Abstract
Background
The Meis1 protein represents an important cofactor for Hox and Pbx1 and is implicated in human and murine leukemias. Though much is known about the role of meis1 in leukemogenesis, its function in normal hematopoiesis remains largely unclear. Here we characterized the role of the proto-oncogene, meis1, during zebrafish primitive and definitive hematopoiesis.
Design and Methods
Zebrafish embryos were stained with o-dianisidine to detect hemoglobin-containing cells and Sudan black to quantify neutrophils. The numbers of other cells (scl-, gata1- and alas2-positive cells) were also quantified by measuring the corresponding stained areas of the embryos. We used anti-Meis1 antibody and whole mount immunohistochemistry to determine the pattern of expression of Meis1 during zebrafish development and then analyzed the functional role of Meis1 by knocking-down the meis1 gene.
Results
Using antisense morpholino oligomers to interrupt meis1 expression we found that, although primitive macrophage development could occur unhampered, posterior erythroid differentiation required meis1, and its absence resulted in a severe decrease in the number of mature erythrocytes. Furthermore a picture emerged that meis1 exerts important effects on later stages of erythrocyte maturation and that these effects are independent of gata1, but under the control of scl. In addition, meis1 morpholino knock-down led to dramatic single arteriovenous tube formation. We also found that knock-down of pbx1 resulted in a phenotype that was strikingly similar to that of meis1 knock-down zebrafish.
Conclusions
These results imply that meis1, jointly with pbx1, regulates primitive hematopoiesis as well as vascular development.
Keywords: Meis1, erythropoiesis
Introduction
Meis homeobox 1 (Meis1) is a three-amino-acid loop extension (TALE) homeodomain protein that was first identified as a common viral integration site in myeloid leukemic cells of BXH-2 mice.1 It is a critical effector, possibly downstream of Scl, that has a rate-limiting regulatory role in mixed lineage leukemia2 and limb development.3 In addition to its role in leukemogenesis, several lines of evidence indicate that meis1 plays a key role in normal hematopoiesis.4 Along with HOXA-9 and PBX1 genes, MEIS1 is transcribed in human CD34+ hematopoietic stem cells and is down-regulated following differentiation5,6 except in the megakaryocytic lineage in which transcripts are abundant.7 Mice lacking meis1 are not viable and die at E11.5–14.5 during development due to a lack of megakaryocytes and extensive hemorrhaging.8,9 Mutant fetal liver cells also fail to protect lethally irradiated animals from the radiation and compete poorly in repopulation assays. In addition, meis1 mutant mice display localized defects in vascular patterning.8,9
The mechanisms by which meis1 exerts its effect on hematopoiesis are, however, relatively poorly understood. Over the years the zebrafish has proven its suitability as a model system for furthering our understanding of the genetic regulation of hematopoiesis in both normal and pathological states.10 As in mammals,11 primitive and definitive hematopoiesis take place in zebrafish in anatomically different locations and can be further distinguished on the basis of cell types produced. Primitive hematopoiesis produces primitive macrophages, which derive from cephalic mesoderm, and primitive erythrocytes from the intermediate cell mass (ICM),12 whereas definitive hematopoiesis gives rise first to erythromyeloid progenitors13 in the posterior blood island and later to hematopoietic stem cells in the aorta-gonad-mesonephros (AGM) region.14,15 From the production site in the AGM, hematopoietic stem cells travel to the caudal hematopoietic tissue, where they expand and finally reach the pronephros and thymus, thereby settling in their final destination, the stem cell niches.16,17
Meis1 is one of the most highly conserved transcription factors in hematopoiesis with over 90% amino acid sequence homology between zebrafish and other vertebrates. We exploited this high level of conservation and that of other key regulators of hematopoiesis, e.g. scl, gata1 and fli1, and determined the effect of morpholino (MO) knockdown of zebrafish meis1 on progenitor and hematopoietic stem cell development. In addition we studied the development of the vascular system where angiogenesis and remodeling processes are responsible for the formation of a functional circulatory system and stem cell niches.
Design and Methods
Quantitative analysis of number of hematopoietic cells
The numbers of cells (erythrocytes, scl-, gata1- and alas2-positive cells) were quantified by measuring the corresponding stained areas of the embryos (e.g. red colored areas for erythrocytes in o-dianisidine-stained embryos).
