Abstract
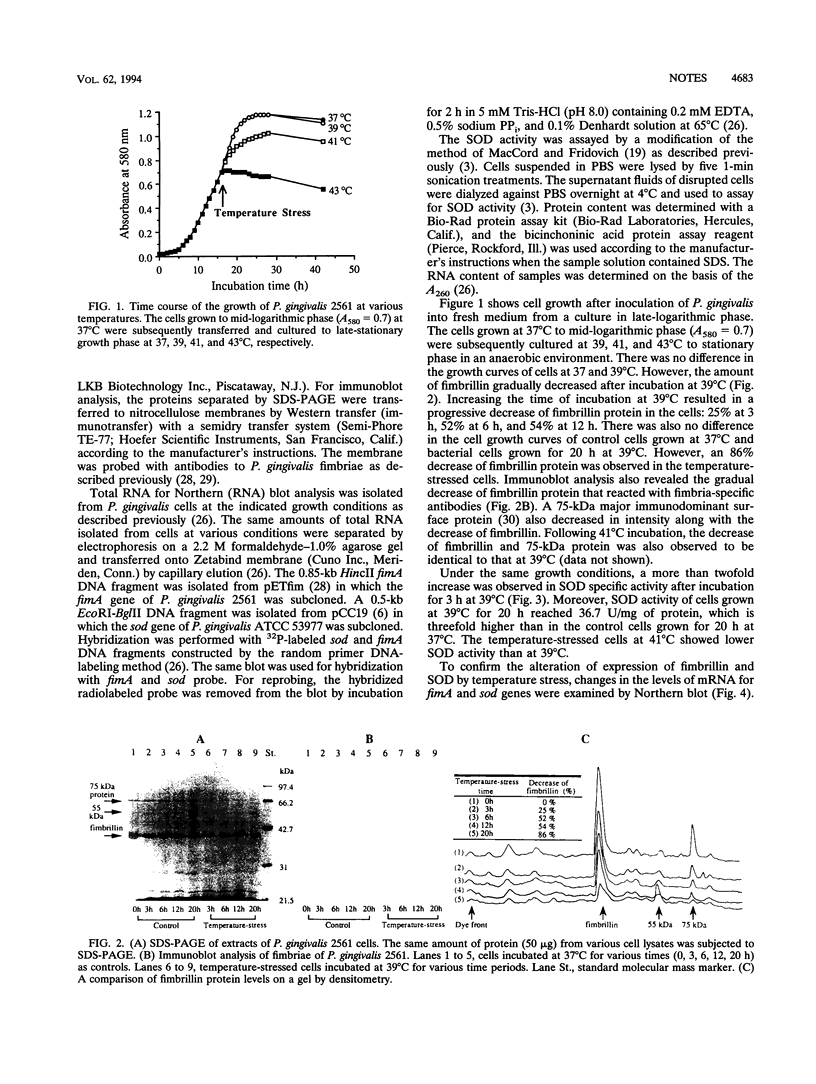
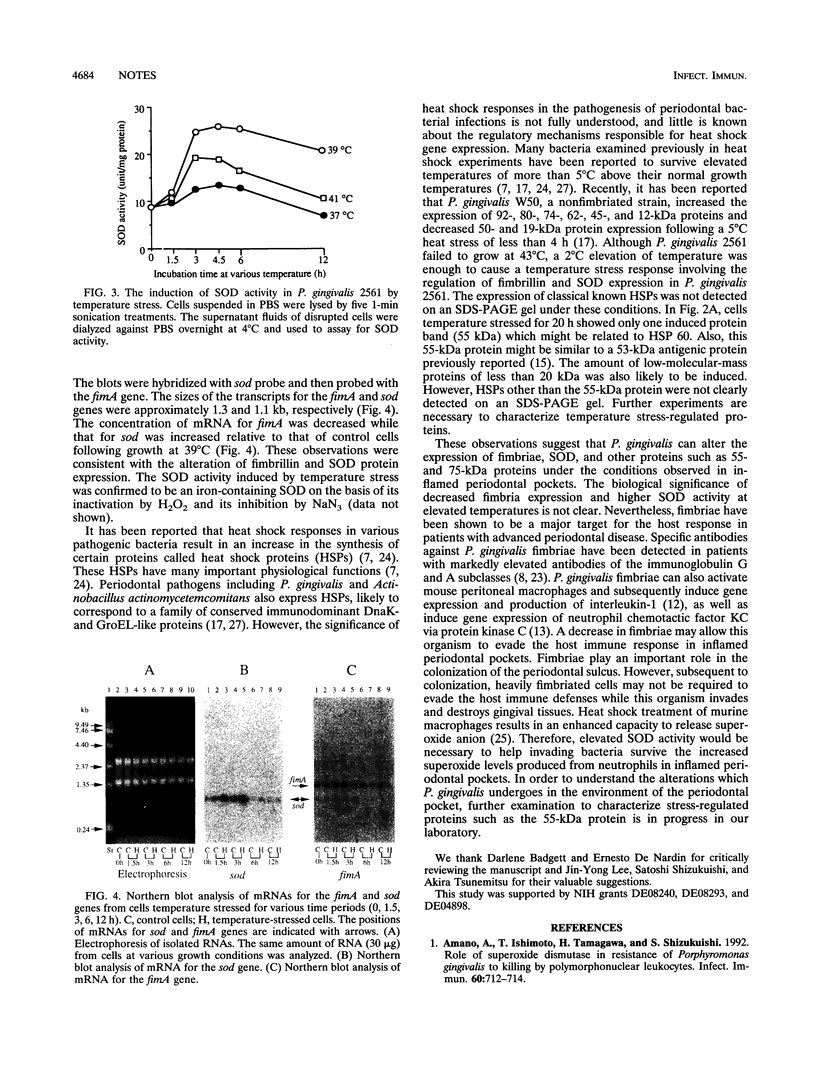
We examined the biosynthesis of fimbriae and superoxide dismutase (SOD) produced by the periodontopathic bacterium Porphyromonas gingivalis in response to elevated temperature. P. gingivalis 2561, grown at 37 degrees C to mid-logarithmic phase, was subsequently incubated at 39, 41, and 43 degrees C, respectively, to stationary phase. There was no difference in the growth of cells at 37 and 39 degrees C. However, at 39 degrees C there was a 54% reduction in the amount of fimbrillin (fimbriae) as well as decreased expression of mRNA for fimA. On the other hand, under the same conditions, a more than twofold increase in the amount of SOD activity, as well as in the levels of SOD mRNA, was observed. Moreover, cells cultured for 20 h at 39 degrees C showed an 86% decrease of fimbrillin protein and a threefold increase in SOD activity. These observations suggest that P. gingivalis may undergo alterations in its virulence and susceptibility to host immune responses as a result of the elevated temperatures found in inflamed periodontal pockets.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano A., Ishimoto T., Tamagawa H., Shizukuishi S. Role of superoxide dismutase in resistance of Porphyromonas gingivalis to killing by polymorphonuclear leukocytes. Infect Immun. 1992 Feb;60(2):712–714. doi: 10.1128/iai.60.2.712-714.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano A., Tamagawa H., Shizukuishi S., Tsunemitsu A. Superoxide dismutase, catalase and peroxidases in oral anaerobic bacteria. J Osaka Univ Dent Sch. 1986 Dec;26:187–192. [PubMed] [Google Scholar]
- Amano A., Tamagawa H., Takagaki M., Murakami Y., Shizukuishi S., Tsunemitsu A. Relationship between enzyme activities involved in oxygen metabolism and oxygen tolerance in black-pigmented Bacteroides. J Dent Res. 1988 Sep;67(9):1196–1199. doi: 10.1177/00220345880670090901. [DOI] [PubMed] [Google Scholar]
- Baab D. A., Oberg A., Lundström A. Gingival blood flow and temperature changes in young humans with a history of periodontitis. Arch Oral Biol. 1990;35(2):95–101. doi: 10.1016/0003-9969(90)90169-b. [DOI] [PubMed] [Google Scholar]
- Beaman L., Beaman B. L. The role of oxygen and its derivatives in microbial pathogenesis and host defense. Annu Rev Microbiol. 1984;38:27–48. doi: 10.1146/annurev.mi.38.100184.000331. [DOI] [PubMed] [Google Scholar]
