Abstract
Anticipatory emotional responses play a crucial role in preparing individuals for impending challenges. They do this by triggering a coordinated set of changes in behavioral, autonomic, and neural response systems. In the present study, we examined the biobehavioral impact of varying levels of anticipatory anxiety, using a shock anticipation task in which unpredictable electric shocks were threatened and delivered to the wrist at variable intervals and intensities (safe, medium, strong). This permitted investigation of a dynamic range of anticipatory anxiety responses. In two studies, 95 and 51 healthy female participants, respectively, underwent this shock anticipation task while providing continuous ratings of anxiety experience and electrodermal responding (Study 1) and during fMRI BOLD neuroimaging (Study 2). Results indicated a step-wise pattern of responding in anxiety experience and electrodermal responses. Several brain regions showed robust responses to shock anticipation relative to safe trials, including the hypothalamus, periaqueductal gray, caudate, precentral gyrus, thalamus, insula, ventrolateral PFC, dorsomedial PFC, and ACC. A subset of these regions demonstrated a linear pattern of increased responding from safe to medium to strong trials, including the bilateral insula, ACC, and inferior frontal gyrus. These responses were modulated by individual differences in neuroticism, such that those high in neuroticism showed exaggerated anxiety experience across the entire task, and reduced brain activation from medium to strong trials in a subset of brain regions. These findings suggest that individual differences in neuroticism may influence sensitivity to anticipatory threat and provide new insights into the mechanism through which neuroticism may confer risk for developing anxiety disorders via dysregulated anticipatory responses.
Keywords: anxiety, anticipation, neuroticism, unpredictability, insula, anterior cingulate, fMRI
INTRODUCTION
One of the principal goals of affective neuroscience is to delineate neurobiological responses to emotional challenges. Typically, this goal has been pursued in laboratory contexts by inducing emotion and then examining changes in subjective experience (Dimberg, 1987; Hubert and de Jong-Meyer, 1991; Magai et al., 2006), peripheral physiology (Sequeira et al., 2009; Vrana et al., 1988) and neural responses (Carretie et al., 2009; Hagemann et al., 2003). For instance, conditioning studies have explored manifestations of fear responses to aversive stimuli, and dozens of studies have demonstrated increased skin conductance and brain activation in response to electric shock, loud noise and other aversive stimuli (e.g. (Cheng et al., 2003; Cheng et al., 2007; Dunsmoor et al., 2007; Fischer et al., 2002; Knight et al., 2004b; LaBar et al., 1998; Phelps et al., 2004); for review see (Sehlmeyer et al., 2009)).
Although this work is of fundamental importance, one limitation has been the frequent confounding of emotional responses inherent in the psychological representation of the event with the cascade of responses that take place once the body is actually enduring a challenge. For example, the anticipatory anxiety that happens while one awaits a visit to the dentist is importantly distinguishable from the responses that occur once the drilling has begun. It is crucial to parse these two components and carefully examine the anticipatory period before the aversive assault because 1) much of our emotional life is spent in the anticipation of future events, and 2) this window allows a purer examination of the impact of the emotional representation of a stressor in the absence of the responses to the stressor itself. Unfortunately, less is known about the anticipatory component of emotional responding than is known about the responses that occur once the demanding situation is actually unfolding. Still less is known about the way that individual difference variables moderate these anticipatory responses.
While the vast majority of fear conditioning studies have employed a strategy in which a cue is presented and then nearly immediately (e.g. less than 500 ms) followed by an aversive stimulus, a handful of neuroimaging studies in humans have employed a “trace” conditioning strategy in which the cue is followed by a waiting period (e.g. from 500 ms to 8 s) before the aversive stimulus is deployed (Buchel et al., 1999; Carter et al., 2006; Cheng et al., 2008; Cheng et al., 2006; Knight et al., 2004a). Unfortunately, most of these paradigms have not analyzed the waiting period separately from the receipt of the shock, making it difficult to interpret which element (anticipation or receipt of shock) is driving the findings. The literature on pain anticipation has addressed anticipatory emotional responses more directly, often using longer anticipatory delay periods, and a variety of aversive stimuli to induce anticipatory responses. Early studies of anticipatory anxiety have typically examined anticipatory effects on autonomic responding. For example, anticipation of electric shock (Chua et al., 1999; Kopacz and Smith, 1971) and venipuncture (Geddes et al., 1993) have been shown to lead to increased skin conductance responses (SCRs), and potentiate protective reflexes such as the eyeblink startle reflex (Grillon et al., 1993; Grillon et al., 1991; Vrana et al., 1988).
A seminal study by Ploghaus and colleagues was the first neuroimaging study to disentangle the anticipation and receipt of pain, and they found a separable network of regions invoked purely by emotional distress in the absence of external stimulation (Ploghaus et al., 1999). Subsequent studies have begun to elucidate the neural correlates of anticipatory anxiety using standard threat of shock tasks in which shocks were delivered (Chua et al., 1999; Schunck et al., 2008; Straube et al., 2009), or not (Kumari et al., 2007), as well as threat of heat stimuli (Wager et al., 2004), or upsetting images (Nitschke et al., 2006; Waugh et al., 2008). These studies typically show increased activation in bilateral insula, anterior cingulate cortex (ACC), dorsomedial prefrontal cortex (dmPFC) and ventrolateral prefrontal cortex (vlPFC) (Mee et al., 2006). These brain regions have been associated with representing visceral body states and conscious feelings related to interoceptive processes (insula), integrating contextual cues and sensory information (ACC), self-referential processing (dmPFC), and representing the affective stimulus value and expectation of negative outcomes (vlPFC). Only one relatively small study (N = 16) has examined the relationship between varying levels of shock intensity and anticipatory responses in the brain (Straube et al., 2009), with findings that suggest that anticipatory processes are not “all or nothing”, but are instead modulated by the intensity of the anticipated threat.
There is considerable individual-related variation in anticipatory anxiety. One particularly important individual-difference dimension for understanding variation in negative emotional responses is neuroticism. Compared to individuals low in neuroticism, individuals high in neuroticism are more prone to anxiety and negative affect, respond to environmental stressors more negatively (Costa and McCrae, 1980), and are particularly concerned with averting possible threats (Zelenski and Larsen, 1999). Moreover, neuroticism has been associated with an avoidant coping style (Bolger, 1990; McCrae and Costa, 1986; Parkes, 1986) and increased levels of suppression (Gross and John, 2003), Neuroimaging studies have focused on the impact of neuroticism on responses during exposure to aversive images and faces, and have found exaggerated neural responses (Canli, 2004; Canli et al., 2001; Carroll et al., 2007; Haas et al., 2007) in the left middle temporal gyrus, middle frontal gyrus, and insula. The autonomic literature, however, has been mixed, with some studies showing increased electrodermal responding to aversive images (Norris et al., 2007) and others showing decreased responding (De Pascalis et al., 2007). It is less clear whether neuroticism affects anticipatory responding before the stimulus actually occurs. Only one study to date has used fMRI to examine neuroticism in response to threat of noxious stimuli (Kumari et al., 2007), finding that neuroticism was negatively correlated with a number of prefrontal and parietal regions during threat greater than safe conditions in a brief task in which shocks were not delivered. Given that neuroticism has been identified as a risk factor for anxiety disorders, many of which are characterized by chronic worry and anxiety about the future (Barlow, 2002; Bienvenu and Stein, 2003), neuroticism may modulate anticipatory anxiety even in healthy individuals.
The goal of the present study was to investigate how anticipation of varying levels of shock intensity impacts anxiety experience, autonomic responding, and neural activity. To address this goal, we employed a task in which a long anticipatory cue period (7-11 s) allowed for robust responses to be generated. To create maximal levels of anxiety with minimal levels of habituation, three layers of unpredictability were embedded into the shock trials: event (whether the shock will occur or not), temporal (when will it occur), and intensity (how strong will it be) unpredictability. These levels of unpredictability have been shown to potentiate emotional reactivity (D'Amato and Gumenik, 1960; Monat et al., 1972), autonomic (Geer and Maisel, 1972) and neural responding (Carlsson et al., 2006). Multiple levels of shock intensity permitted investigation of a dynamic range of responses. In order to disentangle anticipatory anxiety from shock response, the anticipatory period was analyzed separately from the receipt of shock. A relatively large number of trials were employed to enhance power to detect effects, and a large sample of healthy women was employed to permit investigation of individual differences in neuroticism without confounding effects of gender or previous history of psychopathology. Multiple output channels were investigated to better elucidate the richness of anticipatory emotional responses.