Results are expressed as means ± standard error of mean (SEM) with the number of experiments. Statistical comparisons of groups were performed by the two-tailed t-test, using Excel. Differences were considered significant when P values were less than 0.05.
O-dianisidine staining
Staining of hemoglobin by o-dianisidine was performed as previously described.18
Fluorescence in situ hybridization
Larvae were processed for fluorescence in situ hybridization with antisense riboprobes for scl, gata1, alas2, c-myb, rag1, flt4 and l-plastin as previously described.19
Sudan black staining of neutrophils
To identify neutrophils Sudan black staining was performed as described previously20,21 and photomicrographs were taken as described above.
Whole-mount immunohistochemistry
Embryos at 3 days post-fertilization (dpf) were fixed in 4% methanol-free formaldehyde, 0.4% Triton X-100 for 2 h at room temperature, then washed four times for 20 min in 1 x phosphate-buffered saline (PBS), 0.4% Triton X-100, and 1% dimethyl sulfoxide (DMSO) at room temperature. Embryos were then incubated in blocking solution (10% goat serum; 0.4% Triton X-100; 1% DMSO; 1 x PBS) for 1 h at room temperautre. Primary antibody (anti-L-plastin; anti-H3P, Upstate Biotechnology Inc., NY, USA; anti-Meis1, Abcam; anti-zebrafish Ephrin B2, R&D systems) was diluted 1:500, 1:500, 1:100, or 1:25, respectively, in blocking solution and incubated with embryos overnight at 4° C. Embryos were then washed four times for 20 min with blocking solution and incubated overnight at 4° C in fresh blocking solution containing goat anti-rabbit IgG secondary antibody coupled to Alexa Fluor 488, at a dilution of 1:250. Finally, embryos were washed four times for 20 min in 1 x PBS, 0.4% Triton X-100 and photomicrographs were taken as described above.
The TdT-mediated dUTP nick end labeling assay
Cell death was detected using the TdT-mediated dUTP nick end labeling (TUNEL) assay (In Situ Cell Death Detection Kit, alkaline phosphatase detection, Roche, Burgess Hill, UK). Embryos were fixed in 4% methanol-free formaldehyde overnight at +4°C, followed by a 20 min wash in PBST (0.1% Tween 20 in 1 x PBS). After proteinase K digestion (5 min at room temperature) embryos were post-fixed in 4% methanol-free formaldehyde and stained in accordance with the manufacturer's protocol. Stained embryos were transferred to 100% glycerol for documentation. Photomicrographs were taken as described above.
Results
Meis1 is expressed at sites of hematopoiesis in zebrafish
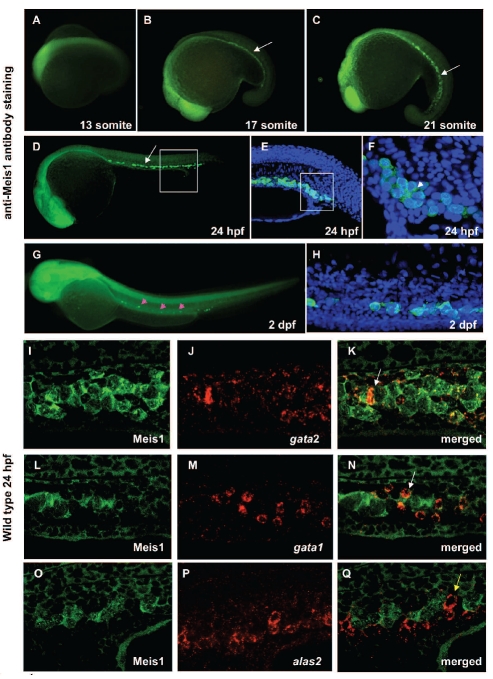
Zebrafish Meis1 protein shows 94% identity at the amino acid level with its human counterpart. In addition, the primary structure of Meis1 in zebrafish has over 90% sequence homology to that of mouse, rat, Xenopus tropicalis, and fugu (Online Supplementary Table S1, Online Supplementary Figure S1). In addition to the reported expression of meis1 in brain, eyes and neural tube of developing embryos,22–24 our whole-mount immunohistochemistry with anti-Meis1 antibody revealed the dynamic expression pattern of this protein throughout development and delineated its presence in hematopoietic progenitor cells (Figure 1A-H). At the 17-somite stage Meis1 was expressed in the ICM, the region overlying the yolk tube and extending caudally (Figure 1B). From 24 hours post-fertilization (hpf) onwards, Meis1 expression in the ICM decreased progressively, until there were only a few cells in the AGM at 2 dpf (Figure 1G–H) and no cells at 3 dpf (data not shown). Moreover, our dual immunohistological detection of Meis1 and in situ hybridization detection of gata2, gata1 and alas2 in wild-type embryos 24 hpf (Figure 1I-Q) showed that gata2- and gata1-positive cells also expressed Meis1 (Figure 1I-N). In contrast Meis1 was absent from more committed progenitors, such as alas2-expressing cells (Figure 1O-Q). The pattern of intracellular immunostaining is compatible with both nuclear and cytoplasmic presence of Meis1 (Figure 1F).
