Abstract
Though incidence of colorectal cancer (CRC) in the US, has declined in recent years, rates remain higher in men than women and the male-to-female incidence rate ratio (MF IRR) increases progressively across the colon from the cecum to the rectum. Rates among races/ethnicities other than Whites or Blacks have not been frequently reported. To examine CRC rates by sex across anatomic subsite, age, and racial/ethnic groups, we used the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program for cases diagnosed among residents of 13 registries during 1992–2006. Incidence rates were expressed per 100,000 person-years and age-adjusted to the 2000 US Standard Population; MF IRR and 95% confidence intervals were also calculated. Among each racial/ethnic group, the MF IRR increased fairly monotonically from close to unity for cecal cancers to 1.81 (Hispanics) for rectal cancers. MF IRRs increased with age most rapidly for distal colon cancers from <1.0 at ages <50 years to 1.4–1.9 at older ages. The MF IRR for rectal cancers also rose with age from about 1.0 to 2.0. For proximal cancer, the MF IRR was consistently <1.5; among American Indian/Alaska Natives it was <1.0 across all ages. The MF IRRs for CRC vary markedly according to subsite and age but less by racial/ethnic group. These findings may partially reflect differences in screening experiences and access to medical care but also suggest that etiologic factors may be playing a role.
Keywords: colorectal cancer, sex ratio, incidence, SEER program, epidemiology, neoplasms
Introduction
Colorectal cancer (CRC) remains the fourth most common cancer diagnosed and the second most common cause of cancer death in the US, accounting for 146,970 new cancer cases and 49,920 cancer deaths in 2009 1. Though CRC incidence rates generally are higher among males, than females, at all anatomic subsites, the male-to-female incidence rate ratio (MF IRR) increases progressively across the colon from the cecum to the rectum 2, 3. This pattern is unexplained and likely a result of a combination of better awareness of screening in women, sex-specific exposure to risk factors, and protective effects of both endogenous and exogenous hormones 4. Blacks have higher incidence rates than Whites and a similar sex ratio pattern across the colon so that Black men have the highest incidence rates of all 4, however, data regarding this subsite-specific sex-ratio pattern in other races are sparse 5, 6.
The increasing MF IRR distally, from cecum to rectum, is particularly relevant given the changing global incidence of CRC: rates in Asia and Eastern Europe, traditionally considered regions of low incidence, have been increasing rapidly in recent years 7, 8. In fact, data from Cancer Incidence in Five Continents indicated that while CRC rates increased significantly from 1983–87 to 1998–2002, these increases were largely confined to economically transitioning countries (Eastern European countries, most parts of Asia and some South American countries) 9. Meanwhile, incidence rates during the same period in the US generally have been declining 10. This overall trend, however, masks emerging and widening CRC racial disparities 4, and recent reports have demonstrated an alarming increase in incidence of CRC among young adults aged 20–49 years in the US11.
We investigated recent CRC incidence data according to anatomic subsite, race/ethnicity and age, focusing on the sex-specific patterns to broaden our understanding and reveal potential clues for etiologic research.
Material and Methods
We used the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program (November 2008 submission). The SEER program began in the 1970s and included nine registries; however, in 1992 SEER expanded to include a total of 13 registries serving the states of Connecticut, Hawaii, Iowa, New Mexico and Utah and the greater San Francisco/Oakland, San Jose/Monterey, Los Angeles (California), Detroit (Michigan), Seattle (Washington) metropolitan areas as well as Atlanta and rural Georgia and the Alaska Native Registry. Further, 1992 marks the first specification of races other than Blacks and Whites (American Indians/Alaska Natives; Asians/Pacific Islanders) and ethnicity (Hispanics and non-Hispanics). For this analysis, counts and rates for American Indians/Alaskan Natives were restricted to the SEER 13 Contract Health Service Delivery Areas. Rates among the following racial groups were examined: Black, Asian/Pacific Islander (A/PI), American Indian/Alaska Native (AI/AN), white non-Hispanics (White) and Hispanics were examined. Cases with race specified other or unknown were excluded.
Malignant tumors of the colon or rectum diagnosed during 1992–2006 were included in this analysis. Lymphomas carcinomas were excluded. Anatomic subsites were defined using the 3rd edition of the International Classification of Diseases for Oncology 12. We defined proximal colon as including the cecum (C18.0), ascending colon (C18.2), and transverse colon (C18.4) including the hepatic (C18.3) and splenic flexures (C18.5); distal colon as including the descending colon (C18.6) and sigmoid (C18.7); and rectum as including the rectum (C20.9) and rectosigmoid junction (C19.9). Total colorectum included these three subsites plus overlapping lesions of the colon (C18.8), colon not otherwise specified (C18.9) and intestine, not otherwise specified (C26.0).