Based on the extant literature on anticipatory anxiety, we hypothesized that as shock intensity increased, anticipatory anxiety would result in stepwise increases in 1) anxiety experience, 2) electrodermal responding, and 3) neural activity in brain regions including the insula, anterior cingulate, thalamus, and prefrontal cortex (but not in the amygdala). Based on studies showing exaggerated reactivity during negative stimulus presentation due to neuroticism, we further hypothesized that individuals high in neuroticism would show increases in anticipatory responding before stimulus presentation. Specifically, we expected greater increases in anxiety, electrodermal responding and brain activity in the middle temporal gyrus, frontal gyrus and insula relative to those low in neuroticism.
Study 1: Experiential and Autonomic Responses to Shock Anticipation
In Study 1, we devised a shock anticipation task that involved anticipating electric shocks to the wrist in three different intensity levels: safe (no shock), medium shock, and strong shock. To increase anxiety and prevent habituation, three layers of uncertainty were embedded into the shock trials: event, temporal, and intensity uncertainty. The task was administered in a sample large enough to examine the potential effect of neuroticism. We sought to examine the effects of shock anticipation on anxiety experience and electrodermal responses, and to test whether neuroticism moderated these responses.
Methods
Participants
All potential participants were screened using an interview based on the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (SCID for DSM-IV) (First et al., 1995). Eligible participants were healthy females that did not meet criteria for any psychiatric disorder within the past year, or for lifetime posttraumatic stress, bipolar, obsessive–compulsive, or psychotic disorders, and were not currently taking psychotropic medications. Ninety-five women (mean age 21.8 ± 2.2) participated in the anticipatory anxiety task. The study was approved by the Stanford University Institutional Review Board, and all subjects gave informed consent and were paid for participation.
Procedure
Participants underwent a shock calibration procedure and training phase before beginning the task. In preparation for the task, participants received a series of brief (50 ms) electric shocks delivered to the left wrist above the median nerve with a Grass SD-9 stimulator (West Warick, RI) and were instructed to set a maximum voltage (MV) that was “painful and difficult to tolerate but not unbearable.” A brief training phase followed which mirrored the task, however without actual shock presentation.
The shock anticipation task consisted of three conditions presented in a pseudorandom order with no more than two sequential trials of the same condition: safe (8 trials), medium shock (13 trials), strong shock (13 trials). Conditions were indicated by the words “safe” (on a green background), “medium” (on an orange background), and “strong” (on a red background). During safe trials, participants were instructed that no shocks would be administered. Instructions for the medium and strong trials featured three levels of uncertainty which were explained during the training phase. Event uncertainty was implemented by indicating that shocks would only be delivered during 85% of the trials. Temporal uncertainty by was implemented by indicating that trials would last between 0-20 seconds and shocks could occur at any time; in actuality, trials ranged between 7-11 s (avg 9 s) and terminated with shock in 85% of the trials; both medium and strong conditions contained a single “quick” trial terminated with a shock at 3 s in order to maintain the claim that shocks could occur at any time. Intensity uncertainty was implemented by leaving the exact strength of the shock unspecified within a 20% window; for medium trials the range was indicated as 40-60% of MV and for strong trials the range was indicated as 70-90% of MV (in actuality, medium trials were always at 55% of MV and strong trials were always at 85% of MV).
Measures
Continuous anxiety ratings
Continuous ratings of emotional experience were obtained using a rating dial similar to that used previously (Mauss et al., 2005; Ruef and Levenson, 2007). The dial allows participants to move a pointer along a 180° scale, with the anchors “no anxiety ‘0’” at 0° and “very high anxiety ‚10’” at 180°. Participants were asked to adjust the dial position as often as necessary to reflect the current amount of anxiety they felt.
Electrodermal responses
Participants’ skin conductance (SC) was recorded from the middle phalange of the index and middle finger of the left hand using 11-mm inner diameter Ag/AgCl electrodes filled with isotonic electrode paste (TD-246, Med Associates, Inc., St. Albans, VT, USA) to provide a continuous index of sympathetic activity (Boucsein, 1992).
Neuroticism
The neuroticism scale (N) of the NEO-PI-R (Costa and McCrae, 1992) was administered on a day prior to the anticipatory anxiety task. The neuroticism scale assesses an individual's tendency to experience psychological distress and sensitivity to negative cues in the environment. In keeping with previous literature showing the utility of dissociating the anxious and depressive aspects of neuroticism, the score for the N1 “anxiety” scale was utilized in subsequent analyses (mean score = 21.4 +/- 5.7 SD) (Haas et al., 2007).
Data Reduction and Analysis
All analyses only examined responses during the anticipatory cue period, up to the point but not including the shock. The two “quick trials” where not analyzed since the 3 second window may not be long enough to obtain meaningful responses, leaving 12 medium and strong trials for analysis respectively. Using ANSLAB software (Wilhelm and Peyk, 2005), we calculated skin conductance responses (SCRs) by determining the maximum SC during each trial (prior to the shocks) from which the average SC during a 1 s baseline before each cue onset was subtracted. SCRs were then averaged across trials within each condition. Negative SCRs were recoded to zero. Rating dial values were averaged across the whole cue phasei.
We first analyzed main effects of the task on continuous anxiety ratings and SCRs in one way ANOVAS (safe, medium, strong). Next, we compared participants with high and low neuroticism (as determined by median split) in their responses to the three conditions in a Condition (safe, medium, strong) X Group (high vs. low neuroticism) ANOVA for anxiety ratings and SCRs.
Results and Discussion
Effects of Task
Continuous anxiety ratings
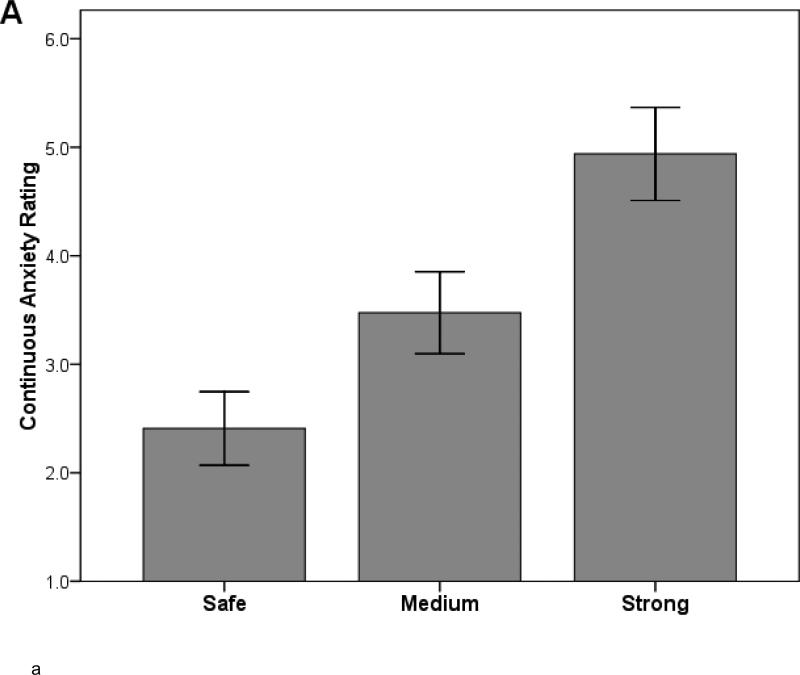
A one-way ANOVA for anxiety ratings yielded an effect of Condition, F(2,178)=228.16, p<.001, partial eta squared = .72 (see Figure 1A). Bonferroni-corrected paired t-tests indicated that anxiety ratings displayed the expected dose-response differences between the three conditions (strong > medium > safe) (all ps<.001).