Figure 1.
Meis1 is expressed at sites of hematopoiesis in zebrafish. (A–H) Lateral view from anterior to the left. Whole mount immunohistochemistry showing Meis1 expression pattern at the 13 (A), 17 (B) and 21 somite stage (C) at 24 (D–F) and 48 hpf (G–H). (B–F) Staining with anti-Meis1 indicates expression in the ICM (white arrow) and (G–H) AGM (pink arrowheads) of the embryos at different stages of development. (E) Higher magnification view of the boxed area in (D) with DAPI staining (blue) to demonstrate the location of nuclei. (F) High magnification view of the boxed area in (E) shows intracellular expression of Meis1 as revealed by a specific antibody (green) and denotes its localization primarily in the cell nucleus (blue) with the white color indicating areas of co-localization, but also in the cytoplasm (white arrowhead). From 24 hpf onwards, Meis1 expression in the ICM decreased progressively, until only a few cells were detectable in the AGM (pink arrowheads) at 2 dpf (G). (H) Higher-magnification view of Meis1-positive cells (green) in the AGM of 2 dpf embryos; DAPI staining (blue) shows the location of nuclei. (I–Q) Dual immunohistological detection of Meis1 and in situ hybridization detection of gata2 (A), gata1 (B) and alas2 (C) in 24 hpf wild-type embryos. The white arrows in (K) and (N) denote a Meis1-positive cell expressing gata2 and gata1, respectively. The yellow arrow in (Q) denotes an alas2-expressing cell that does not co-express Meis1. Anterior to the right in all images.
Meis1-depleted embryos show reduced numbers of circulating blood cells
To analyze the functional role of Meis1 in zebrafish, we performed knock-down analysis of the meis1 gene. We designed a MO complementary to the translation initiation site of the meis1 gene to block its translation (meis1 atg MO), and injected this into zebrafish embryos at the one-to two-cell stage. To test functionality, we co-injected a meis1-EGFP reporter construct, which is robustly expressed in embryos injected with control MO, but not in those injected with meis1 atg MO (Online Supplementary Figure S2Ai-iii). The effectiveness of the Meis1 knockdown was further verified in atg MO-injected and control embryos by immunostaining which showed that Meis1-positive cells were reduced by 55% (46.7±6.1, ncontrol=40 versus 20.8±4.1, nMO=40; P=1.9x10−4) in ICM at 24 hpf (Online Supplementary Figure S2B-C). An additional meis1 MO (meis1 splice MO) was designed which creates aberrant splicing between exons 1 and 2. Reverse transcriptase polymerase chain reaction and cDNA sequencing results showed that exons 2 to 7 were removed in splice MO-injected embryos (Online Supplementary Figure S2D-F). Loss of the six exons is predicted to create a truncated Meis1 protein lacking most of the amino terminal domain while its carboxy terminal domain, including the homeodomain, remains intact. This modified Meis1 loses the Pbx1 interaction motif and, therefore, lacks the capacity to interact with its direct interaction partner, Pbx1 (Online Supplementary Figure S2F).
