Structural fibrils constitute a physiological component of the bone marrow stromal microenvironment and contribute to providing a connective tissue structure and a support for hematopoietic progenitor cells.1 The most common fibers in the bone marrow are reticulin and collagen type I/III. Bone marrow biopsy sections can be examined for stromal fibers using a silver impregnation technique such as Gomori’s stain, reticulin fibers having a smaller diameter and a greater content of interfibrillar material compared to collagen.2
A wide variety of benign conditions and malignant disorders are associated with a pathological increase in bone marrow stromal fibers.1 Among hematologic malignancies, several myeloid neoplasms, as defined by the World Health Organization (WHO) classification, are associated with an increase in bone marrow fibrosis, including myeloproliferative disorders (primary myelofibrosis, myelofibrosis secondary to thrombocythemia or polycythemia, BCR-ABL1+ chronic myelogenous leukemia), myelodysplastic/myeloproliferative (MDS/MPN) disorders (chronic myelomonocytic leukemia, refractory anemia with ring sideroblasts and thrombocytosis) and acute leukemia (acute megakaryoblastic leukemia, acute pan-myelosis with myelofibrosis). In addition, bone marrow fibrosis is described in a proportion of patients with myelodysplastic syndromes (MDS).3
The pathophysiology of bone marrow fibrosis in these disorders is just beginning to be elucidated. Most diseases with increased bone marrow fibrosis are associated with abnormalities of the number and/or function of megakaryocytes and platelets.4 Cytokines from megakaryocytes and platelets appear to be necessary, but perhaps not sufficient, for fibrosis to occur. A growing body of evidence suggests that transforming growth factor-β, a potent stimulator of fibroblast collagen synthesis, plays a key role in determining a pathologically increased deposition of bone marrow stromal fibers, but it is likely that other cell types, cytokines and growth factors are also involved.4
The clinical implications of increased reticulin seem to be different from those of increased collagen: the amount of bone marrow reticulin shows little correlation with the severity of the underlying hematologic disease while the presence and amount of collagen fibers are strongly correlated with abnormal blood counts and poor outcome. Moreover, reticulin fibrosis is often reversible after therapeutic intervention, while collagen fibrosis is less likely to be modified by treatment.1
The use of a clear histological terminology in the definition of bone marrow fibrosis is very important. Until recently the assessment of bone marrow fibrosis was mainly based on subjective evaluations by individual pathologists using different grading systems and methods of processing the trephine biopsies.1,5 In 2005, a group of European experts (European Myelofibrosis Network, EUM-NET) formulated a consensus-based proposal for a semi-quantitative evaluation of bone marrow fibrosis with the aims of avoiding excessive overlapping and achieving a high degree of reproducibility in clinical practice.6 Grading of myelofibrosis was simplified by introducing four categories (including normal reticulin density) and differentiating between reticulin and collagen fibers. Given the high reproducibility documented in the original study as well as by independent investigations in the setting of hematologic malignancies,7 the EUMNET criteria represent a reliable instrument to evaluate the impact of pathologically increased bone marrow structural fibers in different clinical conditions.
The evaluation of bone marrow fibrosis in myeloid neoplasms is relevant not only in the diagnostic work-up but also in prognostic assessment. In primary myelofibrosis the presence of type I collagen in the bone marrow is associated with a poorer prognosis.7 In essential thrombocythemia, elevated reticulin levels at presentation predict high rates of thrombosis, major bleeding and myelofibrotic transformation.8 Finally, myelofibrosis was shown to be a significant predictor of therapeutic efficacy in chronic myelogenous leukemia, including engraftment after transplantation.9
The clinical significance of bone marrow fibrosis in MDS patients remains to be clarified. The current classification of MDS is based on morphological evaluation of bone marrow dysplasia and does not take into account histological features.3 However, histological information complements the morphological information obtained from a marrow aspirate and diagnostic guidelines recommend that a biopsy should be performed in all cases of suspected MDS in which bone marrow examination is indicated.10
Histological parameters are promising candidates to improve the diagnostic and prognostic accuracy of the WHO classification. Indeed, the occurrence of abnormal localization of immature myeloid precursors (ALIP) as well as the presence of CD34+ cell aggregates have been shown to be associated with an increased risk of leukemic transformation in MDS.