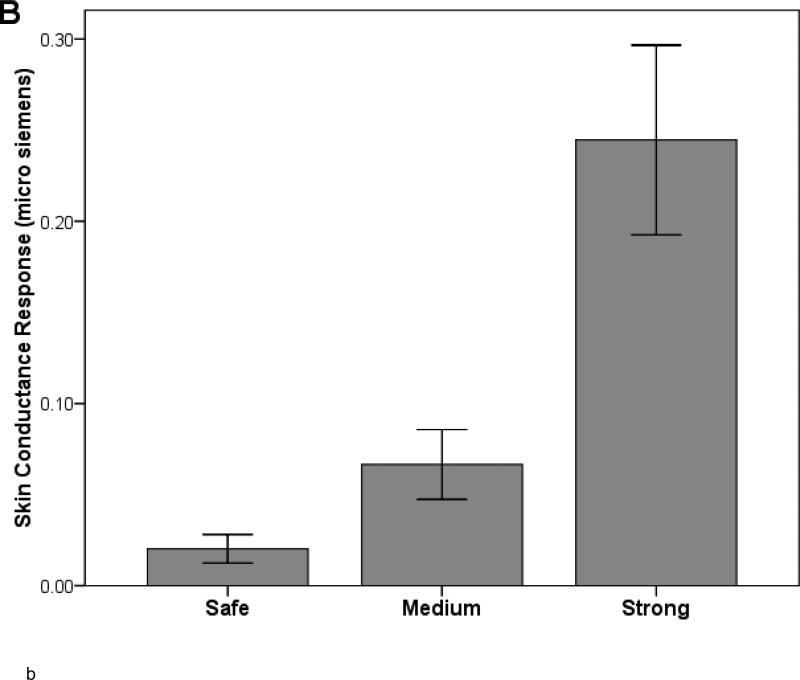
Figure 1.
Study 1: Effects of shock anticipation on (A) continuous ratings of anxiety and (B) skin conductance response across three levels of shock intensity. Paired t-tests indicated significant differences between the conditions for both (A) and (B) (all ps < .001). Error bars = SEM.
Electrodermal responses
A one-way ANOVA for SCRs resulted in an effect of Condition, F(2, 188)=81.57, p<.001, partial eta squared=.47 (see Figure 1B). Bonferroni-corrected paired t-tests indicated that SCRs displayed the expected dose-response differences between the three conditions (strong > medium > safe) (all ps<.001).
Effects of Neuroticism
Continuous anxiety ratings
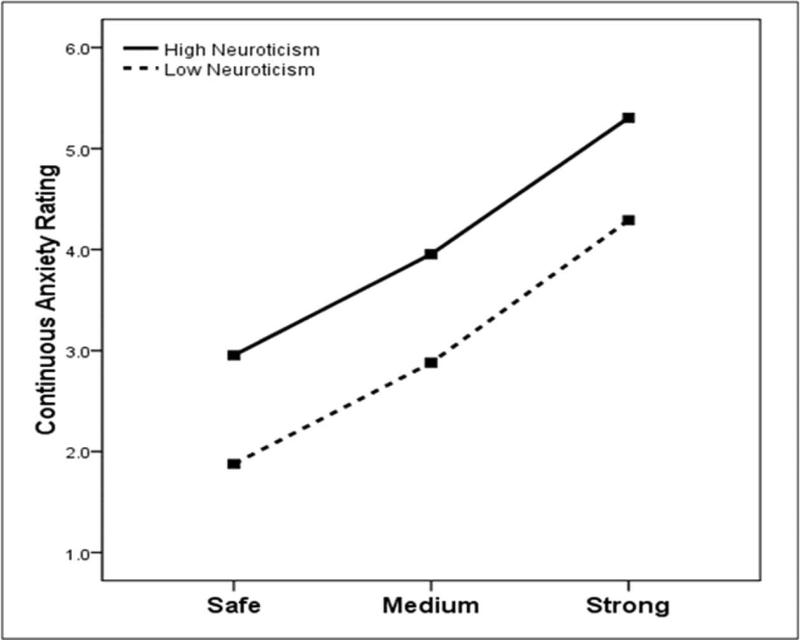
In addition to the task main effects reported above, the Condition (safe, medium, high) X Group (high neuroticism, low neuroticism) ANOVA for continuous anxiety ratings revealed significant main effect for Group F(1, 88)=8.58, p=.004, partial eta squared=.09 (see Figure 2), but not their interaction F(2, 176)=0.466, p=.54, partial eta squared=.005. High neuroticism participants showed elevated levels of subjective anxiety compared to their low neuroticism counterparts across conditions.
Figure 2.
Study 1: The effect of neuroticism (median split) on continuous ratings of anxiety. Repeated-measures ANOVAs showed a significant effect of neuroticism on anxiety ratings (p=.004).
Electrodermal responses
The Condition (safe, medium, high) X Group (high neuroticism, low neuroticism) ANOVA for SCRs was not significant for Group, F(1,93)=.247, p=.621, partial eta squared=.003 or their interaction, F(2, 186)=.025, p=.9, partial eta squared=0.
Summary
As expected, the results from Study 1 revealed a stepwise increase in both anxiety reports and electrodermal responding from safe to medium to strong trials. This suggests that the shock anticipation task is sufficiently potent to induce anticipatory anxiety and engage preparatory responses while awaiting an aversive event. It is also clear that individuals higher in neuroticism showed elevated anxiety reports in all three task conditions, but there were no differences in electrodermal responses.
Study 2: fMRI BOLD Responses During Threat Anticipation
In Study 2, we sought to extend the findings from Study 1 by examining the effects of shock anticipation on neural responses during BOLD neuroimaging. We hypothesized that increasing levels of threat would be associated with increasing neural responses in brain regions implicated in visceral perception (insula and thalamus), motor preparation (caudate), assigning affective stimulus value, and self-referential processing (vlPFC and dmPFC). In this independent sample, we further sought to investigate whether neuroticism moderated neural responses to the shock anticipation task. We hypothesized that individuals higher in neuroticism would show enhanced neural responses during shock anticipation.
Methods
Participants
In Study 2, 51 right-handed healthy female individuals (mean age 22.2 +/- 2.4) participated in the shock anticipation task during fMRI scanning. Participants met the same eligibility criteria described in Study 1, as well as additional criteria to ensure magnet compatibility. The study was approved by the Stanford University Institutional Review Board, and all subjects gave informed consent and were paid for participation.
Procedure
The procedure for the fMRI task was the same as described in Study 1. The task varied slightly in that it contained 12 safe, 13 medium, and 13 strong trials, and the inter stimulus interval (ISI) varied from 3 to 6s. As with Study 1, the “quick” trials were dropped from analysis, leaving 12 trials per each of the three conditions. Rather than providing continuous ratings of anxiety, at the end of each medium and strong shock trial, participants provided ratings of the anxiety that they experienced during the anticipatory period prior to the delivery of the shock, using a scale of 1=not at all to 5=very much. The ratings for 3 subjects were not obtained due to software malfunction. The trial structure was as follows: condition cue (7-11 s), shock (50 ms, only on a subset of trials), rating (6 s, only after medium and strong trials), ISI (3 to 6 s).
Measures
Imaging
Imaging was performed on a GE 3 Tesla Signa magnet (General Electric Medical System, Milwaukee, Wisconsin). Blood oxygenation-level dependent (BOLD) signal was acquired with a T2*-weighted gradient echo spiral-in/out pulse sequence (Glover and Law, 2001) and a custom-built quadrature “dome” elliptical bird cage head coil. Head movement was minimized using a bite-bar. Four hundred and forty-six functional volumes were obtained during the functional run from 22 sequential axial slices (TR/TE = 1500/30 msec, flip angle = 70°, FOV = 22 cm, matrix = 64 × 64, single-shot, in-plane resolution = 3.438 mm2, and slice thickness =4.5 mm). Three-dimensional high-resolution anatomical scans were acquired using fast spin-echo spoiled gradient recalled (SPGR) (.859 x 1.2 mm; FOV = 22 cm, frequency encoding = 256).
Neuroticism
Neuroticism scores were obtained using the same method described in Study 1. In Study 2, the mean neuroticism N1 score = 20.71 +/- 4.75 SD. Neuroticism scores did not differ significantly between participants in Study 1 and Study 2.
Data Reduction and Analysis
Functional data were analyzed with Analysis of Functional NeuroImages (AFNI) software (Cox, 1996). Preprocessing included coregistration, motion correction, 4 mm3 isotropic Gaussian spatial smoothing, high-pass filtering (.011 Hz), and linear detrending. Only volumes which demonstrated less than 1 mm of motion in the x, y, and z directions were included. Three participants exhibited volumes with motion above this threshold; these subjects were removed from all subsequent analyses, leaving 48 participants for these analyses. There was no evidence of stimulus-correlated motion when conducting correlations of condition-specific reference functions and x, y, or z motion correction parameters (all ps > .05).