The optimal dose for microinjection of translation and splice blocking MO was determined so that specific defects were achieved in the absence of gross lethality and possible off target defects (Online Supplementary Figure S2Ga-f). From 24 hpf onwards, compared to control embryos, meis1 atg MO-injected embryos showed reduced numbers of the blood cells in circulation in the presence of a beating heart, although with a lower beat rate (control=90 beats/min versus meis1MO=42 beats/min; Online Supplementary Movies 3 and 4). Interestingly, at 30 hpf there were no blood cells present in the circulation and negligible or fewer blood cells were seen inside the heart and blood vessels at 48 hpf (Online Supplementary Movies 1 and 2). This phenotype was observed in 92% (n=126) of translation and splice blocking MO-injected embryos and, in addition, mild pericardial edema developed over time (Online Supplementary Figure S2Gd,f).
Erythrocyte differentiation is perturbed in meis1 morpholino-injected embryos
The reduced number of circulating blood cells of meis1 MO-injected embryos prompted us to test whether meis1 plays an important role in hematopoiesis during early zebrafish development. To examine the requirement of the meis1 transcript during hematopoiesis, the expression of multiple marker genes for both primitive and definitive hematopoiesis were analyzed using in situ hybridization and immunohistochemistry. We first tested whether depletion of Meis1 affects the number of erythroid cells using o-dianisidine staining. In control embryos, hemoglobin-positive cells were found soon after circulation started at 30 hpf, whereas meis1 MO-injected embryos lacked staining completely (ncontrol=35 versus nMO=35) (Figure 2A,B). Furthermore, in control embryos at 48 hpf erythrocytes were robustly stained with o-dianisidine and were found to be prominent in the ducts of Cuvier, over the yolk sac (Figure 2C,F). Conversely, meis1 atg MO-injected embryos exhibited a 70% decrease in the number of erythroid cells (6413±694pixels, ncontrol=41 versus 2096±491pixels, nMO=28; P=7x10−4) (Figure 2D,F). Consistently, meis1 splice MO-injected embryos had the same lack of red blood cells (6413±694pixels, ncontrol=41 versus 2785±439pixels, nMO=51; P=9×10−6) (Figure 2E,F). A possible defect in primitive erythropoiesis attributable to the slower circulation in meis1 MO-treated embryos was excluded by analyzing embryos that completely lacked circulation. We found that silent heart (sih), that is, troponin T2 (tnnt2) MO-injected embryos, showed no alteration in o-dianisidine staining at either 30 hpf or 48 hpf (Online Supplementary Figure S3A-E).25 Although lack of blood circulation in tnnt2 MO-injected embryos led to accumulation of erythrocytes in the trunk there were no changes in their number and time of maturation in comparison to the control embryos. This demonstrates that meis1 is an essential regulator of primitive erythropoiesis in zebrafish.
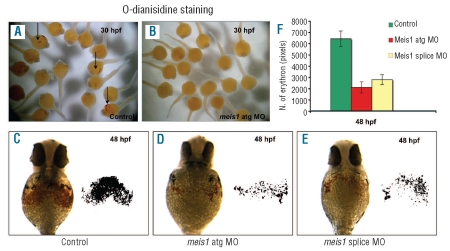
Figure 2.
Number of mature erythrocytes is affected in meis1 MO injected embryos. (A–F) O-dianisidine staining was used to detect the distribution and number of hemoglobin-positive cells in control and meis1 MO-injected embryos at 30 and 48 hpf. (A–B) In control embryos (A) hemoglobin-positive cells (black arrows) were found on the yolk sac soon after circulation started at 30 hpf; however meis1 MO-injected embryos (B) lacked staining completely. (C–F) At 48 hpf the number of o-dianisidine-positive cells was severely reduced in meis1 atg (D) and splice (E) MO embryos in comparison with the control (C). The number of erythrocytes was quantitatively examined by measuring the o-dianisidine stained areas (red colored areas) on the yolk of the embryos (shown on the left side of C, D, E), using the equivalent binary patterns, obtained by image analysis and thresholding (shown on the right side of C, D, E).
(F) Graph to illustrate the observed difference in number of erythrocytes (i.e. the size of o-dianisidine stained areas) in control and meis1 MO-injected embryos. Error bars are the standard error of the mean (SEM).