In the era of the French-American-British classification, several studies evaluated the presence of bone marrow fibrosis in MDS and its clinical relevance, leading however to conflicting results due to the heterogeneity of patients included in each series and of the grading systems adopted. Fibrosis was reported in 12–50% of cases, and some authors suggested that the presence of stromal fiber abnormalities may identify a group of patients with a negative prognosis.11
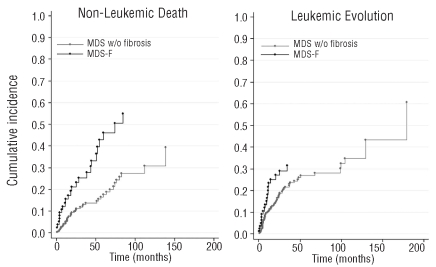
Recent investigations clarified the prevalence and the clinical impact of bone marrow fibrosis in MDS classified according to the WHO criteria.12,13 The occurrence of mild fibrosis (defined as a loose network of reticulin fibers by EUMNET grading on myelofibrosis) is a common feature at diagnosis in these patients and does not correlate with specific clinical features. Conversely, moderate-to-severe fibrosis (defined as a diffuse and dense increase in reticulin with bundles of collagen and/or osteosclerosis) is present in about 10–20% of patients and is closely associated with multilineage dysplasia, profound cytopenia, leading to high red cell/platelet transfusion needs, and poor cytogenetics (Figure 1).12 The survival of patients with moderate-to-severe fibrosis is significantly worse than that of patients with no or mild fibrosis, both because of an increase of non-leukemic death (mainly a consequence of profound marrow failure) and because of the increased rate of leukemic evolution (Figure 2).12,13
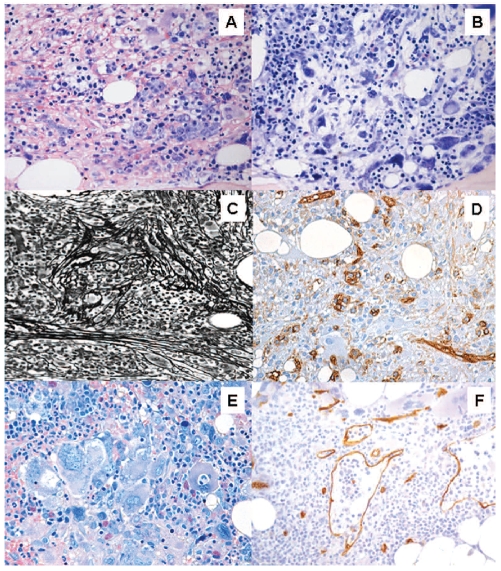
Figure 1.
Typical histological findings in myelodysplastic syndrome with bone marrow fibrosis (MDS-F). These include: (A) increased bone marrow cellularity with erythroid hyperplasia, (B) dysplastic megakaryocytes (such as hypolobulated megakaryocytes) with uncommon sizeable clusters, (C) bone marrow fibrosis (Gomori’s silver impregnation) and (D) the presence of clusters of CD34+ cells. A critical issue in clinical practice is the differential diagnosis from primary myelofibrosis. High bone marrow cellularity, increased bone marrow CD34+ cells and multilineage dysplasia are closely associated with MDS-F. Distinctive features of primary myelofibrosis are (E) megakaryocytic hyperplasia with megakaryocyte clusters and cloud-like or balloon-shaped megakaryocytic nuclei, and (F) dilation of marrow sinuses with intrasinusoidal hematopoiesis (Courtesy of Emanuela Boveri).
Figure 2.