A multiple regression model implemented with AFNI 3dDeconvolve included baseline parameters to remove polynomial trends to the fifth order, as well as individual motion-related variance in the BOLD signal in six orientations. The model also included the following regressors: safe period, medium anticipation period, strong anticipation period, medium shock, strong shock, medium quick trial, strong quick trial, ratings after medium trials, and ratings after strong trials, all of which were convolved with the gamma variate model of the hemodynamic response function. Contrasts were computed by weighting the appropriate columns in the design matrix accordingly.
As with Study 1, brain analysis only examined the anticipatory period, up to but not including the shock (the separate shock regressors accounted for variation due to the shock itself). The first contrast compared safe trials vs. shock anticipation (medium and strong anticipation periods grouped together). The second contrast compared medium vs. strong anticipation trials. In order to determine which regions had a true linear effect across all three conditions, a conjunction analysis was performed on the second contrast such that the main effects from the first contrast (thresholded at corrected p < 0.05) were used to mask the results from the second contrast. The resultant map showed brain regions where responses increased on average from safe to medium to strong trials. Statistical maps were resampled to 3.438 mm3 and converted to Talairach atlas space (Talairach and Tournoux, 1988), and second-level statistical parametric maps were produced according to a random-effects analysis to enhance the generalizability of the results.
To correct for multiple comparisons, AlphaSim, a Monte Carlo simulation bootstrapping program in the AFNI library (Cox, 1996), was employed to identify a joint probability consisting of a voxel-wise threshold and a minimum cluster volume threshold to establish a cluster-wise p-value that protects against false-positive detection of activation clusters (Forman et al., 1995). A voxel-wise threshold of p = .001 (t = 3.50) resulted in a minimum cluster volume threshold of 343 mm3 (8 voxels) to protect against false-positive detection of clusters of activation at p < .05. All clusters cited in this paper survived this correction.
The relationship between neuroticism and BOLD responses was investigated using a whole-brain regression analysis across participants in which individual neuroticism scores were entered as the regressor of interest and examined for the contrast of (strong shock anticipation > medium shock anticipation) using 3dRegAna from the AFNI library (Cox, 1996). The same multiple comparisons threshold was employed as above.
Results and Discussion
Effects of Task
Anxiety ratings
A paired samples t-test was used to compare anxiety ratings made in the medium and strong conditions, and results indicated that ratings in strong trials were significantly higher than medium trials (medium M=2.3, SD =0.65, strong M=3.5, SD =0.89) t(45)= -15.69, p < .001. There were no ratings provided after safe trials.
Neural responses
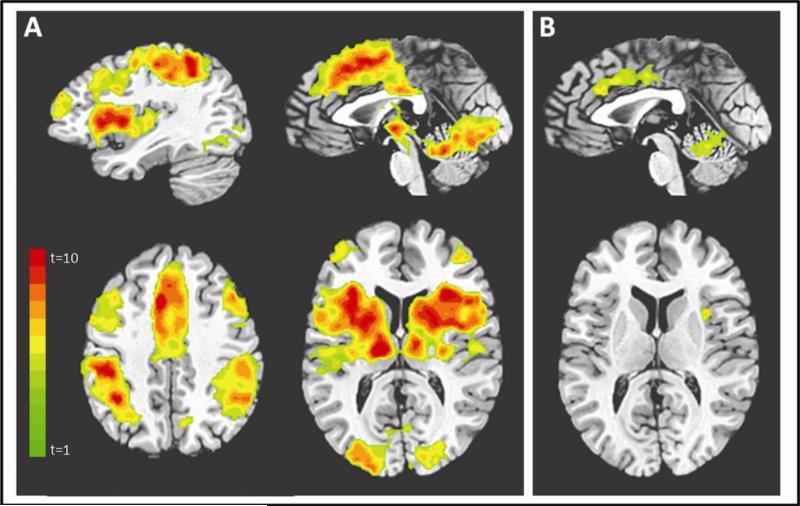
The contrast (shock anticipation > safe) revealed brain regions including bilateral anterior insula, mid cingulate cortex, anterior cingulate cortex, bilateral dorsomedial PFC, bilateral ventrolateral PFC, bilateral thalamus, bilateral caudate, bilateral precentral gyrus, hypothalamus, and periaqueductal gray. The contrast (strong shock anticipation > medium shock anticipation) masked by the contrast (safe vs. shock anticipation) revealed a subset of regions including bilateral anterior insula, mid cingulate cortex, anterior cingulate cortex, inferior frontal gyrus and culmen (see Figure 3 and Table 1).
Figure 3.
Study 2: Effects of shock anticipation on neural responses for (A) the contrast shock anticipation > safe (starting top left and moving clockwise, slices are displayed at x=36, x=0, z=40 , z=11) and (B) the conjunction analysis with strong shock anticipation > medium shock anticipation (top slice is displayed at x=0, bottom slice at z=11). Contrasts displayed at corrected p<.05, t=3.5, color bar indicates t statistic. Coordinates for all regions showing a main effect of shock anticipation are displayed in Table 1.
Table 1.
Study 2: Neural regions exhibiting an effect of shock anticipation for the contrast shock anticipation > safe (top), and the conjunction analysis with strong shock anticipation > medium shock anticipation (bottom) at the threshold p < .05 corrected, t=3.5. Coordinates in Talairach space. Asterisks indicate a subpeak within the cluster listed directly above; voxels are 3.44 mm3.
| SHOCK ANTICIPATION > SAFE | |||||||
|---|---|---|---|---|---|---|---|
| Region | Side | BA | x | y | z | t | Voxels |
| Thalamus | L | - | -10 | -10 | 1 | 12.6 | 6396 |
| Insula | L | 13 | -31 | 21 | 8 | 11.4 | * |
| Insula | R | 13 | 38 | 18 | 5 | 11.4 | * |
| Cingulate Gyrus | L | 24 | -7 | 18 | 36 | 11.3 | * |
| Inferior Parietal Lobule | L | 40 | -34 | -48 | 39 | 10.2 | * |
| Postcentral Gyrus | L | 40 | -58 | -27 | 19 | 8.5 | * |
| Postcentral Gyrus | R | 2 | 48 | -27 | 43 | 8.4 | * |
| Precentral Gyrus | L | 6 | -31 | -6 | 50 | 7.9 | * |
| Middle Frontal Gyrus | R | 8 | 41 | 18 | 43 | 7.7 | * |
| Inferior Frontal Gyrus | L | 45 | -55 | 11 | 25 | 7.6 | * |
| Middle Frontal Gyrus | R | 6 | 24 | -6 | 53 | 7.5 | * |
| Hypothalamus | L | - | -10 | -7 | -1 | 6.8 | * |
| Middle Frontal Gyrus | R | 10 | 38 | 45 | 19 | 6.8 | * |
| Periaqueductal Gray | M | - | 1 | -24 | -10 | 4.4 | * |
| Lingual Gyrus | L | 18 | -7 | -75 | -6 | 10.9 | 1532 |
| Cerebellar Lingual | R | - | 3 | -44 | -16 | 8.8 | * |
| Fusiform Gyrus | L | - | -31 | -44 | -19 | 6.6 | * |
| Superior Frontal Gyrus | L | 10 | -24 | 49 | 29 | 6.2 | 155 |
| Middle Frontal Gyrus | L | 10 | -45 | 49 | 5 | 5.9 | * |
| Precuneus | R | 7 | 7 | -61 | 43 | 5.0 | 36 |
| Superior Temporal Gyrus | R | 21 | 48 | -27 | -2 | 4.9 | 26 |
| Postcentral Gyrus | R | 5 | 31 | -41 | 60 | 5.0 | 8 |
| STRONG SHOCK ANTICIPATION > MEDIUM SHOCK ANTICIPATION | |||||||
|---|---|---|---|---|---|---|---|
| Region | BA | Side | x | y | z | T | Voxels |
| Lingual Gyrus | - | R | 10 | -68 | -6 | 5.9 | 162 |
| Culmen | - | L | -7 | -55 | -6 | 5.6 | * |
| Cingulate Gyrus | 32 | M | 0 | 21 | 26 | 5.0 | 149 |
| Cingulate Gyrus | 24 | M | 0 | -10 | 39 | 5.2 | * |
| Insula | - | R | 31 | 7 | 12 | 6.0 | 25 |
| Insula | - | L | -41 | 11 | 1 | 4.6 | 11 |
| Insula | - | R | 34 | 7 | -6 | 3.8 | 9 |
| Inferior Frontal Gyrus | 45 | R | 45 | 28 | 5 | 4.5 | 8 |
| Cingulate Gyrus | 24 | L | -10 | -20 | 43 | 4.4 | 4 |
Effects of Neuroticism
Anxiety ratings
A one-way ANOVA for anxiety ratings indicated that anxiety ratings did not differ based on neuroticism (median split) for medium or strong trials (ps > .55).