Statistical Methods
Rates were calculated using SEER*Stat 6.4.4 (13), expressed per 100,000 person-years and age-adjusted to the 2000 US Standard Population (19 age groups - Census P25-1130). Ninety-five percent confidence intervals (95% CI) for age-adjusted rates and ratios were calculated using the modification of Tiwari et al.13. Male-to-female (MF) incidence rate ratios (IRRs) were calculated for total CRC and for each anatomic subsite by using the unrounded rates. Graphs showing the incidence rates and MF IRRs were plotted by age of diagnosis for age groups 0–9, 10–19, 20–29,30–39, 40–49, 50–59, 60–69, 70–79 and ≥ 80 years. Only data points based on at least 25 cases for each sex were used in plotting rates and IRRs. Stata (StataCorp LP, 2007, release 10.1) was used for data processing and analysis. Data were graphed using SigmaPlot (SPSS, 2002, version 11.0).
Results
Male rates were higher than female rates at all subsites for all racial/ethnic groups, with the single exception of proximal CRC sites for AI/AN, where female rates were slightly higher than male (Table 1). Among both males and females, total CRC rates were highest among Blacks (71.0 and 54.8 per 100,000 person-years, respectively) and lowest among Hispanics (47.8 and 32.4) (Table 1). Rates were intermediate and similar among Whites and AI/AN while somewhat lower among A/PI. The Black predominance relative to White was particularly notable for proximal cancers, less so for distal colon cancers, and non-existent for rectal cancers. The deficit in incidence rates among Hispanics, relative to Whites, was evident across the entire colorectum, whereas that among A/PI was restricted to the proximal colon. These spatterns were quite consistent for both males and females. Among every sex/race/ethnic group, except A/PI males, the rate of proximal CRC was higher than those for both distal and rectal sites. Further sub-division of the time-period included in the analysis (1992–1999 and 2000–2006) did not provide any appreciable differences in sex-specific patterns of cancer incidence by subsite or by racial/ethnic group (Supplementary Table).
Table 1. Colorectal cancer incidence by racial/ethnic group, sex and subsite, SEER 13, 1992–2006.
Rates are per 100,000 person-years and age-adjusted to the 2000 US standard population (19 age groups). Counts and incidence rates for American Indians/Alaskan Natives were restricted to the SEER 13 Contract Health Service Delivery Areas. Proximal colon included the cecum (C18.0), ascending colon (C18.2), hepatic flexure (18.3), transverse colon (18.4) and splenic flexure (C18.5), distal colon included descending colon (C18.6) and sigmoid colon (C18.7). Rectal included rectosigmoid junction (C19.9) and rectum not otherwise specified (C20.9). Total includes C18.0, C18.2–C20.9, C26.0 (intestinal NOS).
| White | Black | Asian/Pacific Islander | American Indian/Alaska Native |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-Hispanic males |
Non-Hispanic females |
Hispanic males | Hispanic females |
Males | Females | Males | Females | Males | Females | |||||||||||
| N | Rate | N | Rate | N | Rate | N | Rate | N | Rate | N | Rate | N | Rate | N | Rate | N | Rate | N | Rate | |