Cumulative incidence of non-leukemic death (A) and evolution into acute myeloid leukemia (B) by competing risk analysis among 590 patients given a diagnosis of primary MDS at the Department of Hematology and Oncology, Policlinico San Matteo, Pavia Italy, 1995–2009, in which a bone marrow biopsy was performed. Patients were stratified according to the presence of moderate-to-severe bone marrow fibrosis (EUMNET criteria on grading myelofibrosis6). This analysis allows an estimate to be made of the cumulative incidence of a specified failure mode, compared to its competing risk over time. MDS w/o fibrosis: MDS with no or mild fibrosis; MDS-F: MDS with moderate-to-severe fibrosis
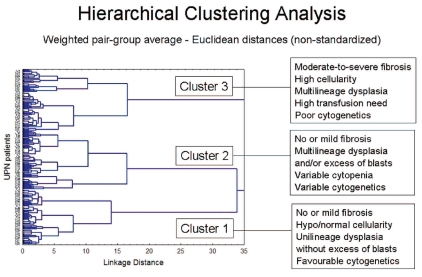
At present, MDS with fibrosis (MDS-F) are not recognized as a distinctive subtype in the WHO classification, and are included under the category “MDS, unclassifiable”.3 However, some evidence suggests that MDS-F should be considered as a distinct clinical entity. By using a clustering analysis, which allows the identification of subgroups of homogeneous patients in an unsupervised manner based on both clinical variables and histological parameters, patients with moderate-to-severe fibrosis were classified into a clearly distinct group characterized by multilineage dysplasia, increased cellularity, high transfusion need and poor prognosis, whereas those with no or mild bone marrow fibrosis segregated into two other subsets including patients with unilineage dysplasia and with multilineage dysplasia or excess blasts, respectively (Figure 3).12
Figure 3.
Unsupervised clustering analysis of MDS patients according to clinical and histological features (data on 301 consecutive patients given a diagnosis of primary MDS at the Department of Hematology Oncology, Policlinico San Matteo, Pavia Italy, 2000–2006). Based on data from Della Porta et al.12
Although MDS-F can share some features with primary myelofibrosis, cytogenetic features and molecular markers differ substantially between the two diseases. JAK/STAT pathway mutations are found in a significant proportion of patients with Philadelphia-negative myeloproliferative neoplasms and MDS/MPN disorders and predict the risk of major clinical events in these patients.3 By contrast, these mutations are found to be a rare event in MDS-F suggesting that they probably do not have a pathogenic role in these patients.12 Whether MDS-F represents a pure myelodysplastic disorder rather than a MDS/MPN disorder remains to be clarified.
In this issue of the journal, Kroger et al., on behalf of the European Group for Blood and Marrow Transplantation (EBMT), addressed the issue of the prognostic effect of bone marrow fibrosis in MDS patients who underwent allogeneic stem cell transplantation (SCT).14
Information regarding the impact of bone marrow fibrosis on outcome after allogeneic SCT for these MDS patients is limited.15 Historical observations suggested that bone marrow fibrosis might affect hematopoietic reconstitution after allogeneic SCT. In this EBMT study, the authors found a higher risk of graft failure and delayed neutrophil engraftment as well as a significantly higher risk of relapse in patients with severe bone marrow fibrosis compared to in those with no or moderate fibrosis, resulting in a significantly reduced disease-free survival in the former.
Although this study did not adopt the EUMNET scoring system and bone marrow fibrosis was assessed using heterogeneous criteria, the results clearly demonstrate that severe bone marrow fibrosis is an independent risk factor for reduced survival after transplantation.