- Choi J. I., Takahashi N., Kato T., Kuramitsu H. K. Isolation, expression, and nucleotide sequence of the sod gene from Porphyromonas gingivalis. Infect Immun. 1991 Apr;59(4):1564–1566. doi: 10.1128/iai.59.4.1564-1566.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A., Gambill B. D., Nelson R. J. Heat shock proteins: molecular chaperones of protein biogenesis. Microbiol Rev. 1993 Jun;57(2):402–414. doi: 10.1128/mr.57.2.402-414.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nardin A. M., Sojar H. T., Grossi S. G., Christersson L. A., Genco R. J. Humoral immunity of older adults with periodontal disease to Porphyromonas gingivalis. Infect Immun. 1991 Dec;59(12):4363–4370. doi: 10.1128/iai.59.12.4363-4370.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. T., Klausen B., Sojar H. T., Bedi G. S., Sfintescu C., Ramamurthy N. S., Golub L. M., Genco R. J. Immunization with Porphyromonas (Bacteroides) gingivalis fimbriae protects against periodontal destruction. Infect Immun. 1992 Jul;60(7):2926–2935. doi: 10.1128/iai.60.7.2926-2935.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedi P. F., Jr, Killoy W. J. Temperature differences at periodontal sites in health and disease. J Periodontol. 1992 Jan;63(1):24–27. doi: 10.1902/jop.1992.63.1.24. [DOI] [PubMed] [Google Scholar]
- Hamada S., Ogawa T., Shimauchi H., Kusumoto Y. Induction of mucosal and serum immune responses to a specific antigen of periodontal bacteria. Adv Exp Med Biol. 1992;327:71–81. doi: 10.1007/978-1-4615-3410-5_9. [DOI] [PubMed] [Google Scholar]
- Hanazawa S., Murakami Y., Hirose K., Amano S., Ohmori Y., Higuchi H., Kitano S. Bacteroides (Porphyromonas) gingivalis fimbriae activate mouse peritoneal macrophages and induce gene expression and production of interleukin-1. Infect Immun. 1991 Jun;59(6):1972–1977. doi: 10.1128/iai.59.6.1972-1977.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanazawa S., Murakami Y., Takeshita A., Kitami H., Ohta K., Amano S., Kitano S. Porphyromonas gingivalis fimbriae induce expression of the neutrophil chemotactic factor KC gene of mouse peritoneal macrophages: role of protein kinase C. Infect Immun. 1992 Apr;60(4):1544–1549. doi: 10.1128/iai.60.4.1544-1549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isogai H., Isogai E., Yoshimura F., Suzuki T., Kagota W., Takano K. Specific inhibition of adherence of an oral strain of Bacteroides gingivalis 381 to epithelial cells by monoclonal antibodies against the bacterial fimbriae. Arch Oral Biol. 1988;33(7):479–485. doi: 10.1016/0003-9969(88)90028-3. [DOI] [PubMed] [Google Scholar]
- Kurihara H., Nishimura F., Nakamura T., Nakagawa M., Tanimoto I., Nomura Y., Kokeguchi S., Kato K., Murayama Y. Humoral immune response to an antigen from Porphyromonas gingivalis 381 in periodontal disease. Infect Immun. 1991 Aug;59(8):2758–2762. doi: 10.1128/iai.59.8.2758-2762.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. Y., Sojar H. T., Bedi G. S., Genco R. J. Synthetic peptides analogous to the fimbrillin sequence inhibit adherence of Porphyromonas gingivalis. Infect Immun. 