Thrombosis in myeloproliferative neoplasms: few answers and many new questions
Life expectancy of patients with myeloproliferative neoplasms (MPNs) and particularly that of subjects with polycythemia vera (PV) and essential thrombocythemia (ET) has significantly increased over the last three decades, largely due to the use of cytoreductive treatments. Currently, polycythemia vera and essential thrombocythemia are considered relatively benign diseases in which the main objective of treatment strategy is the prevention of thrombotic events. Widespread use of routine hematologic screening and novel diagnostic tools greatly facilitate disease recognition and treatment. This helps to prevent a significant number of early vascular events which still constitute the first disease manifestation in approximately one-third of patients.1 We can also expect that new therapeutic options and appropriate use of aspirin will result in a further reduction of morbidity and mortality. The unmet needs of polycythemia vera and essential thrombocythemia subjects, however, remain significant. These include the availability of more safe and effective therapeutic agents and of validated tools for vascular risk stratification. The evaluation of the thrombotic risk in the individual patients, as underlined by Barbui et al. in their paper in this issue of the Journal,2 is still approximate and generally relies on a limited number of variables. The role of all disease-related abnormalities and of their interaction with vascular risk factors remains largely obscure and difficult to explore in limited size epidemiological studies. In this complex scenario a significant scientific effort has recently been concentrated on studies testing new diagnostic and prognostic tools or aimed at clarifying the pathogenetic mechanisms of myeloproliferative neoplasm associated thrombophilia. This has several particular characteristics which include microcirculatory disturbances and thromboses at atypical sites often manifesting several years before diagnosis.3
Pathophysiology of thrombosis in myeloproliferative neoplasms: what do we know?
The pathogenesis of thrombosis in myeloproliferative neoplasms has been extensively investigated by focusing in particular on the possible contribution of disease related hemostatic abnormalities. However, the pathogenesis of thrombosis is multifactorial and even the relative role of these abnormalities, as compared with that of other individual and environmental factors, is controversial. Quantitative and qualitative red blood cell, platelet, and leukocyte abnormalities are likely to play a key-role in myeloproliferative neoplasm thrombophilia. High shear stress of the vessel wall, due to blood hyperviscosity, accounts for chronic endothelial dysfunction and platelet and leukocyte activation.
The increase of the thrombotic risk observed at progressively higher hematocrit values parallels blood viscosity, although also biochemical changes in the cell membrane and content could contribute to rheological abnormalities.4 In this view, aggregates of red cells, platelets, leukocytes and endothelial cells have already been demonstrated to disturb blood flow and cause ischemia in the cerebral vessels.5 Thrombocytosis can contribute to the vascular events in myeloproliferative neoplasms but qualitative platelet changes are likely to be even more important. Both polycythemia vera and essential thrombocythemia subjects were found to have an increased urinary excretion of the two major thromboxane A2 metabolites, 11-dehydro- and 2,3-dinor-Thromboxane B2, which can be suppressed by low-dose aspirin.6 This finding constituted the biological background for assessing the efficacy of low-dose aspirin in the ECLAP trial.7
Platelets and endothelial cells play a pivotal role in regulating blood flow, both cells might contribute to determine a prothrombotic microenvironment in myeloproliferative neoplasm patients by producing more soluble selectins and less nitric oxide, likely as a consequence of inflammation (see below).8
Leukocytosis has been shown to represent an independent risk factor for thrombosis.9–10 In a retrospective analysis of the ECLAP study, a leukocyte count greater than 10×109/L, adjusted for potential confounders such as cytoreductive and antithrombotic treatment, was associated with an increased rate of thrombosis.9 A similar association was demonstrated in essential thrombocythemia patients.11
However, as for platelets, qualitative abnormalities might play a more critical role in the activation of the hemostatic system. Leukocyte activation in myeloproliferative neoplasm patients is demonstrated by overexpression of CD11b antigen and by the raised plasma levels of serine proteases cathepsin G, elastase and myeloperoxidase.12 Expression of integrins and selectins increases the leukocyte adhesion to endothelium and platelets, stimulates the formation of mixed aggregates and the production of reactive oxygen species and inflammatory cytokines.13
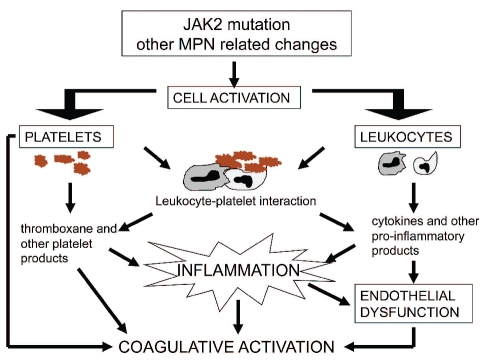
These events link blood cell activation to inflammation and the latter is likely to play a significant role in the thrombotic risk of myeloproliferative neoplasms. Some mechanisms linking cell activation, inflammation and thrombosis are summarized in Figure 1.