The balance between progenitor and differentiated blood cell populations is crucial for the long-term maintenance of functional blood cell lineages. Progenitor cells maintain this balance by choosing one of several alternate paths: self-renewal through proliferation, commitment to differentiation, and senescence or cell death. Thus, we tested whether depletion of Meis1 could have a regulatory effect on proliferation of progenitor blood cells. Proliferating cells were examined in the ICM of zebrafish embryos by immunohistochemistry using the H3P antibody. H3P-positive cells were detectable in the ICM of wild-type embryos at 24 hpf (n=25). The rate of H3P-positive cells was not affected by injection of either the meis1 atg MO (n=20) or the meis1 splice MO (n=24) (Online Supplementary Figure S4A-C). Apoptosis in the ICM was quantified by the TUNEL assay. Background numbers of apoptotic cells were detected in the ICM of both 24 hpf wild-type embryos and 24 hpf meis1 MO-injected embryos (n=30, Online Supplementary Figure S4D-F). Altogether, these findings indicate that Meis1 is not essential for cell proliferation or apoptosis of progenitor blood cells in the ICM.
To further explore the hematopoietic defect in meis1 MO-injected embryos, we examined the expression of several hematopoietic markers, namely: scl, gata2, gata1, alas2 and hbbe3 (n=30). Levels of all five markers appeared indistinguishable from those in control embryos at the 10-somite stage (Online Supplementary Figure S5A-D, A’-D’). However, at 24 hpf, in situ hybridization of gata1 revealed a 1.5-fold increase (2891±356pixels, ncontrol=33 versus 4372±582pixels, nMO=36; P=3.8×10–3) in the number of gata1-positive cells in meis1 atg MO-injected embryos in comparison with control ones (Figure 3E–F, Online Supplementary Figure S6C). Whereas in control embryos gata1 expression slowly decreased and localized mostly to the posterior blood island at 30 hpf (26/27 embryos), in meis1 atg MO-injected ones it persisted at high levels and in 74% of these it was distributed throughout the whole length of the ICM (nMO=38) (Figure 3G–H). Furthermore, at 48 hpf 70% of meis1 MO-injected embryos cells were still expressing high levels of gata1 in the ICM (nMO=30) (Figure 3I–J). In addition, at 24 hpf we observed a 1.5-fold increase in the expression of scl in meis1 MO-injected embryos (4622±752pixels, ncontrol=26 versus 6815±648pixels, nMO=23; P=1.1×10−2), (Figure 3A–B, Online Supplementary Figure S6A) and a 77.5% increase at 30 hpf in comparison with expression in the control (2381±301pixels, ncontrol=20 versus 4229±574pixels, nMO=24, P=4.8×10−3) (Figure 3C–D, Online Supplementary Figure S6B). As primitive erythroblasts mature, they start to express erythroid-specific genes necessary for hemoglobin synthesis, including alas2. Alas2 expression in meis1 MO-injected embryos increased 3-fold (3034±766pixels, ncontrol=36 versus 10172±234pixels, nMO=40; P=1.7×10−3) in comparison to the expression in control embryos at 30 hpf (Figure 3K–L, Online Supplementary Figure S6D).
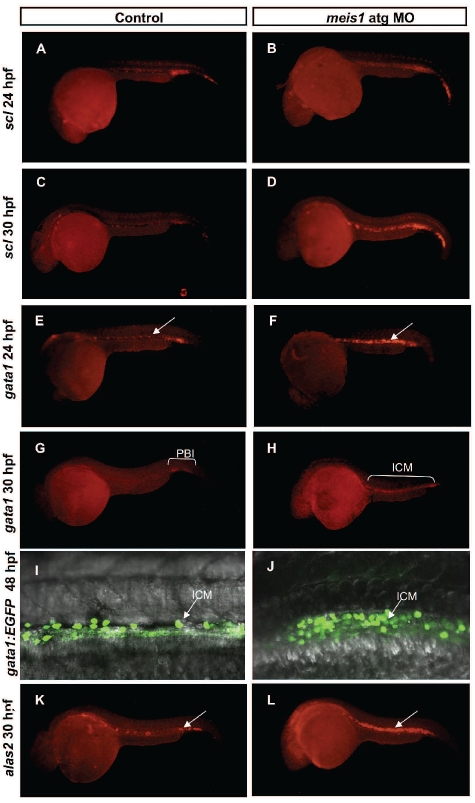
Figure 3.