1992 Apr;60(4):1662–1670. doi: 10.1128/iai.60.4.1662-1670.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B., McBride B. C. Stress response of Porphyromonas gingivalis. Oral Microbiol Immunol. 1994 Jun;9(3):166–173. doi: 10.1111/j.1399-302x.1994.tb00054.x. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Malek R., Fisher J. G., Caleca A., Stinson M., van Oss C. J., Lee J. Y., Cho M. I., Genco R. J., Evans R. T., Dyer D. W. Inactivation of the Porphyromonas gingivalis fimA gene blocks periodontal damage in gnotobiotic rats. J Bacteriol. 1994 Feb;176(4):1052–1059. doi: 10.1128/jb.176.4.1052-1059.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrand D., Holt S. C. Biology of asaccharolytic black-pigmented Bacteroides species. Microbiol Rev. 1988 Mar;52(1):134–152. doi: 10.1128/mr.52.1.134-152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Nakayama K. Rapid viability loss on exposure to air in a superoxide dismutase-deficient mutant of Porphyromonas gingivalis. J Bacteriol. 1994 Apr;176(7):1939–1943. doi: 10.1128/jb.176.7.1939-1943.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Shimauchi H., Kusumoto Y., Hamada S. Humoral immune response to Bacteroides gingivalis fimbrial antigen in mice. Immunology. 1990 Jan;69(1):8–13. [PMC free article] [PubMed] [Google Scholar]
- Polla B. S., Kantengwa S. Heat shock proteins and inflammation. Curr Top Microbiol Immunol. 1991;167:93–105. doi: 10.1007/978-3-642-75875-1_6. [DOI] [PubMed] [Google Scholar]
- Reddy M. V., Gangadharam P. R. Heat shock treatment of macrophages causes increased release of superoxide anion. Infect Immun. 1992 Jun;60(6):2386–2390. doi: 10.1128/iai.60.6.2386-2390.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Sojar H. T., Lee J. Y., Genco R. J. Expression of a functional Porphyromonas gingivalis fimbrillin polypeptide in Escherichia coli: purification, physicochemical and immunochemical characterization, and binding characteristics. Infect Immun. 1993 Aug;61(8):3570–3573. doi: 10.1128/iai.61.8.3570-3573.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sojar H. T., Lee J. Y., Bedi G. S., Cho M. I., Genco R. J. Purification, characterization and immunolocalization of fimbrial protein from Porphyromonas (bacteroides) gingivalis. Biochem Biophys Res Commun. 1991 Mar 15;175(2):713–719. doi: 10.1016/0006-291x(91)91624-l. [DOI] [PubMed] [Google Scholar]
- Yoshimura F., Watanabe K., Takasawa T., Kawanami M., Kato H. Purification and properties of a 75-kilodalton major protein, an immunodominant surface antigen, from the oral anaerobe Bacteroides gingivalis. Infect Immun. 1989 Nov;57(11):3646–3652. doi: 10.1128/iai.57.11.3646-3652.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]