Figure 1.
Mechanisms which, in myeloproliferative neoplasms (MPN), can increase the thrombotic risk through cell activation and inflammation.
Seeking novel vascular biomarkers in myeloproliferative neoplasms, the role of inflammatory parameters
The role of inflammation among the pathogenetic mechanisms of myeloproliferative neoplasm thrombophilia is also intriguing for the possible use of inflammatory parameters as vascular biomarkers. Barbui et al.2 tested the hypothesis that blood levels of the inflammatory biomarkers C-reactive protein (CRP) and pentraxin-3 (PTX3) could be correlated with thrombosis and JAK2V617F allele burden in essential thrombocythemia and polycythemia vera subjects.
Within the limitations of the retrospective nature of the study and of the limited number of events, the results do suggest that C-reactive protein and pentraxin-3 measurements deserve scientific attention and might be helpful both for a more accurate risk stratification and for exploring the possible link between inflammation and thrombosis in myeloproliferative neoplasms.
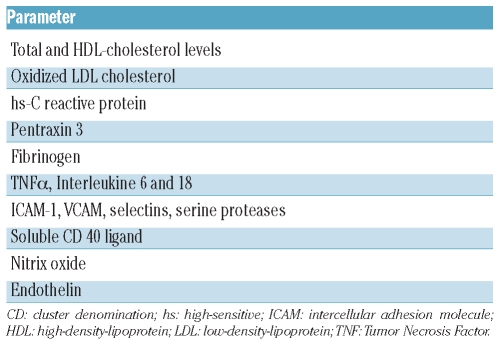
In the general population this link is well established.14–15 Over the last decade, a number of inflammatory biomarkers have been investigated as tools for identifying subjects at high risk for arterial vascular events (Table 1).14–16
Table 1.
More recently, high C-reactive protein levels have been shown to also influence the risk of venous thromboembolism and this has led to the hypothesis of a direct causative role of C-reactive protein on venous thrombophilia.17 A recent study, however, ruled out this role.18 Thus, one has to conclude that an increased inflammatory state can play a causative role in both arterial and venous thromboses.
In the study of Barbui et al.,2 C-reactive protein plasma levels were interestingly related with JAK2 mutation allelic burden thus suggesting a role of this mutation on inflammation and cytokine production, as reflected by C-reactive protein plasma levels. Raised levels of this protein were associated with an increased thrombotic risk but, unfortunately, the size of the study did not allow a comparison to be made on the predictive value of C-reactive protein on venous and arterial thromboses. Also pentraxin-3, like C-reactive protein, was significantly correlated with JAK2 allele burden. But in this case, pentraxin-3 levels higher than 4.5 ng/mL were associated with a lower thrombotic risk.
How can we interpret these data?
For a convincing interpretation of Barbui’s data further investigations both in larger myeloproliferative neoplasm cohorts and in the general population are needed. In fact, compared to C-reactive protein, pentraxin 3 has undergone less extensive investigation and its role is still uncertain.19 Moreover, while C-reactive protein is produced by the liver, pentraxin 3 is produced at the site of inflammation by various cell types, including endothelial cells, neutrophils, monocytes and macrophages, and its level increases in several pathological conditions (sepsis, autoimmune disorders, small vessel vasculitis).19–20
Pentraxin 3 is induced by IL-1, TNF-α, oxidized low-density lipoprotein and lipopolysaccaride and its plasma levels are not correlated with C-reactive protein levels.