Neural responses
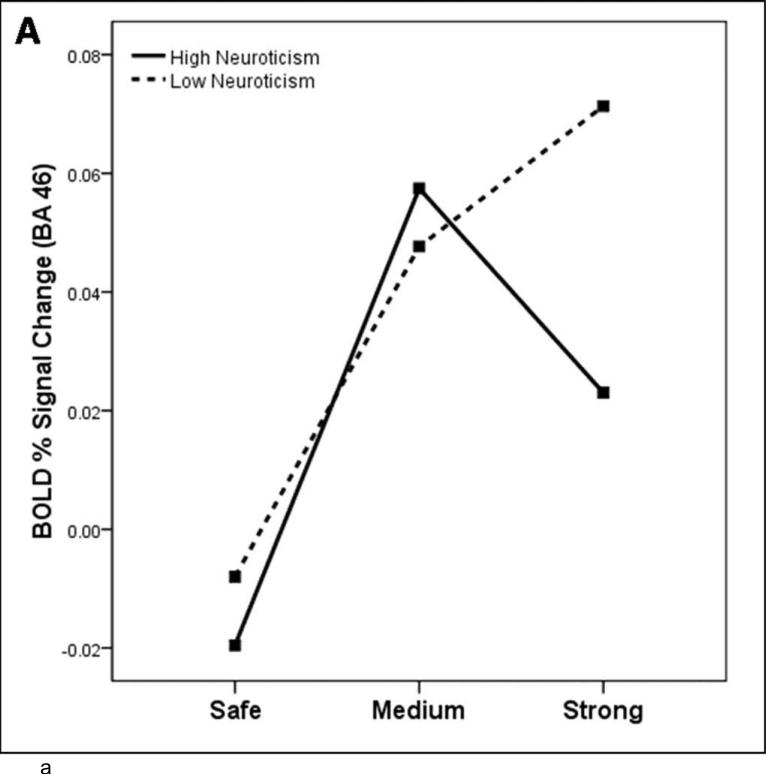
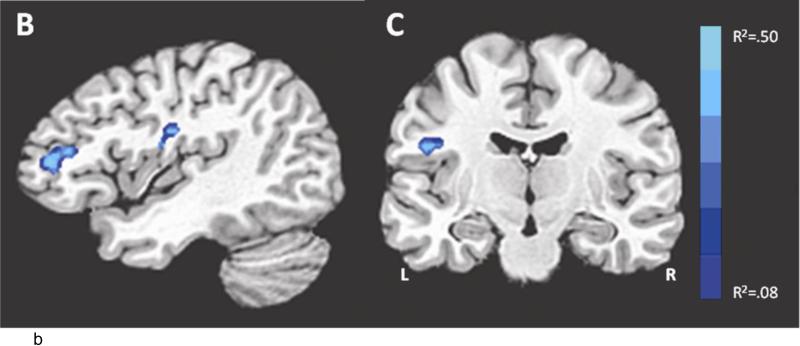
We examined the association between neuroticism and neural responses during the shock anticipation task using a regression with the contrast (strong shock anticipation > medium shock anticipation). We found that neuroticism was negatively correlated with activation in left BA 46 and left insula (see Table 2 and Figure 4). For display purposes, individual subjects’ mean contrast value in BA 46 for each condition are plotted based on neuroticism, defined by a median split (Figure 4a).
Table 2.
Study 2: Neural regions exhibiting a correlation with neuroticism at corrected p<.05, T=3.5. A whole-brain regression analysis was performed on the contrast strong shock anticipation > medium shock anticipation. Coordinates in Talairach space; r value obtained from AFNI for the peak voxel in each functional cluster. Voxels are 3.44 mm3.
| Region | BA | Side | x | y | z | r |
|---|---|---|---|---|---|---|
| Inferior Frontal Gyrus | 46 | L | -41 | 42 | 8 | -0.59 |
| Insula | - | L | -45 | -13 | 22 | -0.65 |
Figure 4.
Study 2: Neural response to threat varies as a function of neuroticism. (A) the relationship between BOLD response to each condition and neuroticism (median split) in right BA 46 (brain slice depicted in (B), the contrast strong shock anticipation > medium shock anticipation). Regions which correlate with neuroticism for the contrast strong shock anticipation > medium shock anticipation, using a whole-brain regression at corrected p < .05, T=3.5, color bar indicates R2 value: (B) right BA 46 (anterior cluster; x=-3, y=-4, z=53), (C) right insula (x=45, y=13, z=22). Coordinates for regions showing a significant correlation with neuroticism are displayed in Table 2.
Summary
As predicted, the results from Study 2 parallel those from Study 1, indicating an increase in anxiety experience from medium to strong shock trials. Study 2 extends these findings by showing that as threat level increases, there are concomitant increases in brain activation in a network of regions involved in visceral perception and integration, motor preparation, context interpretation, and assigning affective value. A subset of these regions show a linear response from safe to medium to strong trials. This study did not show an effect of neuroticism on anxiety ratings, likely due to the reduced sensitivity of the ratings used in Study 2 (continuous ratings vs. punctuate ratings on a five point scale). The results do indicate, however, that relative to individuals who are low in neuroticism, those high in neuroticism show a reduction in brain activation based on threat level in key brain regions.
GENERAL DISCUSSION
The present study utilized a laboratory task to examine how threat anticipation influences experiential, autonomic, and neural responses. Importantly, this study examined the anticipatory responses independently from the receipt of the noxious stimuli and utilized multiple layers of unpredictability to maximize anxiety and minimize habituation. Using an optimized task in which participants anticipated electric shocks to the wrist at three intensity levels, across two studies, our findings indicated that as threat increased from safe to medium to strong intensity, parallel changes occurred in 1) reports of anxiety experience, 2) electrodermal responses, and 3) brain activation in a network of prefrontal, temporal, mid-brain and brainstem regions. These results showed a clear pattern across multiple emotion “output” channels, with each channel showing a similar dose-response pattern as threat level increased. The results also indicated the individual differences in neuroticism modulated anticipatory effects on anxiety experience and brain activation.
The Neural Dynamics of Threat Anticipation
When a threatening event is impending, a number of preparatory mechanisms are engaged which are related to the nature of the threat. Study 1 showed that as threat level increased, subjects reported feeling greater levels of anxiety and exhibited higher skin conductance responses. Study 2 showed that threat of impending shock resulted in increased neural activation in a network of regions including the hypothalamus, periaqueductal gray (PAG), caudate, precentral gyrus, thalamus, insula, vlPFC, dmPFC, and ACC. A subset of these regions demonstrated a linear increase in activation from safe to medium to strong trials including a large portion of the ACC, bilateral anterior insula, and BA 45. This coordinated set of responses suggests a number of functional components underlying anticipatory processes. Specifically, systems involved in threat detection and coordination of defensive behaviors, integration of sensory and emotional responses, and expectation and coping strategies likely came online during the shock anticipation task.
In particular, several regions are likely related to threat detection and coordination of defensive behaviors. Regions in the brainstem and diencephalon such as the PAG and hypothalamus showed increased activation to threat, which is consistent with previous work by Mobbs indicating increased activation in the PAG in response to imminent threat (Mobbs et al., 2007). It is likely that these responses are involved in automatic processing of threat, and coordinating defensive strategies for responding (e.g. freezing) (Bandler et al., 2000; Hsieh et al., 1999). The hypothalamus may be involved in triggering the peripheral electrodermal responses via its communication with the autonomic nervous system (Buijs and Van Eden, 2000). The caudate may be involved in preparing a motor response (White, 2009), along with activation in the precentral gyrus. Even though participants are inside the scanner and have been instructed not to move, this activation may reflect unconscious or automatic motor preparation (e.g. withdrawal).