| Total | 98,573 | 61.8 | 98,140 | 45.3 | 9,783 | 47.8 | 8,592 | 32.4 | 12,396 | 71.0 | 13,841 | 54.8 | 12,314 | 54.0 | 10,790 | 37.7 | 768 | 57.3 | 787 | 48.3 |
| Proximal* | 38,382 | 24.6 | 46,667 | 21.1 | 3,287 | 17.0 | 3,506 | 13.8 | 5,356 | 31.9 | 6,782 | 27.2 | 3,423 | 15.7 | 3,860 | 13.9 | 241 | 19.6 | 349 | 22.3 |
| Cecum | 14,991 | 9.6 | 19,378 | 8.7 | 1,284 | 6.7 | 1,531 | 6.0 | 2,116 | 12.5 | 2,839 | 11.4 | 1,039 | 4.8 | 1,300 | 4.7 | 86 | 7.0 | 137 | 8.9 |
| Ascending | 10,605 | 6.8 | 13,194 | 5.9 | 932 | 4.9 | 977 | 3.9 | 1,458 | 8.8 | 1,916 | 7.7 | 989 | 4.6 | 1,213 | 4.4 | 60 | 4.8 | 87 | 5.5 |
| Transverse | 12,786 | 8.2 | 14,095 | 6.4 | 1,071 | 5.4 | 998 | 3.9 | 1,782 | 10.6 | 2,027 | 8.1 | 1,395 | 6.3 | 1,347 | 4.8 | 95 | 7.8 | 125 | 7.9 |
| Distal^ | 26,716 | 16.6 | 23,570 | 11.2 | 2,652 | 12.8 | 2,269 | 8.3 | 3,165 | 18.0 | 3,433 | 13.5 | 4,037 | 17.8 | 3,461 | 11.9 | 253 | 19.0 | 222 | 13.4 |
| Descending | 4,429 | 2.8 | 3,870 | 1.8 | 382 | 1.7 | 319 | 1.2 | 722 | 4.2 | 783 | 3.1 | 683 | 3.0 | 565 | 2.0 | 21 | 1.5 | 23 | 1.4 |
| Sigmoid | 22,287 | 13.8 | 19,700 | 9.3 | 2,270 | 11.0 | 1,950 | 7.1 | 2,443 | 13.8 | 2,650 | 10.4 | 3,354 | 14.8 | 2,896 | 10.0 | 232 | 17.5 | 199 | 11.9 |
| Rectal† | 30,031 | 18.4 | 23,694 | 11.3 | 3,493 | 16.1 | 2,479 | 8.9 | 3,296 | 17.6 | 2,983 | 11.5 | 4,560 | 19.2 | 3,205 | 10.9 | 254 | 17.1 | 198 | 11.4 |
| Rectosigmoid junction | 9,202 | 5.7 | 7,578 | 3.6 | 951 | 4.5 | 740 | 2.7 | 1,019 | 5.6 | 958 | 3.7 | 1,358 | 5.8 | 978 | 3.3 | 68 | 4.2 | 52 | 3.1 |
| Rectum | 20,829 | 12.8 | 16,116 | 7.7 | 2,542 | 11.6 | 1,739 | 6.2 | 2,277 | 12.0 | 2,025 | 7.8 | 3,202 | 13.4 | 2,227 | 7.6 | 186 | 12.8 | 146 | 8.3 |
% of total CRC which were proximal: Non-Hispanic M: 39%, F: 48%; Hispanic M: 34%, F: 41%; Black M: 43%, F: 49%; A/PI M: 28%, F:36%; AI/AN M:31%, F: 44%
% of total CRC which were distal: Non-Hispanic M: 27%, F: 24%; Hispanic M: 27%, F: 25%; Black M: 25%, F: 25%; A/PI M: 33%, F: 32%; AI/AN M: 33%, F: 28%
% of total CRC which were rectal: Non-Hispanic M: 31%, F: 24%; Hispanic M: 36%, F: 29%; Black M: 27%, F: 22%; A/PI M:37%, 30%; AI/AN M:33%, F: 25%
% will not sum to 100 because C26 was included in total CRC but not in CRC subsites.
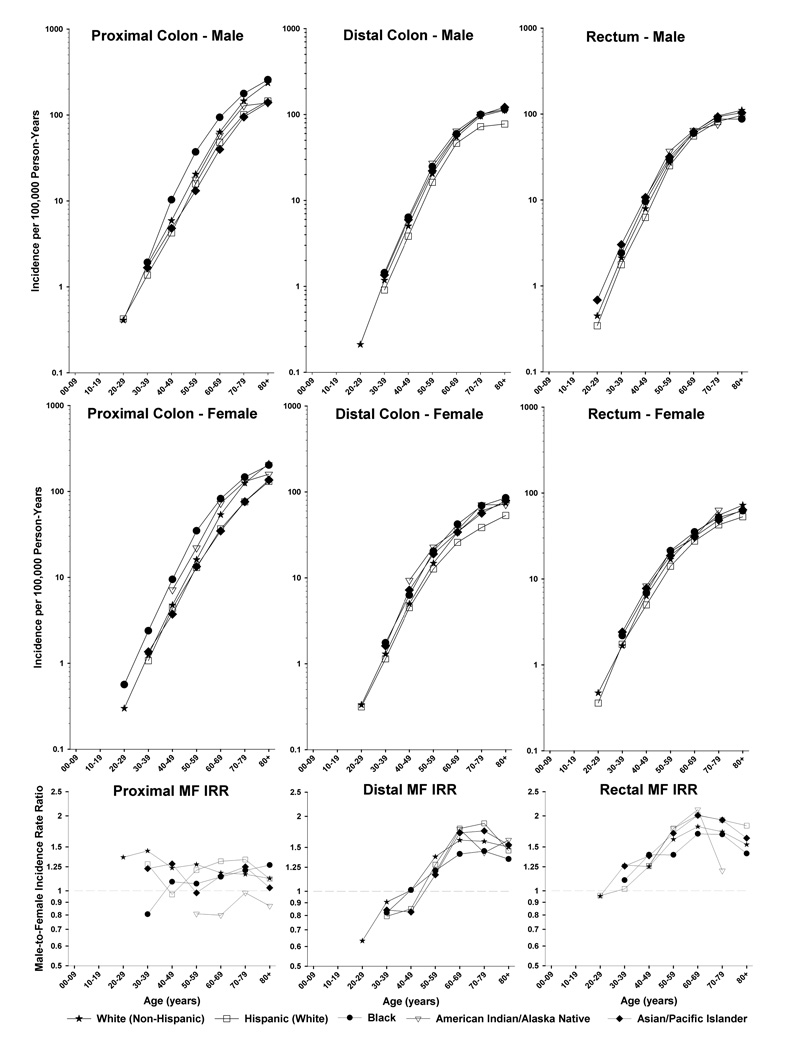
Female cases generally were older (both mean and median age) than males cases (by 0–3 years) for each subsite within each race, and the white CRC cases were the oldest and the AI/AN cases were the youngest for both male and female cases at each site (data not shown); these patterns are largely driven by population variations in life expectancy related to noncancer mortality rather than CRC risk. Taking into account the populations at risk, rates of proximal, distal and rectal CRC increased monotonically with age among both males and females (Figure 1). CRC rates were higher for the proximal than distal or rectal sites across all age groups for males and females. Age-specific rates varied little by race/ethnicity except for the black predominance for proximal cancer among both males and females and the deficit of distal colon cancer among Hispanic males and females.
