Although Hodgkin’s lymphoma (HL) can be considered a successful paradigm of modern treatment strategies, about 15–20% of patients with advanced-stage HL still die following relapse or progressive disease and a similar proportion of patients are over-treated,1,2 leading to treatment-related late sequelae including solid tumors and end-organ dysfunction.3,4 To help guide treatment decisions, the distinction between classical HL and nodular lymphocyte-predominant HL and the separation of limited and advanced-stage disease are widely used in clinical practice. Most patients present with advanced-stage classical HL and for this group of patients the International Prognostic Score was introduced more than a decade ago to improve risk stratification.5 However, neither the International Prognostic Score itself nor any of the individual clinical variables can predict the majority of patients in whom standard therapy will fail to eradicate the disease. Novel biological markers that improve on primary treatment outcome prediction across all clinical stages will, therefore, be critical to advancing the field.
The histological hallmark of HL is the presence of the malignant mononuclear Hodgkin and polynucleated Reed Sternberg (HRS) cells in classical HL and so-called lymphocyte predominant cells in nodular lymphocyte-predominant HL.6 These malignant cells are, however, greatly outnumbered by the reactive cells in the tumor microenvironment.7 Because of the prominent and abnormal immune reaction that creates this variable microenvironment, the biology of HL can be considered unique among lymphomas. Many studies in HL have, therefore, focused on the cellular composition of the microenvironment, not only to gain more insight into the pathobiology of the disease, but also to explore whether these immune-related cells in some way contribute to outcome prediction.
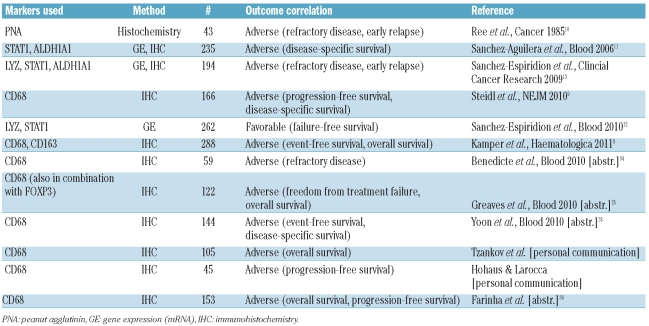
In this issue of the journal, Kamper et al. used immunohistochemistry to investigate a large number (n = 288) of pretreatment biopsies from patients with HL and found a significant correlation between tumor-associated macrophages and adverse treatment outcome. Moreover, they demonstrated a previously unrecognized association of CD68+ macrophages with latent Epstein-Barr virus (EBV) infection of the tumor cells.8 This study, together with others listed in Table 1, convincingly validates similar findings of an earlier study suggesting that the number of CD68+ macrophages can be used as a reliable biomarker in HL.9
Table 1.
Studies on the prognostic value of tumor-associated macrophages in classical Hodgkin’s lymphoma.
Tumor-associated macrophages predict unfavorable treatment outcomes in classical Hodgkin’s lymphoma
Including the study by Kamper et al., multiple reports now support the value of enumerating tumor-associated macrophages in pretreatment biopsies for outcome prediction in classical HL (Table 1). As early as 1985, Ree and Kadin suggested that macrophages in HL biopsies had a prognostic value when they found the number of peanut agglutinin-binding macrophages was correlated with the presence of B symptoms and primary treatment failure.10 However, the authors restricted their analysis to nodular lymphocyte-predominant and mixed cellularity HL. Further evidence of the importance of tumor-associated macrophages was suggested from gene expression profiling studies of larger cohorts of patients, including those with all subtypes of classical HL: these studies identified macrophage signatures as being associated with primary treatment failure.9,11 From these studies, CD68 in particular emerged as the first routinely used immunohistochemical marker detecting a macrophage-associated antigen that was independent from other clinical parameters in multivariate analysis. Of note, the number of CD68+ cells in pretreatment biopsies was not only associated with primary treatment failure, but also with failure of subsequent secondary therapies including autologous stem cell transplantation. Thus far, only one published study has found a correlation of gene expression of macrophage-associated genes with favorable outcome.12 However, in two earlier studies, the authors of this study reported that the same genes, namely LYZ and STAT1, were linked to unfavorable outcome, findings that the authors also validated by immunohistochemistry.11,13 Clarification of these contradictory results is needed.