Results also suggest that participants are integrating sensory and emotional information during shock anticipation. While participants did not experience painful sensations during the anticipation phase, it is possible that the mere expectation of future pain activates the sensory machinery underlying pain perception (Koyama et al., 2005). Activation in the thalamus, particularly the dorsal medial nucleus, may act as a point of integration between bottom-up sensory expectation and cortical input, helping to coordinate awareness, attention, and planning. The task induces strong activation in the insula, particularly the anterior region, which has been associated with interoceptive processing, processing negative emotions, anticipating pain and imagining oneself experiencing pain (Carlsson et al., 2006; Ogino et al., 2007; Ploghaus et al., 1999). In the context of the current task, activation in the anterior insula may reflect the connection of interceptive awareness of body responses with the emotionally salient context of threat anticipation in producing subjective emotional experience. This region shows a linear activation pattern from safe to medium to strong trials, suggesting that it is differentially sensitive to the level of threat, which perhaps results in greater interceptive awareness and resultant subjective experience for strong trials.
Regions consistent with higher-order cognitive processes were also active during the anticipation phase. The dorsal ACC may integrate information about relevant contextual variables with sensory information to generate context-appropriate affective responses and behaviors (Salomons et al., 2004). Specifically, in the shock conditions, the embedded levels of uncertainty (event, temporal and intensity) may engage the ACC in an effort to resolve ambiguity (Carlsson et al., 2006). The ACC may be differentially engaged in the strong relative to medium condition in order to further maintain vigilant attention to the increased level of danger of the upcoming shock and prepare context-appropriate responses. This interpretation aligns with the behavioral and electrodermal data showing increased anxiety and SCR from medium to strong trials. These results are also consistent with previous research showing that activation in the mid-cingulate is linked to increased attention in preparation of painful stimuli (Munafo et al., 2008). Activation in prefrontal regions may represent the engagement of resources to regulate bottom-up emotional responding suggested by increased anxiety ratings and SCR. Indeed, previous work suggests automatic emotion regulation is associated with increased prefrontal activation (Drabant et al., 2009).
It bears comment that we did not find significant amygdala activation as a function of increasing threat intensity. In our view, this is not surprising, for a number of reasons. Activation in the amygdala is typically not demonstrated in pure anticipatory tasks (Chua et al., 1999; Schunck et al., 2008; Simpson et al., 2001), arguably because 1) these tasks do not include a learning or acquisition phase like Pavlovian tasks which typically elicit amygdala activation, 2) no pain is occurring during the anticipatory period, which is a characteristic associated with alterations in amygdala activation (Petrovic et al., 2004) and 3) amygdala activation rapidly habituates (Buchel et al., 1998) or is down-regulated by higher order structures (Phelps et al., 2001).
These results indicate that healthy individuals show differential neural patterns of responding to varying levels of threat. Importantly, all these effects were seen during the anticipatory phase of the task, in the absence of any physical input other than a visual cue. The widespread nature of the activation may reflect both the intensity with which the task affected participants, as well as the complex nature of anticipatory processes themselves, involving the coordination of multiple brain systems and cognitive and affective processes.
Anticipatory Processes in Personality and Psychopathology
Based on evidence showing that neuroticism is related to increased responding during aversive stimuli (Canli, 2004; Canli et al., 2001; Haas et al., 2007), we examined whether neuroticism might also be linked to alterations in anticipatory processes preceding threat. Indeed, despite the fact that there were powerful main effects of the anticipatory task, there were substantial individual differences in responding. The relatively large sample size of the current study allowed for meaningful investigation of the modulatory effects of neuroticism, a primary index of negative affect and threat sensitivity.
The results from Study 1 indicated that individuals high in neuroticism had greater anxiety ratings relative to those low in neuroticism, suggesting that they were generally more anxious during the entire context of the shock task. In Study 2, the patterns of brain responding indicated that high neuroticism individuals demonstrated a blunted neural response during strong relative to medium trials in several brain regions. Specifically, we found an association between neuroticism and level of threat, such that those high in neuroticism showed a decrease in activation from medium to strong trials in the insula and BA 46 relative to those low in neuroticism. These results suggest that high neuroticism individuals are particularly sensitive to the intensity of the threat, and these findings are consistent with the proposition that at a sufficiently high threshold, they shut down and switch to a different processing strategy. These effects are in line with the vast literature which suggests that those high in neuroticism employ an avoidance (Bolger, 1990; McCrae and Costa, 1986; Parkes, 1986) and/or suppression (Canli et al., 2001) strategy in the face of high levels of stress. It is possible that an avoidance response to this shock anticipation task results in decreased visceral reactivity and reduced cognitive implementation of emotion regulation. Reduced activation in the insula and BA 46 aligns with this proposition. But ultimately those high in neuroticism show higher overall ratings of anxiety across the task, suggesting that their strategy is ineffective at reducing anxiety. Importantly, these effects would not have been detected in a simple safe/shock task and highlight the utility of employing multiple levels of threat to explore the complexity of individual differences in anticipatory responses.
One puzzle is why we did not observe an effect of neuroticism on electrodermal responses in Study 1. The only other study to date examining the effect of neuroticism on anticipatory responding to noxious stimuli found a similar pattern, such that those high in neuroticism did not show differences in SCR, but did show a reduction in brain activation in several regions from safe relative to threat trials . Overall, these findings suggest that the way an individual interprets a threatening event is importantly tied to their reactions and preparations.
These results extend previous individual-difference work in several ways. First, the current study employed a significantly larger sample than previous studies, particularly relative to other brain imaging studies of neuroticism, which have often have studied fewer than 15 participants (Britton et al., 2007; Canli, 2004; Canli et al., 2001; Kumari et al., 2007). Second, the current study examined the anticipatory phase in preparation for negative stimuli rather than reactions to negative stimuli after they've been presented. Third, this study builds on work by Kumari and colleagues, by employing a potently anxiogenic task in which shocks were actually delivered and a larger number of trials per condition were employed.
While preparatory responses to impending aversive events can be adaptive, when these responses cross a certain threshold they can become maladaptive. Indeed, many anxiety disorders are characterized by elevated and sustained levels of anticipatory anxiety in response to forecasted negative events, both real and imagined. One of the advantages of using a symptom provocation model such as this one where the task provokes responses which are manifested in clinical disorders (perhaps to a greater extreme) is that it permits for investigation of mechanisms underlying the particular disorder.
The modulation of anticipatory responses to varying levels of threat by neuroticism may have clinical significance. Neuroticism has been shown as a consistent risk factor for developing clinical anxiety disorders in both longitudinal and cross-sectional studies (Bienvenu and Stein, 2003), and therefore can inform our understanding of the mechanism underlying these disorders. These personality trait-based differences may be part of what leads to increased vulnerability to psychopathology seen in these individuals. For example, as anticipatory anxiety mounts, individuals may overestimate the frequency and/or intensity of feared future events. Chronically engaging preparatory mechanisms which were intended for short-term activation may have devastating long-term consequences. Research in animals and humans has demonstrated that when anxiety responses are sustained over long periods of time, they can have profoundly deleterious effects on the organism by causing alterations in neuroendocrine feedback systems which can result in far-reaching cascading effects on central and peripheral functioning (Sapolsky, 2005).
Limitations and Future Directions
This study has several limitations. First, while we prioritized studying a relatively homogenous sample in order to decrease error variance, this sampling decision limits the generalizablity of the findings. Future studies should extend this work to examine anticipatory responses in men and populations with a wider age range. Second, while focusing on a healthy population allowed us to investigate how neuroticism might confer risk for anxiety disorders, we cannot directly infer whether individuals with current anxiety disorders have maladaptive anticipatory processes, and thus future research should examine clinical populations. Third, the current study only examined anticipatory processes in preparation for a negative event. Future research should explore whether anticipatory processes in preparation for positive events show similar patterns. Indeed, the preparatory mechanisms associated with avoidant versus appetitive behaviors may be separable but overlapping. It would also be interesting to see whether anticipation of positive events is similarly affected by individual differences in neuroticism, or whether this represents a distinct aspect of the emotion processing stream. Fourth, in Study 2 anxiety ratings were only solicited after medium and strong trials, but not safe trials. Future fMRI research should include ratings after all trials of interest. Lastly, this task represents a potent stressor that is a stable platform for examining individual differences, yet the current study only examined the modulatory effects of a single individual difference measure; namely neuroticism. We believe that it is very likely that many other factors bias anticipatory responses in both the peripheral and central nervous system, including variation in genetic background. One direction for future research would be to use this shock anticipation task as a model of exposure to environmental stress in the context of an examination of gene by environment (GxE) interactions. Using a laboratory based procedure for modeling stress exposure has a number of distinct advantages over obtaining self-report indices of exposure to stressful life events, which are often hampered by memory biases, self-presentation management, idiosyncrasies in interpretation of items, and variability across instruments. In contrast, the current task can be administered in a rigorously controlled atmosphere and titrated in a dose-dependent fashion that is replicable and uniform across subjects.