Figure 1.
Colorectal cancer incidence rates by sex, racial/ethnic group, age and subsite and male-female incidence rate ratios by age and subsite, SEER 13, 1992–2006.
The MF IRR for total CRC was highest among Hispanics (IRR= 1.48) and lowest for AI/AN (IRR= 1.19) (Table 2). Among each racial/ethnic group, the MF IRR increased fairly monotonically from close to unity for cecum cancers to 1.81 (Hispanics) for the rectum.
Table 2. Colorectal cancer male-to-female incidence rate ratios by racial/ethnic group and subsite, SEER 13, 1992–2006.
IRRs are based on unrounded rates per 100,000, age-adjusted to the 2000 US standard population (19 age groups).
| White | Black | Asian/Pacific Islander | American Indian/Alaska Native |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-Hispanic | Hispanic | |||||||||
| IRR | 95% CI | IRR | 95% CI | IRR | 95% CI | IRR | 95% CI | IRR | 95% CI | |
| Total | 1.37 | 1.35, 1.38 | 1.48 | 1.43, 1.52 | 1.30 | 1.26, 1.33 | 1.43 | 1.39, 1.47 | 1.19 | 1.07, 1.32 |
| Proximal | 1.17 | 1.15, 1.18 | 1.23 | 1.17, 1.30 | 1.17 | 1.13, 1.22 | 1.13 | 1.08, 1.18 | 0.88 | 0.73, 1.04 |
| Cecum | 1.10 | 1.08, 1.13 | 1.12 | 1.03, 1.21 | 1.10 | 1.03, 1.16 | 1.02 | 0.94, 1.11 | 0.79 | 0.59, 1.05 |
| Ascending | 1.15 | 1.12, 1.18 | 1.26 | 1.14, 1.38 | 1.14 | 1.06, 1.22 | 1.04 | 0.95, 1.13 | 0.86 | 0.60, 1.22 |
| Transverse | 1.27 | 1.24, 1.31 | 1.39 | 1.27, 1.52 | 1.32 | 1.23, 1.41 | 1.32 | 1.22, 1.42 | 0.99 | 0.74, 1.32 |
| Distal | 1.49 | 1.46, 1.51 | 1.55 | 1.46, 1.64 | 1.33 | 1.27, 1.40 | 1.49 | 1.42, 1.56 | 1.43 | 1.18, 1.73 |
| Descending | 1.53 | 1.46, 1.59 | 1.52 | 1.29, 1.78 | 1.36 | 1.23, 1.52 | 1.52 | 1.36, 1.71 | 1.04 | 0.53, 2.00 |
| Sigmoid | 1.48 | 1.45, 1.51 | 1.55 | 1.46, 1.65 | 1.32 | 1.25, 1.40 | 1.48 | 1.41, 1.56 | 1.47 | 1.20, 1.81 |
| Rectal | 1.63 | 1.60, 1.66 | 1.81 | 1.71, 1.91 | 1.53 | 1.46, 1.61 | 1.76 | 1.68, 1.84 | 1.50 | 1.23, 1.84 |
| Rectosigmoid junction | 1.56 | 1.51, 1.61 | 1.68 | 1.52, 1.86 | 1.50 | 1.36, 1.64 | 1.74 | 1.60, 1.90 | 1.37 | 0.93, 2.03 |
| Rectum | 1.66 | 1.63, 1.70 | 1.87 | 1.75, 1.99 | 1.55 | 1.46, 1.65 | 1.76 | 1.67, 1.86 | 1.55 | 1.23, 1.96 |
MF IRRs are plotted by age at diagnosis for each of the three sites in Figure 1. Proximal colon cancer rates were higher among females than males for young Blacks <age 40 years and for AI/AN regardless of age; the MF IRR ranged between 0.92 and 1.23 among Whites, Hispanics, and A/PI, with some suggestion of declines with age among Whites; MF IRRs in other racial/ethnic groups did not vary dramatically with age. The patterns for distal colon and rectum were quite different to patterns for the proximal colon. Among all races, the distal colon MF IRR rose rapidly with age from less than one at ages <50 years to peak between 60–79 years at 1.5 among Blacks, 1.6 among Whites, 1.8 among A/PI and AI/AN, and 1.9 among Hispanics. The MF IRR for rectal cancer rose from approximately one at young ages to peak between ages 60–69 at 1.7 for Blacks, 1.8 for Whites, 2.0 for Hispanics and A/PI, and 2.1 for AI/AN.
Discussion
Total CRC rates for the period from 1992–2006 were highest in Blacks and lowest in Hispanics. The largest number of CRCs for all races/ethnicities occurred in the proximal colon. Among each racial/ethnic group, MF IRR increased monotonically from the cecum to the rectum. MF IRRs rose with age more rapidly for distal cancers than for rectal cancers, peaking at ages 60–69 for rectal cancers and peaking between ages 60–79 years for distal cancers. The MF IRR for proximal colon cancers did not vary dramatically with age, and among Whites, actually declined from 1.4 to 1.2 (though this difference was not significant).