Lack of reproducibility and inconsistency of scoring have been repeatedly named as potential pitfalls for the routine use of immunohistochemical biomarkers. In the present study Kamper et al. address this issue by employing a semi-quantitative computer-assisted stereological analysis system that records the percentage of positively stained cells in an unbiased way that is free of inter- and intra-observer variability. Although it is reassuring that the correlation of tumor-associated macrophages with adverse treatment outcome was validated using this more objective methodology, the standard use of semi-quantitative methods for the assessment of immunohistochemical biomarkers will be confounded by the general lack of availability of such technologies in the routine laboratory setting.
The phenotype of tumor-associated macrophages and correlation with latent Epstein-Barr virus infection
The authors of the present study also used CD163 as a reportedly more specific marker for alternatively activated macrophages (M2) and found similar correlations with overall and event-free survival as for CD68. Interestingly, both CD68 and CD163, determined by immunohistochemistry, were associated with latent EBV infection of the malignant cells as detected by the presence of EBV-encoded RNA. However, when comparing both immunohistochemical stains, the number of CD163+ cells seemed to be more closely correlated with EBV infection. These associations have not been previously described and warrant further investigations aiming to define the specific phenotypes of tumor-associated macrophages in HL which are possibly linked to EBV infection. In a re-analysis of our data we were able to confirm a relationship between increased tumor-associated macrophages and EBV positivity; however, virtually all of our cases were of the nodular sclerosis subtype and EBV alone was not associated with treatment outcome (unpublished observations, 2010). EBV infection of HRS cells has been reported in up to 60% of patients and is more frequent in mixed cellularity subtype, although varying with geographical location, age, gender, clinical stage and histological subtype.14 The impact of EBV infection on outcome remains controversial, but appears to be dependent on age. Accordingly, many studies support an association between EBV positivity and adverse outcome in older adult patients,15–17 while in younger and, specifically in pediatric patients, EBV positivity has been linked to better outcome.15,18,19 It will be of interest in subsequent studies to assess the interaction between EBV infection and tumor-associated macrophages as outcome predictors in cHL.
The study in this issue of the journal also raises the question of which marker should be used to reliably and reproducibly identify tumor-associated macrophages and which provides the best information for clinical use. Kamper et al. used two different antibodies against macrophage-associated antigens, namely CD68 and CD163, but no firm conclusions could be reached concerning which marker should be preferred. While CD68 is reported to be a pan-macrophage marker with less specificity, CD163 expression is considered more specific for tumor-infiltrating macrophages.20 However, in their study only CD68 staining retained a significant influence on overall survival in multivariate analysis, rather suggesting that CD68 staining could be used for outcome prediction. Further study is needed to determine the optimal antigen (e.g. CD68 versus CD163), anti-CD68 antibody clone (e.g. KP1 versus PGM1) and scoring thresholds (e.g. manual versus computer-assisted) for detecting HL-associated macrophages.
Combination of CD68 with other biomarkers
The prognostic value of individual biomarkers, especially those based on immunohistochemistry, has been repeatedly demonstrated in lymphoid malignancies. In HL, several biomarkers other than CD68 have been reported to be associated with treatment outcome, in particular markers expressed by certain T-cell subsets.21–24 It, therefore, appears to be a logical next step to explore whether multi-gene predictors combining different markers can exceed the performance of individual biomarkers alone with the aim of better outcome prediction following primary treatment in HL. This approach was already tried in some studies using low-density gene expression techniques12,13 or genome-wide gene-expression profiling, developing predictors for favorable and unfavorable treatment outcome.9 The results show some promise; however, widespread application in routine practice is doubtful given the challenges of technical reproducibility. The preliminary results of an immunohistochemistry study combining two markers, CD68 and FOXP3 (a marker for regulatory T cells), were presented at the ASH 2010 meeting. The authors showed that a combined FOXP3/CD68 immunohistochemistry score was an improvement over the predictive value of the individual markers alone and that this score was applicable to both limited and advanced-stage disease. The value of this combined biomarker strategy must await publication of the complete results of the study.25
Perspective
The number of tumor-associated macrophages has been identified as an adverse prognostic factor in many solid tumors.26 In lymphoid cancers, studies of follicular lymphoma and the most recent data from HL confirm that the presence of macrophages as part of the tumor microenvironment reflects specific tumor biology linked to treatment failure.27 However, many questions remain regarding the exact mechanisms of how HRS cells can attract and induce differentiation of monocytes/macrophages, how macrophages contribute to HRS cell growth and how HRS cells might gain immune privilege through this unique interaction. Besides further detailed molecular characterization of HRS cells, it appears that cellular enrichment and characterization of macrophages from primary clinical material will be critical to gain further insight into the unique phenotype and gene expression profile of HL-associated macrophages. Recently, several molecules that have been implicated in macrophage chemotaxis and signaling have been linked to HL pathobiology, including CSF1R, fractalkine, MIF and CD74.28–30 Some of these molecules might be promising targets for novel drug therapies aiming at macrophages directly or at the interface between HRS cells and their tumor microenvironment.