ACKNOWLEDGMENTS
This research was supported by the National Alliance for Research on Schizophrenia and Depression (NARSAD) Young Investigator Award 34676 to Wiveka Ramel, the National Institutes of Health (NIH) R01 Grant MH58147 awarded to James Gross, and the National Defense Science and Engineering Graduate Fellowship (NDSEG) awarded to Emily Drabant. The authors thank Isabel Edge, Whitney Lynch, and Michael Mehler for help with participant recruitment, screening, and data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This research was conducted at Stanford University.
Five subjects were excluded from these analyses as they failed to move the rating dial for more than half of the trials
-
-A shock anticipation task was designed to maximally drive anticipatory anxiety
-
-Results indicated a clear dose-response pattern across multiple emotion “output” channels
-
-Specifically, increased anxiety experience, SCR and brain activation
-
-High neuroticism was related to increased anxiety and decreased brain activation
-
-Neuroticism risk for developing anxiety disorders via dysregulated anticipatory responses
REFERENCES
- Bandler R, Keay KA, Floyd N, Price J. Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Res Bull. 2000;53:95–104. doi: 10.1016/s0361-9230(00)00313-0. [DOI] [PubMed] [Google Scholar]
- Barlow DH. Anxiety and Its Disorders. Second ed. Guilford Press; New York: 2002. [Google Scholar]
- Bienvenu OJ, Stein MB. Personality and anxiety disorders: a review. J Pers Disord. 2003;17:139–151. doi: 10.1521/pedi.17.2.139.23991. [DOI] [PubMed] [Google Scholar]
- Bolger N. Coping as a personality process: a prospective study. J Pers Soc Psychol. 1990;59:525–537. doi: 10.1037//0022-3514.59.3.525. [DOI] [PubMed] [Google Scholar]
- Boucsein W. Electrodermal Activity. Plenum; New York: 1992. [Google Scholar]
- Britton JC, Ho SH, Taylor SF, Liberzon I. Neuroticism associated with neural activation patterns to positive stimuli. Psychiatry Res. 2007;156:263–267. doi: 10.1016/j.pscychresns.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Buchel C, Dolan RJ, Armony JL, Friston KJ. Amygdala-hippocampal involvement in human aversive trace conditioning revealed through event-related functional magnetic resonance imaging. J Neurosci. 1999;19:10869–10876. doi: 10.1523/JNEUROSCI.19-24-10869.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Van Eden CG. The integration of stress by the hypothalamus, amygdala and prefrontal cortex: balance between the autonomic nervous system and the neuroendocrine system. Prog Brain Res. 2000;126:117–132. doi: 10.1016/S0079-6123(00)26011-1. [DOI] [PubMed] [Google Scholar]
- Canli T. Functional brain mapping of extraversion and neuroticism: learning from individual differences in emotion processing. J Pers. 2004;72:1105–1132. doi: 10.1111/j.1467-6494.2004.00292.x. [DOI] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Desmond JE, Kang E, Gross J, Gabrieli JD. An fMRI study of personality influences on brain reactivity to emotional stimuli. Behav Neurosci. 2001;115:33–42. doi: 10.1037/0735-7044.115.1.33. [DOI] [PubMed] [Google Scholar]
- Carlsson K, Andersson J, Petrovic P, Petersson KM, Ohman A, Ingvar M. Predictability modulates the affective and sensory-discriminative neural processing of pain. Neuroimage. 2006;32:1804–1814. doi: 10.1016/j.neuroimage.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Carretie L, Albert J, Lopez-Martin S, Tapia M. Negative brain: an integrative review on the neural processes activated by unpleasant stimuli. Int J Psychophysiol. 2009;71:57–63. doi: 10.1016/j.ijpsycho.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Carroll JC, Boyce-Rustay JM, Millstein R, Yang R, Wiedholz LM, Murphy DL, Holmes A. Effects of mild early life stress on abnormal emotion-related behaviors in 5-HTT knockout mice. Behav Genet. 2007;37:214–222. doi: 10.1007/s10519-006-9129-9. [DOI] [PubMed] [Google Scholar]
- Carter RM, O'Doherty JP, Seymour B, Koch C, Dolan RJ. Contingency awareness in human aversive conditioning involves the middle frontal gyrus. Neuroimage. 2006;29:1007–1012. doi: 10.1016/j.neuroimage.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Cheng DT, Disterhoft JF, Power JM, Ellis DA, Desmond JE. Neural substrates underlying human delay and trace eyeblink conditioning. Proc Natl Acad Sci U S A. 2008;105:8108–8113. doi: 10.1073/pnas.0800374105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng DT, Knight DC, Smith CN, Helmstetter FJ. Human amygdala activity during the expression of fear responses. Behav Neurosci. 2006;120:1187–1195. doi: 10.1037/0735-7044.120.5.1187. [DOI] [PubMed] [Google Scholar]
- Cheng DT, Knight DC, Smith CN, Stein EA, Helmstetter FJ. Functional MRI of human amygdala activity during Pavlovian fear conditioning: stimulus processing versus response expression. Behav Neurosci. 2003;117:3–10. doi: 10.1037//0735-7044.117.1.3. [DOI] [PubMed] [Google Scholar]
- Cheng DT, Richards J, Helmstetter FJ. Activity in the human amygdala corresponds to early, rather than late period autonomic responses to a signal for shock. Learn Mem. 2007;14:485–490. doi: 10.1101/lm.632007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua P, Krams M, Toni I, Passingham R, Dolan R. A functional anatomy of anticipatory anxiety. Neuroimage. 1999;9:563–571. doi: 10.1006/nimg.1999.0407. [DOI] [PubMed] [Google Scholar]
- Costa PT, Jr., McCrae RR. Influence of extraversion and neuroticism on subjective well-being: happy and unhappy people. J Pers Soc Psychol. 1980;38:668–678. doi: 10.1037//0022-3514.38.4.668. [DOI] [PubMed] [Google Scholar]
- Costa PT, Jr., McCrae RR. Normal personality assessment in clinical practice: The NEO Personality Inventory. Psychol Assessment. 1992;4:5–13. [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- D'Amato MR, Gumenik WE. Some effects of immediate versus randomly delayed shock on an instrumental response and cognitive processes. J Abnorm Soc Psychol. 1960;60:64–67. doi: 10.1037/h0041397. [DOI] [PubMed] [Google Scholar]
- De Pascalis V, Valerio E, Santoro M, Cacace I. Neuroticism-Anxiety, Impulsive-Sensation Seeking and autonomic responses to somatosensory stimuli. Int J Psychophysiol. 2007;63:16–24. doi: 10.1016/j.ijpsycho.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Dimberg U. Facial reactions, autonomic activity and experienced emotion: a three component model of emotional conditioning. Biol Psychol. 1987;24:105–122. doi: 10.1016/0301-0511(87)90018-4. [DOI] [PubMed] [Google Scholar]
- Drabant EM, McRae K, Manuck SB, Hariri AR, Gross JJ. Individual differences in typical reappraisal use predict amygdala and prefrontal responses. Biol Psychiatry. 2009;65:367–373. doi: 10.1016/j.biopsych.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Bandettini PA, Knight DC. Impact of continuous versus intermittent CS-UCS pairing on human brain activation during Pavlovian fear conditioning. Behav Neurosci. 2007;121:635–642. doi: 10.1037/0735-7044.121.4.635. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders. Washington, DC: 1995. [Google Scholar]
- Fischer H, Andersson JL, Furmark T, Wik G, Fredrikson M. Right-sided human prefrontal brain activation during acquisition of conditioned fear. Emotion. 2002;2:233–241. doi: 10.1037/1528-3542.2.3.233. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a clustersize threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Geddes SM, Gray WM, Millar K, Asbury AJ. Skin conductance responses to auditory stimuli and anticipatory responses before venepuncture in patients premedicated with diazepam or morphine. Br J Anaesth. 1993;71:512–516. doi: 10.1093/bja/71.4.512. [DOI] [PubMed] [Google Scholar]