Regarding overall rates of CRC, our data update and expand on previous findings 4, 6. Total CRC rates for Black males and females are substantially higher than those reported for other races. While CRC test use is known to be greater in Whites than Blacks, this difference is eliminated when the data are adjusted for health-care coverage and other factors 14. Interestingly, a recent analysis suggested Blacks were less likely to undergo diagnostic evaluation for screening-detected abnormalities when compared with Whites, but there was little difference in the yield of colorectal neoplasia 15. The racial differences we observed are likely a result of a complex interplay between screening access (such as health-care coverage) and uptake, and etiologic factors across different racial and ethnic populations. The higher prevalence of type 2 diabetes mellitus (a known risk factor for CRC) among Blacks, for example, may possibly explain the higher rate of CRC among Blacks than Whites, particularly for proximal CRC 4, 16, 17.
With the exception of a recent paper 6, nationally representative data relating to CRC incidence rates in minority populations is notable in it’s rarity. Our data also suggest that distal CRC is more common in AI/AN and, worryingly, evidence from the Behavioral Risk Factor Surveillance Survey 2000–2006 indicated lower rates of fecal occult blood testing and colonoscopy in these groups 18. Even in an equal access setting, ethnic discrepancies in screening persist 19, suggesting that some ethnic groups might have greater resistance to the screening process or that the importance of screening is not as effectively conveyed in minority populations. As regards to etiologic factors, high body mass index/body fatness and alcohol intake have been consistently associated with increased risk and physical activity with decreased risk of CRC, and these factors in turn are known to vary by race, sex and socioeconomic status 20, 21.
The monotonic pattern we report of increasing MF IRR from the cecum to the rectum is consistent for all races; the exception noted for AI/AN MF IRR (particularly in the proximal colon) is likely due to lower case numbers lending greater instability to estimates for this group. This general pattern has been noted previously, but little has been hypothesized by way of explanation. Using age-period-cohort (APC) modeling of data from the Danish Cancer Registry, Dubrow et al.,22 described relatively small differences in APC patterns between males and females for the cecum, ascending colon, transverse colon and descending colon but large differences between males and females for the sigmoid colon and rectum. Within sexes, the APC patterns among males suggested a clear distinction between tumors of the colon and those of the rectum, among females, patterns for tumors of the cecum and ascending colon were clearly distinct from those of the other subsites. The authors concluded that given “a sex difference in period trends (which are usually due to changes in cancer registration or diagnostic practices) is unlikely and therefore probably cannot explain sex differences”, they argued that the differences they observed were likely reflective of etiologic distinctions among subsites and between sexes. The consensus from the National Health Interview Survey in the US suggests that, in general, sex does not dictate patient adherence with CRC screening 14; however, earlier results using the same data suggested differences, in that men reported higher use of endoscopy (sigmoidoscopy and colonoscopy) than women generally 23,24. This difference in use of endoscopy may reflect differential referral or acceptance of these tests by sex. Looking at localized, regional and distant SEER CRC stage over time 4 incidence rates by stage track in parallel for White males and females: localized CRC rates increased briefly during the late 1980s and early 1990s (attributed to an increase in awareness and screening following President Ronald Reagan’s CRC diagnosis in 1985) and then stabilize; regional CRC rates for both White males and females decrease gradually from 1985 onwards while distant CRC rates decrease more sharply, again in parallel for White males and females. The more apparent change over the time period is the disparity in distant CRC rates for Black males, compared to White males and Black females compared to White females 4.
It is possible that sex differences in exposure to certain risk factors may modify risk for tumor development at certain sites; however, evidence to support this is sparse. Associations between diet and CRC risk seem to differ very little by sex but some differences have been noted 25. Red meat has been associated with an increased risk of distal (distal colon or rectum), relative to proximal CRC26. Sex stratification has produced equivocal results, in that some studies suggest the effect of red meat on CRC risk is stronger in men than in women 27, while others suggest that risk is higher in women 28, while a recent analysis, in a large cohort, reported no significant interaction by sex for either red or processed meat26. Similarly inconclusive results for a difference in etiology by sex have been noted for vitamin D, calcium and fiber 25. Alcohol consumption has been associated with increased CRC risk in males and, though evidence is mixed, the association may be stronger for rectal cancer than colon cancer29–31. Smoking also appears to be a stronger risk factor for rectal, than for colon, cancer29. The inverse association between physical activity and risk of CRC is well documented 30, and evidence from a large cohort of men and women in Sweden suggests that this association may differ by both sex and subsite, in that the protective effect in women is greatest in the proximal and middle/transverse colon, whereas the protective effect in men is largely confined to the distal colon 32. Taken together, these data suggest that exposure to dietary and lifestyle related risk factors for CRC may differ by sex, however, these exposures are likely acting differently at various locations across the colon as a result of subsite differences in morphology, enzyme expression, fermentation, transit time and metabolism of bile acids33.