The research towards the goal of better outcome prediction in HL is dominated by two important questions. First, can information on tumor-associated macrophages and other biomarkers be integrated into routine clinical practice in order to facilitate the decision to escalate or de-escalate primary treatment? Secondly, can the same biomarker profile be used to guide the choice of salvage therapy? To resolve these issues it seems reasonable to combine biomarkers such as CD68 with other immunohistochemical and clinical markers (e.g. regulatory T cells, stage) on the basis of pre-existing data derived from retrospective studies. However, integration of biomarkers into prospective clinical trials is desperately needed to convincingly show an improvement compared to risk stratification based solely on clinical parameters. Finally, future studies should examine whether the approach of identifying biomarkers prior to therapy is equal or superior to assessing disease markers during and after treatment, as realized by interim positron emission tomography scanning31 and measuring serum markers (e.g. TARC).32,33 However, based on ten of the 11 studies listed in Table 1, the prognostic relevance of tumor-associated macrophages in HL has been retrospectively validated and now deserves to be analyzed going forward at both biological and clinical levels in the context of prospective clinical trials.
Acknowledgments
This work is supported by a postdoctoral fellowship of the Cancer Research Society (CRS, Steven E. Drabin Fellowship), by the Michael Smith Foundation for Health Research (MSFHR), and the Lymphoma Research Foundation (LRF) to CS. RDG is supported by the Canadian Institutes for Health Research (CIHR #178536).
Footnotes
Financial and other disclosures provided by the author using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are available with the full text of this paper at www.haematologica.org.
References
- 1.Bjorkholm M, Axdorph U, Grimfors G, Merk K, Johansson B, Landgren O, et al. Fixed versus response-adapted MOPP/ABVD chemotherapy in Hodgkin's disease. A prospective randomized trial. Ann Oncol. 1995;6(9):895–9. doi: 10.1093/oxfordjournals.annonc.a059356. [DOI] [PubMed] [Google Scholar]
- 2.Aleman BM, van den Belt-Dusebout AW, Klokman WJ, Van't Veer MB, Bartelink H, van Leeuwen FE. Long-term cause-specific mortality of patients treated for Hodgkin's disease. J Clin Oncol. 2003;21(18):3431–9. doi: 10.1200/JCO.2003.07.131. [DOI] [PubMed] [Google Scholar]
- 3.Salloum E, Doria R, Schubert W, Zelterman D, Holford T, Roberts KB, et al. Second solid tumors in patients with Hodgkin's disease cured after radiation or chemotherapy plus adjuvant low-dose radiation. J Clin Oncol. 1996;14(9):2435–43. doi: 10.1200/JCO.1996.14.9.2435. [DOI] [PubMed] [Google Scholar]
- 4.van Leeuwen FE, Klokman WJ, Veer MB, Hagenbeek A, Krol AD, Vetter UA, et al. Long-term risk of second malignancy in survivors of Hodgkin's disease treated during adolescence or young adulthood. J Clin Oncol. 2000;18(3):487–97. doi: 10.1200/JCO.2000.18.3.487. [DOI] [PubMed] [Google Scholar]
- 5.Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin's disease. International Prognostic Factors Project on Advanced Hodgkin's Disease. N Engl J Med. 1998;339(21):1506–14. doi: 10.1056/NEJM199811193392104. [DOI] [PubMed] [Google Scholar]
- 6.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC; 2008. [Google Scholar]
- 7.Kuppers R. The biology of Hodgkin's lymphoma. Nat Rev Cancer. 2009;9(1):15–27. doi: 10.1038/nrc2542. [DOI] [PubMed] [Google Scholar]