The results of this study may have relevant implications and may contribute to improving our decision-making for young patients with MDS. In fact, although allogeneic SCT is the only curative treatment for MDS, there is still considerable uncertainty regarding the best timing and transplant modalities. Careful selection of candidate recipients and evidence-based evaluation of risks and benefits are mandatory in order to improve transplant outcome.16,17 This is crucial for patients with low or intermediate-1 risk according to the International Prognostic Scoring Systemic (IPSS), most of whom enjoy a long period after diagnosis without obvious disease progression. In general, for these patients, the risk of immediate morbidity and mortality associated with transplantation is unacceptably high and a delayed transplantation strategy is commonly adopted.18 However, this subset of patients is extremely heterogeneous, and selection of those at high risk of disease progression is essential in order not to put at risk their eligibility for the transplant procedure or to jeopardize its potential benefit.
Recently multilineage dysplasia, transfusion-dependency and moderate-to-severe bone marrow fibrosis were proven to identify subsets of patients with low or intermediate-1 IPSS risk with a significantly worse survival and higher risk of leukemic progression.12,19,20 The study by Kroger et al. appears to substantiate the concept that MDS-F is an aggressive disease, and shows that despite the poor outcome, allogeneic SCT remains a potentially curative option for these patients.
Collectively, these data suggest that in patients with low or intermediate-1 IPSS risk and multilineage dysplasia, transfusion-dependency or moderate-to-severe bone marrow fibrosis, an early transplant should be considered when possible.
Much work does, however, remain to be done. The poor results observed in patients with severe bone marrow fibrosis in the study by Kroger et al. (18% 3-year disease-free survival) strongly support the need for further investigations aimed at improving this outcome. Such investigations must encompass an evaluation of the role of pre-transplant treatments, including hypomethylating agents and intensive chemotherapy, the most appropriate intensity of the preparative regimen and possible post-transplantation interventions aimed at preventing relapse.
Footnotes
Financial and other disclosures provided by the author using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are available with the full text of this paper at www.haematologica.org.
References
- 1.Kuter DJ, Bain B, Mufti G, Bagg A, Hasserjian RP. Bone marrow fibrosis: pathophysiology and clinical significance of increased bone marrow stromal fibres. Br J Haematol. 2007;139(3):351–62. doi: 10.1111/j.1365-2141.2007.06807.x. [DOI] [PubMed] [Google Scholar]
- 2.Gomori G. Silver impregnation of reticulum in paraffin sections. Am J Pathol. 1937;13(6):993–1002. 5. [PMC free article] [PubMed] [Google Scholar]
- 3.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;1145(5):937–51. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 4.Tefferi A. Pathogenesis of myelofibrosis with myeloid metaplasia. J Clin Oncol. 2005;23(33):8520–30. doi: 10.1200/JCO.2004.00.9316. [DOI] [PubMed] [Google Scholar]
- 5.Bauermeister DE. Quantitation of bone marrow reticulin--a normal range. Am J Clin Pathol. 1971;56(1):24–31. doi: 10.1093/ajcp/56.1.24. [DOI] [PubMed] [Google Scholar]
- 6.Thiele J, Kvasnicka HM, Facchetti F, Franco V, van der Walt J, Orazi A. European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica. 2005;90(8):1128–32. [PubMed] [Google Scholar]
- 7.Vener C, Fracchiolla NS, Gianelli U, Calori R, Radaelli F, Iurlo A, et al. Prognostic implications of the European consensus for grading of bone marrow fibrosis in chronic idiopathic myelofibrosis. Blood. 2008;111(4):1862–5. doi: 10.1182/blood-2007-09-112953. [DOI] [PubMed] [Google Scholar]