The differing embryological origin of the proximal (embryonic midgut) and distal (embryonic hindgut) colon has led to suspicions that colonic regions may be molecularly distinct. Gene expression profiling has suggested only modest differences between the mucosal epithelium from the proximal and distal regions 34. Interestingly, normal colorectal mucosa has been shown to exhibit sex- and subsite-specific susceptibility to DNA methylation, specifically at the promoter region of hMLH1 and MGMT, genes critical to the maintenance of DNA stability 35. High-level microsatellite instability (MSI-H), as demonstrated in approximately 15% of sporadic colorectal cancers, also displays sex-specificity in that MSI-H (sporadic) tumors occur predominantly in older females and 90% of these sporadic MSI-H tumors occur in the proximal colon36, 37. Differential expression of hormonal and other receptors across the length of the colon and rectum could conceivably modulate risk in a sex- and subsite-specific manner, which may change with age (female menopause), and some investigations have focused on expression of estrogen receptors α and β across the colon 38–40 however the etiological role of these receptors is not well understood.
Our study is an analysis of registry data and as such is subject to the usual limits incurred with these data: non-review of histopathologic diagnoses, the potential for incomplete data collection and minor inconsistencies in tumor classification as a result of changing staging systems over time. Likewise, SEER data are descriptive only and as such do not allow for any assessment of etiology/causality. While we aimed to investigate CRC rates by sex across anatomic subsite, in a number of racial/ethnic groups, the number of AI/AN CRC cases is small, particularly in the context of sub-group analyses. We chose to present MF IRR in addition to absolute incidence rates as they are less likely to be affected by changes in diagnostic techniques, tumor definitions and coding practices 41.
It seems likely that the increasing MF IRR from cecum to rectum may result from a myriad of interactions between changing colonic histology across subsites, related genetic and molecular changes, and possible sex-specific exposure to, or metabolism of, environmental risk factors (such as red meat or physical activity) for CRC. It is likely that differences in screening experiences and access to medical care may also have a role to play in this sex-specific pattern of incidence. The same remarkable monotonic trend in MF IRR is observed across each of racial/ethnic groups studied and could have consequences for targeted screening approaches as well as for future etiologic investigations. Large scale studies or consortial efforts are required to investigate the relationship between CRC risk by subsite and sex.
Supplementary Material
Acknowledgements
The authors would like to express their sincere gratitude to Sabah Quraishi at the Hormonal and Reproductive Epidemiology Branch of the Division of Cancer Epidemiology and Genetics at the National Cancer Institute for her technical assistance with the figure included in this manuscript.
Funding: Intramural Research Program, Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, Department of Health and Human Services.
Abbreviations
- AI/AN
American Indian/Alaska Native
- A/PI
Asian/Pacific Islander
- APC
age period cohort
- CRC
colorectal cancer
- IRR
incidence rate ratio
- MF
male-female
- SEER
Surveillance, Epidemiology, and End Results
Footnotes
Disclosure of Potential Conflicts of Interest: None to declare.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA: a cancer journal for clinicians. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Cheng X, Chen VW, Steele B, Ruiz B, Fulton J, Liu L, Carozza SE, Greenlee R. Subsite-specific incidence rate and stage of disease in colorectal cancer by race, gender, and age group in the United States, 1992–1997. Cancer. 2001;92:2547–2554. doi: 10.1002/1097-0142(20011115)92:10<2547::aid-cncr1606>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 3.Devesa SS, Chow WH. Variation in colorectal cancer incidence in the United States by subsite of origin. Cancer. 1993;71:3819–3826. doi: 10.1002/1097-0142(19930615)71:12<3819::aid-cncr2820711206>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 4.Irby K, Anderson WF, Henson DE, Devesa SS. Emerging and widening colorectal carcinoma disparities between Blacks and Whites in the United States (1975–2002) Cancer Epidemiol Biomarkers Prev. 2006;15:792–797. doi: 10.1158/1055-9965.EPI-05-0879. [DOI] [PubMed] [Google Scholar]
