010;42:10–16. doi: 10.1007/s12035-010-8126-4. [DOI] [PubMed] [Google Scholar]
- Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, Musto A, Hardy M, Gimenez JM, Chiang N, Serhan CN, Bazan NG. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- McFarland CT, Fan YY, Chapkin RS, Weeks BR, McMurray DN. Dietary polyunsaturated fatty acids modulate resistance to Mycobacterium tuberculosis in guinea pigs. J Nutr. 2008;138:2123–2128. doi: 10.3945/jn.108.093740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan ME, van Bilsen JH, Bakker AM, Heemskerk B, Schilham MW, Hartgers FC, Elferink BG, van der Zanden L, de Vries RR, Huizinga TW, Ottenhoff TH, Toes RE. Expression of FOXP3 mRNA is not confined to CD4+CD25+ T regulatory cells in humans. Hum Immunol. 2005;66:13–20. doi: 10.1016/j.humimm.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Ouellet M, Emond V, Chen CT, Julien C, Bourasset F, Oddo S, LaFerla F, Bazinet RP, Calon F. Diffusion of docosahexaenoic and eicosapentaenoic acids through the blood-brain barrier: An in situ cerebral perfusion study. Neurochem Int. 2009;55:476–482. doi: 10.1016/j.neuint.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Palacios-Pelaez R, Lukiw WJ, Bazan NG. Omega-3 essential Fatty acids modulate initiation and progression of neurodegenerative disease. Mol Neurobiol. 2010;41:367–374. doi: 10.1007/s12035-010-8139-z. [DOI] [PubMed] [Google Scholar]
- Schwerbrock NM, Karlsson EA, Shi Q, Sheridan PA, Beck MA. Fish oil-fed mice have impaired resistance to influenza infection. J Nutr. 2009;139:1588–1594. doi: 10.3945/jn.109.108027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN. Resolution phase of inflammation: novel endogenous antiinflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual antiinflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008a;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Yacoubian S, Yang R. Anti-inflammatory and proresolving lipid mediators. Annu Rev Pathol. 2008b;3:279–312. doi: 10.1146/annurev.pathmechdis.3.121806.151409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinto L, Marracci G, Baldauf-Wagner S, Strehlow A, Yadav V, Stuber L, Bourdette D. Omega-3 fatty acid supplementation decreases matrix metalloproteinase-9 production in relapsing-remitting multiple sclerosis. Prostaglandins Leukot Essent Fatty Acids. 2009;80:131–136. doi: 10.1016/j.plefa.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavin A, Kelly-Modis L, Labadia M, Ryan K, Brown ML. Pathogenic mechanisms and experimental models of multiple sclerosis. Autoimmunity. 2010 doi: 10.3109/08916931003674733. [DOI] [PubMed] [Google Scholar]
- Tiesset H, Pierre M, Desseyn JL, Guery B, Beermann C, Galabert C, Gottrand F, Husson MO. Dietary (n-3) polyunsaturated fatty acids affect the kinetics of pro-and antiinflammatory responses in mice with Pseudomonas aeruginosa lung infection. J Nutr. 2009;139:82–89. doi: 10.3945/jn.108.096115. [DOI] [PubMed] [Google Scholar]
- Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou SS, Chiu WC, Yeh CL, Hou YC, Yeh SL. Effects of omega-3 fatty acids on inflammatory mediators and splenocyte cytokine mRNA expressions in rats with polymicrobial sepsis. Nutrition. 2008;24:484–491. doi: 10.1016/j.nut.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Wallace FA, Miles EA, Evans C, Stock TE, Yaqoob P, Calder PC. Dietary fatty acids influence the production of Th1- but not Th2-type cytokines. J Leukoc Biol. 2001;69:449–457. [PubMed] [Google Scholar]
- Wang H, Hao Q, Li QR, Yan XW, Ye S, Li YS, Li N, Li JS. Omega-3 polyunsaturated fatty acids affect lipopolysaccharide-induced maturation of dendritic cells through mitogen-activated protein kinases p38. Nutrition. 2007;23:474–482. doi: 10.1016/j.nut.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Weinstock-Guttman B, Baier M, Park Y, Feichter J, Lee-Kwen P, Gallagher E, Venkatraman J, Meksawan K, Deinehert S, Pendergast D, Awad AB, Ramanathan M, Munschauer F, Rudick R. Low fat dietary intervention with omega-3 fatty acid supplementation in multiple sclerosis patients. Prostaglandins Leukot Essent Fatty Acids. 2005;73:397–404. doi: 10.1016/j.plefa.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Weylandt KH, Nadolny A, Kahlke L, Kohnke T, Schmocker C, Wang J, Lauwers GY, Glickman JN, Kang JX. Reduction of inflammation and chronic tissue damage by omega-3 fatty acids in fat-1 transgenic mice with pancreatitis. Biochim Biophys Acta. 2008;1782:634–641. doi: 10.1016/j.bbadis.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yessoufou A, Ple A, Moutairou K, Hichami A, Khan NA. Docosahexaenoic acid reduces suppressive and migratory functions of CD4+CD25+ regulatory T-cells. J Lipid Res. 2009;50:2377–2388. doi: 10.1194/jlr.M900101-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata-Gonzalez F, Rueda F, Petriz J, Domingo P, Villarroya F, Diaz-Delfin J, de Madariaga MA, Domingo JC. Human dendritic cell activities are modulated by the omega-3 fatty acid, docosahexaenoic acid, mainly through PPAR(gamma):RXR heterodimers: comparison with other polyunsaturated fatty acids. J Leukoc Biol. 2008;84:1172–1182. doi: 10.1189/jlb.1007688. [DOI] [PubMed] [Google Scholar]
- Zeyda M, Saemann MD, Stuhlmeier KM, Mascher DG, Nowotny PN, Zlabinger GJ, Waldhausl W, Stulnig TM. Polyunsaturated fatty acids block dendritic cell activation and function independently of NF-kappaB activation. J Biol Chem. 2005;280:14293–14301. doi: 10.1074/jbc.M410000200. [DOI] [PubMed] [Google Scholar]
- Zhang F, Meng G, Strober W. Interactions among the transcription factors Runx1, RORgammat and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nat Immunol. 2008;9:1297–1306. doi: 10.1038/ni.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]