- 8.Campbell PJ, Bareford D, Erber WN, Wilkins BS, Wright P, Buck G, et al. Reticulin accumulation in essential thrombocythemia: prognostic significance and relationship to therapy. J Clin Oncol. 2009;27 (18):2991–9. doi: 10.1200/JCO.2008.20.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buesche G, Hehlmann R, Hecker H, Heimpel H, Heinze B, Schmeil A, et al. Marrow fibrosis, indicator of therapy failure in chronic myeloid leukemia - prospective long-term results from a randomized-controlled trial. Leukemia. 2003;17(12):2444–53. doi: 10.1038/sj.leu.2403172. [DOI] [PubMed] [Google Scholar]
- 10.Bowen D, Culligan D, Jowitt S, Kelsey S, Mufti G, Oscier D, et al. Guidelines for the diagnosis and therapy of adult myelodysplastic syndromes. Br J Haematol. 2003;120(2):187–200. doi: 10.1046/j.1365-2141.2003.03907.x. [DOI] [PubMed] [Google Scholar]
- 11.Lambertenghi-Deliliers G, Orazi A, Luksch R, Annaloro C, Soligo D. Myelodysplastic syndrome with increased marrow fibrosis: a distinct clinico-pathological entity. Br J Haematol. 1991;78(2):161–6. doi: 10.1111/j.1365-2141.1991.tb04411.x. [DOI] [PubMed] [Google Scholar]
- 12.Della Porta MG, Malcovati L, Boveri E, Travaglino E, Pietra D, Pascutto C, et al. Clinical relevance of bone marrow fibrosis and CD34-positive cell clusters in primary myelodysplastic syndromes. J Clin Oncol. 2009;27(5):754–62. doi: 10.1200/JCO.2008.18.2246. [DOI] [PubMed] [Google Scholar]
- 13.Buesche G, Teoman H, Wilczak W, Ganser A, Hecker H, Wilkens L, et al. Marrow fibrosis predicts early fatal marrow failure in patients with myelodysplastic syndromes. Leukemia. 2008;22(2):313–22. doi: 10.1038/sj.leu.2405030. [DOI] [PubMed] [Google Scholar]
- 14.Kroger N, Zabelina T, van Biezen A, Brand R, Niederwieser D, Martino R, et al. Allogeneic stem cell transplantation for myelodysplastic syndromes with bone marrow fibrosis. Haematologica. 2010;96(2):291–7. doi: 10.3324/haematol.2010.031229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott BL, Storer BE, Greene JE, Hackman RC, Appelbaum FR, Deeg HJ. Marrow fibrosis as a risk factor for posttransplantation outcome in patients with advanced myelodysplastic syndrome or acute myeloid leukemia with multilineage dysplasia. Biol Blood Marrow Transplant. 2007;13(3):345–54. doi: 10.1016/j.bbmt.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 16.Alessandrino EP, Della Porta MG, Bacigalupo A, Van Lint MT, Falda M, Onida, et al. WHO classification and WPSS predict posttransplantation outcome in patients with myelodysplastic syndrome: a study from the Gruppo Italiano Trapianto di Midollo Osseo (GITMO) Blood. 2008;112(3):895–902. doi: 10.1182/blood-2008-03-143735. [DOI] [PubMed] [Google Scholar]
- 17.Della Porta MG, Malcovati L, Strupp C, et al. Risk stratification based on both disease status and extra-hematologic comorbidities in patients with myelodysplastic syndrome. Haematologica. 2010 doi: 10.3324/haematol.2010.033506. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cutler CS, Lee SJ, Greenberg P, Deeg HJ, Pérez WS, Anasetti C, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood. 2004;104(2):579–85. doi: 10.1182/blood-2004-01-0338. [DOI] [PubMed] [Google Scholar]
- 19.Malcovati L, Della Porta MG, Pascutto C, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol. 2005;23(30):7594–603. doi: 10.1200/JCO.2005.01.7038. [DOI] [PubMed] [Google Scholar]
- 20.Alessandrino EP, Della Porta MG, Bacigalupo A, Malcovati L, Angelucci E, Van Lint MT, et al. Prognostic impact of pre-transplantation transfusion history and secondary iron overload in patients with myelodysplastic syndrome undergoing allogeneic stem cell transplantation: a GITMO study. Haematologica. 2010;95(3):476–84. doi: 10.3324/haematol.2009.011429. [DOI] [PMC free article] [PubMed] [Google Scholar]