- 8.Kamper P, Bendix K, Hamilton-Dutoit S, Honore B, Nyengaard J, d'Amore F. Tumor-infiltrating macrophages correlate with adverse prognosis and Epstein-Barr virus status in classical Hodgkin’s lymphoma. Haematologica. 2011;96(2):269–76. doi: 10.3324/haematol.2010.031542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, et al. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. N Engl J Med. 2010;362(10):875–85. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ree HJ, Kadin ME. Macrophage-histiocytes in Hodgkin's disease. The relation of peanut-agglutinin-binding macrophage-histiocytes to clinicopathologic presentation and course of disease. Cancer. 1985;56(2):333–8. doi: 10.1002/1097-0142(19850715)56:2<333::aid-cncr2820560222>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez-Aguilera A, Montalban C, de la Cueva P, Sanchez-Verde L, Morente MM, Garcia-Cosio M, et al. Tumor microenvironment and mitotic checkpoint are key factors in the outcome of classic Hodgkin lymphoma. Blood. 2006;108(2):662–8. doi: 10.1182/blood-2005-12-5125. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Espiridion B, Montalban C, Lopez A, Menarguez J, Sabin P, Ruiz-Marcellan C, et al. A molecular risk score based on four functional pathways for advanced classical Hodgkin lymphoma. Blood. 2010;116(8):e12–7. doi: 10.1182/blood-2010-02-270009. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez-Espiridion B, Sanchez-Aguilera A, Montalban C, Martin C, Martinez R, Gonzalez-Carrero J, et al. A TaqMan low-density array to predict outcome in advanced Hodgkin's lymphoma using paraffin-embedded samples. Clin Cancer Res. 2009;15(4):1367–75. doi: 10.1158/1078-0432.CCR-08-1119. [DOI] [PubMed] [Google Scholar]
- 14.Glaser SL, Lin RJ, Stewart SL, Ambinder RF, Jarrett RF, Brousset P, et al. Epstein-Barr virus-associated Hodgkin's disease: epidemiologic characteristics in international data. Int J Cancer. 1997;70(4):375–382. doi: 10.1002/(sici)1097-0215(19970207)70:4<375::aid-ijc1>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 15.Keegan TH, Glaser SL, Clarke CA, Gulley ML, Craig FE, Digiuseppe JA, et al. Epstein-Barr virus as a marker of survival after Hodgkin's lymphoma: a population-based study. J Clin Oncol. 2005;23(30):7604–13. doi: 10.1200/JCO.2005.02.6310. [DOI] [PubMed] [Google Scholar]
- 16.Jarrett RF, Stark GL, White J, Angus B, Alexander FE, Krajewski AS, et al. Impact of tumor Epstein-Barr virus status on presenting features and outcome in age-defined subgroups of patients with classic Hodgkin lymphoma: a population-based study. Blood. 2005;106(7):2444–51. doi: 10.1182/blood-2004-09-3759. [DOI] [PubMed] [Google Scholar]
- 17.Diepstra A, van Imhoff GW, Schaapveld M, Karim-Kos H, van den Berg A, Vellenga E, Poppema S. Latent Epstein-Barr virus infection of tumor cells in classical Hodgkin's lymphoma predicts adverse outcome in older adult patients. J Clin Oncol. 2009;27(23):3815–21. doi: 10.1200/JCO.2008.20.5138. [DOI] [PubMed] [Google Scholar]
- 18.Engel M, Essop MF, Close P, Hartley P, Pallesen G, Sinclair-Smith C. Improved prognosis of Epstein-Barr virus associated childhood Hodgkin's lymphoma: study of 47 South African cases. J Clin Pathol. 2000;53(3):182–6. doi: 10.1136/jcp.53.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon JM, Park YH, Kang JH, Kim K, Ko YH, Ryoo BY, et al. The effect of Epstein-Barr virus status on clinical outcome in Hodgkin's lymphoma. Ann Hematol. 2006;85(7):463–8. doi: 10.1007/s00277-006-0081-9. [DOI] [PubMed] [Google Scholar]
- 20.Lau SK, Chu PG, Weiss LM. CD163: a specific marker of macrophages in paraffin-embedded tissue samples. Am J Clin Pathol. 2004;122(5):794–801. doi: 10.1309/QHD6-YFN8-1KQX-UUH6. [DOI] [PubMed] [Google Scholar]
- 21.Oudejans JJ, Jiwa NM, Kummer JA, Ossenkoppele GJ, van Heerde P, Baars JW, et al. Activated cytotoxic T cells as prognostic marker in Hodgkin's disease. Blood. 1997;89(4):1376–82. [PubMed] [Google Scholar]