- 5.Perdue DG, Perkins C, Jackson-Thompson J, Coughlin SS, Ahmed F, Haverkamp DS, Jim MA. Regional differences in colorectal cancer incidence, stage, and subsite among American Indians and Alaska Natives, 1999–2004. Cancer. 2008;113:1179–1190. doi: 10.1002/cncr.23726. [DOI] [PubMed] [Google Scholar]
- 6.Rim SH, Seeff L, Ahmed F, King JB, Coughlin SS. Colorectal cancer incidence in the United States, 1999–2004: an updated analysis of data from the National Program of Cancer Registries and the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115:1967–1976. doi: 10.1002/cncr.24216. [DOI] [PubMed] [Google Scholar]
- 7.Gao YT, Jin F, Xiang Y, Zhang W, Lu W, Zheng Y, Gu K, Bao P, Song G, Han M. Cancer Incidence in Shanghai, China (1998–2002) IARC. 2007 [Google Scholar]
- 8.Ji BT, Devesa SS, Chow WH, Jin F, Gao YT. Colorectal cancer incidence trends by subsite in urban Shanghai, 1972–1994. Cancer Epidemiol Biomarkers Prev. 1998;7:661–666. [PubMed] [Google Scholar]
- 9.Center MM, Jemal A, Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2009;18:1688–1694. doi: 10.1158/1055-9965.EPI-09-0090. [DOI] [PubMed] [Google Scholar]
- 10.Troisi RJ, Freedman AN, Devesa SS. Incidence of colorectal carcinoma in the U.S.: an update of trends by gender, race, age, subsite, and stage, 1975–1994. Cancer. 1999;85:1670–1676. [PubMed] [Google Scholar]
- 11.Siegel RL, Jemal A, Ward EM. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18:1695–1698. doi: 10.1158/1055-9965.EPI-09-0186. [DOI] [PubMed] [Google Scholar]
- 12.ICD-O-3 Coding Materials. http://seer.cancer.gov/icd-o-3/
- 13.Tiwari RC, Ghosh K, Jemal A, Hachey M, Ward E, Thun MJ, Feuer EJ. A new method of predicting US and state-level cancer mortality counts for the current calendar year. CA: a cancer journal for clinicians. 2004;54:30–40. doi: 10.3322/canjclin.54.1.30. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro JA, Seeff LC, Thompson TD, Nadel MR, Klabunde CN, Vernon SW. Colorectal cancer test use from the 2005 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2008;17:1623–1630. doi: 10.1158/1055-9965.EPI-07-2838. [DOI] [PubMed] [Google Scholar]
- 15.Laiyemo AO, Doubeni C, Pinsky PF, Doria-Rose VP, Bresalier R, Lamerato LE, Crawford ED, Kvale P, Fouad M, Hickey T, Riley T, Weissfeld J, Schoen RE, Marcus PM, Prorok PC, Berg CD. Race and Colorectal Cancer Disparities: Health-Care Utilization vs Different Cancer Susceptibilities. J. Natl. Cancer Inst. 2010 doi: 10.1093/jnci/djq068. djq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiderpass E, Gridley G, Nyren O, Ekbom A, Persson I, Adami HO. Diabetes mellitus and risk of large bowel cancer. Journal of the National Cancer Institute. 1997;89:660–661. doi: 10.1093/jnci/89.9.660. [DOI] [PubMed] [Google Scholar]
- 17.Hu FB, Manson JE, Liu S, Hunter D, Colditz GA, Michels KB, Speizer FE, Giovannucci E. Prospective study of adult onset diabetes mellitus (type 2) and risk of colorectal cancer in women. Journal of the National Cancer Institute. 1999;91:542–547. doi: 10.1093/jnci/91.6.542. [DOI] [PubMed] [Google Scholar]
- 18.Steele CB, Cardinez CJ, Richardson LC, Tom-Orme L, Shaw KM. Surveillance for health behaviors of American Indians and Alaska Natives-findings from the behavioral risk factor surveillance system, 2000–2006. Cancer. 2008;113:1131–1141. doi: 10.1002/cncr.23727. [DOI] [PubMed] [Google Scholar]
- 19.Brounts LR, Lehmann RK, Lesperance KE, Brown TA, Steele SR. Improved rates of colorectal cancer screening in an equal access population. Am J Surg. 2009;197:609–612. doi: 10.1016/j.amjsurg.2008.12.006. discussion 12-3. [DOI] [PubMed] [Google Scholar]
- 20.Parker SL, Davis KJ, Wingo PA, Ries LA, Heath CW., Jr Cancer statistics by race and ethnicity. CA: a cancer journal for clinicians. 1998;48:31–48. doi: 10.3322/canjclin.48.1.31. [DOI] [PubMed] [Google Scholar]
- 21.Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Ghafoor A, Thun M. Cancer disparities by race/ethnicity and socioeconomic status. CA: a cancer journal for clinicians. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 22.Dubrow R, Johansen C, Skov T, Holford TR. Age-period-cohort modelling of large-bowel-cancer incidence by anatomic sub-site and sex in Denmark. International journal of cancer. 1994;58:324–329. doi: 10.1002/ijc.2910580303. [DOI] [PubMed] [Google Scholar]
- 23.Breen N, Wagener DK, Brown ML, Davis WW, Ballard-Barbash R. Progress in cancer screening over a decade: results of cancer screening from the 1987, 1992, and 1998 National Health Interview Surveys. Journal of the National Cancer Institute. 2001;93:1704–1713. doi: 10.1093/jnci/93.22.1704. [DOI] [PubMed] [Google Scholar]
- 24.Meissner HI, Breen N, Klabunde CN, Vernon SW. Patterns of colorectal cancer screening uptake among men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2006;15:389–394. doi: 10.1158/1055-9965.EPI-05-0678. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs ET, Thompson PA, Martinez ME. Diet, gender, and colorectal neoplasia. J Clin Gastroenterol. 2007;41:731–746. doi: 10.1097/MCG.0b013e3180338e56. [DOI] [PubMed] [Google Scholar]