Recent studies on humans and mice reported that high levels of pentraxin 3 in cardiovascular diseases seem to reflect a protective response to severe ischemic injury.19–21 Moreover, pentraxin 3 deficiency in mice is associated with increased atherosclerosis and increased macrophage accumulation in atherosclerotic lesions. In addition, myocardial infarcts are significantly larger in PTX3-deficient than in wild-type mice and the use of recombinant pentraxin 3 can reduce heart damage and inflammation and block neointimal thickening after balloon injury of rat carotid arteries.19
It has been hypothesized that an increased pentraxin 3 production is a feedback mechanism which inhibits leukocyte recruitment, modulates complement activation and balances the overactivation of a pro-inflammatory and pro-atherogenic cascade.22 This hypothesis suggests that the increased levels of pentraxin 3 in subjects with cardiovascular diseases could reflect both inflammation and a protective response. On the basis of the model proposed above one can hypothesize that when the pentraxin 3 response to inflammation is blunted by genetic or acquired factors the thrombophilic state linked to inflammation is further enhanced. The hypothesis is too simplistic to apply to all clinical settings but Barbui’s data suggest that it could fit myeloproliferative neoplasm patients.
Perspectives
The study by Barbui et al. raises several questions which need to be addressed by future studies. These questions include the type of thromboses which are more prevalent in subjects having high C-reactive protein, low pentraxin 3 plasma levels or both conditions. This issue seem relevant since inflammation in the general population seems particularly linked with the risk of acute coronary syndromes and also the increased risk associated with high leukocyte count in polycythemia vera was found to be particularly linked with coronary events.9 Another interesting issue is the association of inflammatory biomarkers with JAK2 allelic burden. This association is biologically plausible12–13 but we do need more extensive clinical data to clarify this field also in view of the rather controversial association between the risk of thrombosis and allele burden. According to the data of Barbui et al.,2 it is intriguing to consider the possibility that pentraxin 3 response to inflammation in subjects with high JAK2 burden might contribute to lower or enhance the thrombotic risk. More generally the association between JAK2 mutation, inflammation and thrombotic risk deserves scientific attention also for other speculative and practical purposes. For example, there is still no convincing explanation for the well-known association between venous thromboses at atypical sites and JAK2 mutation in patients who do not meet criteria for myeloproliferative neoplasm diagnosis and this also raises the question of interventional strategies. To date, this relationship suggests a direct influence of the JAK2 mutation on the hemostatic system and raises further expectations from molecules that selectively target JAK2 kinase.
Footnotes
Financial and other disclosures provided by the author using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are available with the full text of this paper at www.haematologica.org.
References
- 1.De Stefano V, Za T, Rossi E, Vannucchi AM, Ruggeri M, Elli E, et al. GIMEMA CMD-Working Party. Recurrent thrombosis in patients with polycythemia vera and essential thrombocythemia: incidence, risk factors, and effect of treatments. Haematologica. 2008;93(3):372–80. doi: 10.3324/haematol.12053. [DOI] [PubMed] [Google Scholar]
- 2.Barbui T, Carobbio A, Finazzi G, Vannucchi AM, Barosi G, Antonioli E, et al. Inflammation and thrombosis in essential thrombocythemia and polycythemia vera: different role of C-reactive protein and Pentraxin 3. Haematologica. 2011;96(2):315–8. doi: 10.3324/haematol.2010.031070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinelli I, De Stefano V. Rare thromboses of cerebral, splanchnic and upper-extremity veins. A narrative review. Thromb Haemost. 2010;103(6):1136–44. doi: 10.1160/TH09-12-0873. [DOI] [PubMed] [Google Scholar]
- 4.Landolfi R, Di Gennaro L, Falanga A. Thrombosis in myeloproliferative disorders: pathogenetic facts and speculation. Leukemia. 2008;22(11):2020–8. doi: 10.1038/leu.2008.253. [DOI] [PubMed] [Google Scholar]