- 22.ten Berge RL, Oudejans JJ, Dukers DF, Meijer JW, Ossenkoppele GJ, Meijer CJ. Percentage of activated cytotoxic T-lymphocytes in anaplastic large cell lymphoma and Hodgkin's disease: an independent biological prognostic marker. Leukemia. 2001;15(3):458–64. doi: 10.1038/sj.leu.2402045. [DOI] [PubMed] [Google Scholar]
- 23.Alvaro T, Lejeune M, Salvado MT, Bosch R, Garcia JF, Jaen J, et al. Outcome in Hodgkin's lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin Cancer Res. 2005;11(4):1467–73. doi: 10.1158/1078-0432.CCR-04-1869. [DOI] [PubMed] [Google Scholar]
- 24.Kelley TW, Pohlman B, Elson P, Hsi ED. The ratio of FOXP3+ regulatory T cells to granzyme B+ cytotoxic T/NK cells predicts prognosis in classical Hodgkin lymphoma and is independent of bcl-2 and MAL expression. Am J Clin Pathol. 2007;128(6):958–65. doi: 10.1309/NB3947K383DJ0LQ2. [DOI] [PubMed] [Google Scholar]
- 25.Greaves P, Clear AJ, Owen DA, Macdougall F, Wilson A, Lister A, et al. An immunohistochemical score based on CD68 and FOXP3 expression in the tumour microenvironment at diagnosis defines prognostic groups in both early and advanced stage classical Hodgkin lymphoma. ASH Annual Meeting Abstracts. 2010;116:750. [Google Scholar]
- 26.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farinha P, Masoudi H, Skinnider BF, Shumansky K, Spinelli JJ, Gill K, et al. Analysis of multiple biomarkers shows that lymphoma-associated macrophage (LAM) content is an independent predictor of survival in follicular lymphoma (FL) Blood. 2005;106(6):2169–74. doi: 10.1182/blood-2005-04-1565. [DOI] [PubMed] [Google Scholar]
- 28.Ma Y, Visser L, Roelofsen H, de Vries M, Diepstra A, van Imhoff G, et al. Proteomics analysis of Hodgkin lymphoma: identification of new players involved in the cross-talk between HRS cells and infiltrating lymphocytes. Blood. 2008;111(4):2339–46. doi: 10.1182/blood-2007-09-112128. [DOI] [PubMed] [Google Scholar]
- 29.Lamprecht B, Walter K, Kreher S, Kumar R, Hummel M, Lenze D, et al. Derepression of an endogenous long terminal repeat activates the CSF1R proto-oncogene in human lymphoma. Nat Med. 2010;16(5):571–9. doi: 10.1038/nm.2129. [DOI] [PubMed] [Google Scholar]
- 30.Hsu SM, Hsu PL. The nature of Reed-Sternberg cells: phenotype, genotype, and other properties. Crit Rev Oncog. 1994;5(2–3):213–45. doi: 10.1615/critrevoncog.v5.i2-3.60. [DOI] [PubMed] [Google Scholar]
- 31.Gallamini A, Hutchings M, Rigacci L, Specht L, Merli F, Hansen M, et al. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin's lymphoma: a report from a joint Italian-Danish study. J Clin Oncol. 2007;25(24):3746–52. doi: 10.1200/JCO.2007.11.6525. [DOI] [PubMed] [Google Scholar]
- 32.Niens M, Visser L, Nolte IM, van der Steege G, Diepstra A, Cordano P, et al. Serum chemokine levels in Hodgkin lymphoma patients: highly increased levels of CCL17 and CCL22. Br J Haematol. 2008;140(5):527–36. doi: 10.1111/j.1365-2141.2007.06964.x. [DOI] [PubMed] [Google Scholar]
- 33.Plattel W, Van Den Berg A, Van der Graaf AM, Vos H, Visser L, Diepstra A, van Imhoff G. Mid-Treatment plasma levels of thymus activated and regulated chemokine (TARC) predict treatment outcome in classical Hodgkin lymphoma patients. ASH Annual Meeting Abstracts. 2010;116:748. [Google Scholar]
- 34.Benedicte Deau, Bachy E, Ribrag V, Delarue R, Rubio M-T, Bosq J, et al. Macrophages and Mast Cells Infiltration Are Biomarkers of Primary Refractory Hodgkin's Lymphoma. ASH Annual Meeting Abstracts. 2010;116:3881. [Google Scholar]
- 35.Yoon DH, Koh YW, Kim S, Park C-S, Lee DH, Kim S-W, et al. Prognostic significance of CD68 expression for Korean patients with Hodgkin lymphoma. ASH Annual Meeting Abstracts. 2010;116:3888. [Google Scholar]