- 26.Cross AJ, Ferrucci LM, Risch A, Graubard BI, Ward MH, Park Y, Hollenbeck AR, Schatzkin A, Sinha R. A large prospective study of meat consumption and colorectal cancer risk: an investigation of potential mechanisms underlying this association. Cancer Res. 70:2406–2414. doi: 10.1158/0008-5472.CAN-09-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chao A, Thun MJ, Connell CJ, McCullough ML, Jacobs EJ, Flanders WD, Rodriguez C, Sinha R, Calle EE. Meat consumption and risk of colorectal cancer. JAMA. 2005;293:172–182. doi: 10.1001/jama.293.2.172. [DOI] [PubMed] [Google Scholar]
- 28.Gaard M, Tretli S, Loken EB. Dietary factors and risk of colon cancer: a prospective study of 50,535 young Norwegian men and women. Eur J Cancer Prev. 1996;5:445–454. [PubMed] [Google Scholar]
- 29.Poynter JN, Haile RW, Siegmund KD, Campbell PT, Figueiredo JC, Limburg P, Young J, Le Marchand L, Potter JD, Cotterchio M, Casey G, Hopper JL, et al. Associations between smoking, alcohol consumption, and colorectal cancer, overall and by tumor microsatellite instability status. Cancer Epidemiol Biomarkers Prev. 2009;18:2745–2750. doi: 10.1158/1055-9965.EPI-09-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Cancer Research Fund/American Institute for Cancer Research, Food, Nutrition, Physical Activity and the Prevention of Cancer: a Global Perspective. AICR. 2007 [Google Scholar]
- 31.Moskal A, Norat T, Ferrari P, Riboli E. Alcohol intake and colorectal cancer risk: a dose-response meta-analysis of published cohort studies. International journal of cancer. 2007;120:664–671. doi: 10.1002/ijc.22299. [DOI] [PubMed] [Google Scholar]
- 32.Moradi T, Gridley G, Bjork J, Dosemeci M, Ji BT, Berkel HJ, Lemeshow S. Occupational physical activity and risk for cancer of the colon and rectum in Sweden among men and women by anatomic subsite. Eur J Cancer Prev. 2008;17:201–208. doi: 10.1097/CEJ.0b013e3282b6fd78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iacopetta B. Are there two sides to colorectal cancer? International journal of cancer. 2002;101:403–408. doi: 10.1002/ijc.10635. [DOI] [PubMed] [Google Scholar]
- 34.Glebov OK, Rodriguez LM, Nakahara K, Jenkins J, Cliatt J, Humbyrd CJ, DeNobile J, Soballe P, Simon R, Wright G, Lynch P, Patterson S, et al. Distinguishing right from left colon by the pattern of gene expression. Cancer Epidemiol Biomarkers Prev. 2003;12:755–762. [PubMed] [Google Scholar]
- 35.Menigatti M, Truninger K, Gebbers JO, Marbet U, Marra G, Schar P. Normal colorectal mucosa exhibits sex- and segment-specific susceptibility to DNA methylation at the hMLH1 and MGMT promoters. Oncogene. 2009;28:899–909. doi: 10.1038/onc.2008.444. [DOI] [PubMed] [Google Scholar]
- 36.Young J, Simms LA, Biden KG, Wynter C, Whitehall V, Karamatic R, George J, Goldblatt J, Walpole I, Robin SA, Borten MM, Stitz R, et al. Features of colorectal cancers with high-level microsatellite instability occurring in familial and sporadic settings: parallel pathways of tumorigenesis. Am J Pathol. 2001;159:2107–2116. doi: 10.1016/S0002-9440(10)63062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soreide K, Janssen EA, Soiland H, Korner H, Baak JP. Microsatellite instability in colorectal cancer. Br J Surg. 2006;93:395–406. doi: 10.1002/bjs.5328. [DOI] [PubMed] [Google Scholar]
- 38.Nussler NC, Reinbacher K, Shanny N, Schirmeier A, Glanemann M, Neuhaus P, Nussler AK, Kirschner M. Sex-specific differences in the expression levels of estrogen receptor subtypes in colorectal cancer. Gend Med. 2008;5:209–217. doi: 10.1016/j.genm.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Barone M, Tanzi S, Lofano K, Scavo MP, Guido R, Demarinis L, Principi MB, Bucci A, Di Leo A. Estrogens, phytoestrogens and colorectal neoproliferative lesions. Genes Nutr. 2008;3:7–13. doi: 10.1007/s12263-008-0081-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Konstantinopoulos PA, Kominea A, Vandoros G, Sykiotis GP, Andricopoulos P, Varakis I, Sotiropoulou-Bonikou G, Papavassiliou AG. Oestrogen receptor beta (ERbeta) is abundantly expressed in normal colonic mucosa, but declines in colon adenocarcinoma paralleling the tumour's dedifferentiation. Eur J Cancer. 2003;39:1251–1258. doi: 10.1016/s0959-8049(03)00239-9. [DOI] [PubMed] [Google Scholar]
- 41.Nicholson WJ, Davis DL. Analyses of changes in the ratios of male-to-female cancer mortality. A hypothesis-generating exercise. Ann N Y Acad Sci. 1990;609:290–297. doi: 10.1111/j.1749-6632.1990.tb32076.x. discussion 7-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.