- Geer JH, Maisel E. Evaluating the effects of the prediction-control confound. J Pers Soc Psychol. 1972;23:314–319. doi: 10.1037/h0033122. [DOI] [PubMed] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Merikangas K, Woods SW, Davis M. Measuring the time course of anticipatory anxiety using the fear-potentiated startle reflex. Psychophysiology. 1993;30:340–346. doi: 10.1111/j.1469-8986.1993.tb02055.x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Woods SW, Merikangas K, Davis M. Fear-potentiated startle in humans: effects of anticipatory anxiety on the acoustic blink reflex. Psychophysiology. 1991;28:588–595. doi: 10.1111/j.1469-8986.1991.tb01999.x. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J Pers Soc Psychol. 2003;85:348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Haas BW, Omura K, Constable RT, Canli T. Emotional conflict and neuroticism: personality-dependent activation in the amygdala and subgenual anterior cingulate. Behav Neurosci. 2007;121:249–256. doi: 10.1037/0735-7044.121.2.249. [DOI] [PubMed] [Google Scholar]
- Hagemann D, Waldstein SR, Thayer JF. Central and autonomic nervous system integration in emotion. Brain Cogn. 2003;52:79–87. doi: 10.1016/s0278-2626(03)00011-3. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Stone-Elander S, Ingvar M. Anticipatory coping of pain expressed in the human anterior cingulate cortex: a positron emission tomography study. Neurosci Lett. 1999;262:61–64. doi: 10.1016/s0304-3940(99)00060-9. [DOI] [PubMed] [Google Scholar]
- Hubert W, de Jong-Meyer R. Autonomic, neuroendocrine, and subjective responses to emotion-inducing film stimuli. Int J Psychophysiol. 1991;11:131–140. doi: 10.1016/0167-8760(91)90005-i. [DOI] [PubMed] [Google Scholar]
- Knight DC, Cheng DT, Smith CN, Stein EA, Helmstetter FJ. Neural substrates mediating human delay and trace fear conditioning. J Neurosci. 2004a;24:218–228. doi: 10.1523/JNEUROSCI.0433-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Smith CN, Cheng DT, Stein EA, Helmstetter FJ. Amygdala and hippocampal activity during acquisition and extinction of human fear conditioning. Cogn Affect Behav Neurosci. 2004b;4:317–325. doi: 10.3758/cabn.4.3.317. [DOI] [PubMed] [Google Scholar]
- Kopacz FM, 2nd, Smith BD. Sex differences in skin conductance measures as a function of shock threat. Psychophysiology. 1971;8:293–303. doi: 10.1111/j.1469-8986.1971.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. The subjective experience of pain: where expectations become reality. Proc Natl Acad Sci U S A. 2005;102:12950–12955. doi: 10.1073/pnas.0408576102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, ffytche DH, Das M, Wilson GD, Goswami S, Sharma T. Neuroticism and brain responses to anticipatory fear. Behav Neurosci. 2007;121:643–652. doi: 10.1037/0735-7044.121.4.643. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Magai C, Consedine NS, Krivoshekova YS, Kudadjie-Gyamfi E, McPherson R. Emotion experience and expression across the adult life span: insights from a multimodal assessment study. Psychol Aging. 2006;21:303–317. doi: 10.1037/0882-7974.21.2.303. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, Gross JJ. The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion. 2005;5:175–190. doi: 10.1037/1528-3542.5.2.175. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Costa PT. Personality, coping and coping effectiveness in an adult sample. J Pers. 1986;54:385–405. [Google Scholar]
- Mee S, Bunney BG, Reist C, Potkin SG, Bunney WE. Psychological pain: a review of evidence. J Psychiatr Res. 2006;40:680–690. doi: 10.1016/j.jpsychires.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Petrovic P, Marchant JL, Hassabis D, Weiskopf N, Seymour B, Dolan RJ, Frith CD. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317:1079–1083. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monat A, Averill JR, Lazarus RS. Anticipatory stress and coping reactions under various conditions of uncertainty. J Pers Soc Psychol. 1972;24:237–253. doi: 10.1037/h0033297. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ. Functional neuroanatomy of aversion and its anticipation. Neuroimage. 2006;29:106–116. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Norris CJ, Larsen JT, Cacioppo JT. Neuroticism is associated with larger and more prolonged electrodermal responses to emotionally evocative pictures. Psychophysiology. 2007;44:823–826. doi: 10.1111/j.1469-8986.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- Ogino Y, Nemoto H, Inui K, Saito S, Kakigi R, Goto F. Inner experience of pain: imagination of pain while viewing images showing painful events forms subjective pain representation in human brain. Cereb Cortex. 2007;17:1139–1146. doi: 10.1093/cercor/bhl023. [DOI] [PubMed] [Google Scholar]
- Parkes KR. Coping in stressful episodes: the role of individual differences, environmental factors, and situational characteristics. J Pers Soc Psychol. 1986;51:1277–1292. doi: 10.1037//0022-3514.51.6.1277. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Carlsson K, Petersson KM, Hansson P, Ingvar M. Context-dependent deactivation of the amygdala during pain. J Cogn Neurosci. 2004;16:1289–1301. doi: 10.1162/0898929041920469. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O'Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci. 2001;4:437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, Rawlins JN. Dissociating pain from its anticipation in the human brain. Science. 1999;284:1979–1981. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- Ruef AM, Levenson RW. Continuous measurement of emotion: The affect rating dial. In: Coan JA, Allen JB, editors. Handbook of Emotion Elicitation and Assessment. Oxford University Press; New York, NY: 2007. pp. 286–297. [Google Scholar]
- Salomons TV, Johnstone T, Backonja MM, Davidson RJ. Perceived controllability modulates the neural response to pain. J Neurosci. 2004;24:7199–7203. doi: 10.1523/JNEUROSCI.1315-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Schunck T, Erb G, Mathis A, Jacob N, Gilles C, Namer IJ, Meier D, Luthringer R. Test-retest reliability of a functional MRI anticipatory anxiety paradigm in healthy volunteers. J Magn Reson Imaging. 2008;27:459–468. doi: 10.1002/jmri.21237. [DOI] [PubMed] [Google Scholar]
- Sehlmeyer C, Schoning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, Konrad C. Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS One. 2009;4:e5865. doi: 10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira H, Hot P, Silvert L, Delplanque S. Electrical autonomic correlates of emotion. Int J Psychophysiol. 2009;71:50–56. doi: 10.1016/j.ijpsycho.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Simpson JR, Jr., Drevets WC, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: II. During anticipatory anxiety. Proc Natl Acad Sci U S A. 2001;98:688–693. doi: 10.1073/pnas.98.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T, Schmidt S, Weiss T, Mentzel HJ, Miltner WH. Dynamic activation of the anterior cingulate cortex during anticipatory anxiety. Neuroimage. 2009;44:975–981. doi: 10.1016/j.neuroimage.2008.10.022. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme; New York: 1988. [Google Scholar]
- Vrana SR, Spence EL, Lang PJ. The startle probe response: A new measure of emotion? J Abnorm Psychol. 1988;97:487–491. doi: 10.1037//0021-843x.97.4.487. [DOI] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Waugh CE, Wager TD, Fredrickson BL, Noll DC, Taylor SF. The neural correlates of trait resilience when anticipating and recovering from threat. Soc Cogn Affect Neurosci. 2008;3:322–332. doi: 10.1093/scan/nsn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NM. Some highlights of research on the effects of caudate nucleus lesions over the past 200 years. Behav Brain Res. 2009;199:3–23. doi: 10.1016/j.bbr.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Wilhelm FH, Peyk P. ANSLAB 4.0. Autonomic Nervous System Laboratory; 2005. [Google Scholar]
- Zelenski JM, Larsen RJ. Susceptibility to affect: A comparison of three personality taxonomies. J Pers. 1999;67:761–791. doi: 10.1111/1467-6494.00072. [DOI] [PubMed] [Google Scholar]