- 5.Wautier MP, El Nemer W, Gane P, Rain JD, Cartron JP, Colin Y, et al. Increased adhesion to endothelial cells of erythrocytes from patients with polycythemia vera is mediated by laminin alpha5 chain and Lu/BCAM. Blood. 2007;110(3):894–901. doi: 10.1182/blood-2006-10-048298. [DOI] [PubMed] [Google Scholar]
- 6.Landolfi R, Ciabattoni G, Patrignani P, Castellana MA, Pogliani E, Bizzi B, et al. Increased thromboxane biosynthesis in patients with polycythemia vera: evidence for aspirin-suppressible platelet activation in vivo. Blood. 1992;80(8):1965–71. [PubMed] [Google Scholar]
- 7.Landolfi R, Marchioli R, Kutti J, Gisslinger H, Tognoni G, Patrono C, et al. Efficacy and safety of low dose aspirin in polycythemia vera. N Engl J Med. 2004;350(2):114–24. doi: 10.1056/NEJMoa035572. [DOI] [PubMed] [Google Scholar]
- 8.Cella G, Marchetti M, Vianello F, Panova-Noeva M, Vignoli A, Russo L, et al. Nitric oxide derivatives and soluble plasma selectins in patients with myeloproliferative neoplasms. Thromb Haemost. 2010;104(1):151–6. doi: 10.1160/TH09-09-0663. [DOI] [PubMed] [Google Scholar]
- 9.Landolfi R, Di Gennaro L, Barbui T, De Stefano V, Finazzi G, Marfisi R, et al. Leukocytosis as a major thrombotic risk factor in patients with Polycythemia Vera. Blood. 2007;109(6):2446–52. doi: 10.1182/blood-2006-08-042515. [DOI] [PubMed] [Google Scholar]
- 10.Carobbio A, Antonioli E, Guglielmelli P, Vannucchi AM, Delaini F, Guerini V, et al. Leukocytosis and risk stratification assessment in essential thrombocythemia. J Clin Oncol. 2008;26(16):2732–6. doi: 10.1200/JCO.2007.15.3569. [DOI] [PubMed] [Google Scholar]
- 11.Carobbio A, Finazzi G, Guerini V, Spinelli O, Delaini F, Marchioli R, et al. Leukocytosis is a risk factor for thrombosis in essential thrombocythemia: interaction with treatment, standard risk factors and JAK2 mutation status. Blood. 2007;109(6):2310–3. doi: 10.1182/blood-2006-09-046342. [DOI] [PubMed] [Google Scholar]
- 12.Falanga A, Marchetti M, Evangelista V, Vignoli A, Licini M, Balicco M, et al. Polymorphonuclear leukocyte activation and hemostasis in patients with essential thrombocythemia and polycythemia vera. Blood. 2000;96(13):4261–6. [PubMed] [Google Scholar]
- 13.Cervantes F, Arellano-Rodrigo E, Alvarez-Larrán A. Blood cell activation in myeloproliferative neoplasms. Haematologica. 2009;94(11):1484–8. doi: 10.3324/haematol.2009.013375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stefanadi E, Tousoulis D, Papageorgiou N, Briasoulis A, Stefanadis C. Inflammatory biomarkers predicting events in atherosclerosis. Curr Med Chem. 2010;17(16):1690–707. doi: 10.2174/092986710791111288. [DOI] [PubMed] [Google Scholar]
- 15.Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355(25):2631–9. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 16.Blankenberg S, Zeller T, Saarela O, Havulinna AS, Kee F, Tunstall-Pedoe H, et al. MORGAM Project. Contribution of 30 biomarkers to 10-year cardiovascular risk estimation in 2 population cohorts: the MONICA, risk, genetics, archiving, and monograph (MORGAM) biomarker project. Circulation. 2010;121(22):2388–97. doi: 10.1161/CIRCULATIONAHA.109.901413. [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, et al. JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 18.Zacho J, Tybjaerg-Hansen A, Nordestgaard BG. C-reactive protein and risk of venous thromboembolism in the general population. Arterioscler Thromb Vasc Biol. 2010;30(8):1672–8. doi: 10.1161/ATVBAHA.109.198473. [DOI] [PubMed] [Google Scholar]
- 19.Norata GD, Garlanda C, Catapano AL. The long pentraxin PTX3: a modulator of the immunoinflammatory response in atherosclerosis and cardiovascular diseases. Trends Cardiovasc Med. 2010;20(2):35–40. doi: 10.1016/j.tcm.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Mantovani A, Garlanda C, Bottazzi B, Peri G, Doni A, Martinez de la Torre Y, et al. The long pentraxin PTX3 in vascular pathology. Vascul Pharmacol. 2006;45(5):326–30. doi: 10.1016/j.vph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Norata GD, Marchesi P, Pulakazhi Venu VK, Pasqualini F, Anselmo A, Moalli F, et al. Deficiency of the long pentraxin PTX3 promotes vascular inflammation and atherosclerosis. Circulation. 2009;120(8):699–708. doi: 10.1161/CIRCULATIONAHA.108.806547. [DOI] [PubMed] [Google Scholar]
- 22.Deban L, Russo RC, Sironi M, Moalli F, Scanziani M, Zambelli V, et al. Regulation of leukocyte recruitment by the long pentraxin PTX3. Nature Immunol. 2010;11(14):328–34. doi: 10.1038/ni.1854. [DOI] [PubMed] [Google Scholar]