Meis1 is required for normal differentiation of primitive erythrocytes. (A–B) scl in situ hybridization delineates hematopoietic and endothelial progenitors and the number of scl-positive cells was increased in morphant embryos (B) in comparison with the control (A) at 24 hpf. (C–D) By 30 hpf scl expression in the ICM was 77.5 % increased in MO-injected embryos (D) in contrast to the expression of scl in control siblings (C). To further validate the hematopoietic defect in meis1 MO-injected embryos, expression of erythroid-specific genes was examined. (E–F) In situ hybridization of gata1 at 24 hpf, revealed the 1.5-fold increase of the expression of gata1 in MO-injected embryos (E) in comparison with the control (F). (G–H) However, whereas in control embryos (G) gata1 expression slowly decreased, as gata1-positive cells entered the circulation, in meis1 atg MO-injected embryos (H) it persisted at high levels and was distributed throughout the whole length of the ICM. (I–J) At 48 hpf strong gata1 expression persisted in meis1 MO-injected embryos (J) whereas it was down-regulated in control embryos (I). (K–L) In situ hybridization of the differentiation marker alas2 showed a 3-fold increase in number of alas2-positive cells (L, white arrows point to the ICM, highlighting the accumulation of extra cells in MO-injected embryos) when compared with the controls (K).
The observed absence of mature erythrocytes in meis1 MO-injected embryos at 30 hpf, as shown by the lack of o-dianisidine staining, and a concomitant presence of gata1 and alas2 staining suggest that meis1 exerts its effect independently of gata1 and alas2. These results are compatible with the notion that, in zebrafish, meis1 is required for terminal erythroid differentiation during primitive hematopoiesis.
Meis1 morpholino-injected embryos exhibit reduced numbers of neutrophils and macrophages
The first leukocytes to appear in developing embryos are primitive macrophages.26 These macrophages can be visualized in Tg(fli1:EGFP)-expressing embryos.27 Embryos in which meis1 was knocked-down appear to have a normal number and distribution of primitive macrophages at 24 hpf (Online Supplementary Figure S7A’-B’). However, by 28 hpf in meis1 atg MO-injected embryos (Online Supplementary Figure S7B,D) a mean value of only 7.1±1.5 (nMO=24, P=3.6×10−10) l-plastin-positive cells was observed in the posterior blood island in comparison to 23.6±1.6 (ncontrol=24) cells in control MO-injected embryos (Online Supplementary Figure S7A,D). A similar phenotype was observed in meis1 splice MO-injected embryos (n=8.5±1.3, nMO=21; P=1.1×10−9) (Online Supplementary Figure S7C-D).
We next tested whether depletion of meis1 affects the number of neutrophils. At 48 hpf we observed 67% (P=1.4×10−7) and 81% (P=2×10−9) reductions in the total number of Sudan black-positive cells in meis1 MO-injected embryos, for atg MO (n=15.3±2.5, nMO=26) and splice (n=8.9±1.7, nMO=26) MO, respectively (Online Supplementary Figure S7F-H) in comparison with control numbers (n=46.8±4.7, ncontrol=30), (Online Supplementary Figure S7E,H). Furthermore, very few of these neutrophils were seen in the caudal hematopoietic tissue at 2 dpf, with a total ablation at 3.5 dpf (ncontrol =30 versus nMO=35), (Online Supplementary Figure S7C’-D’).
Meis1 knock-down leads to severe disruption of vessel lumen formation
Vasculature provides not only a conduit for mature hematopoietic cells to the peripheral circulation but also a site where hematopoietic progenitors differentiate and set the stage for the full reconstitution of hematopoiesis. Our studies have shown that endothelial cells were specified in meis1 MO-injected embryos as demonstrated by fli1 expression in Tg(fli1:EGFP) embryos, but we confirmed poor segregation of artery and vein, evident in the trunk (Online Supplementary Figure S8A-F). Unlike the situation in both control embryos (Online Supplementary Figure S8A,D) and silent heart embryos (Online Supplementary Figure S8B,E), in which dorsal aorta and posterior caudal vein form two distinct tubes by 30 hpf, in meis1 MO-injected embryos a single tube with intersegmental vessels was formed (Online Supplementary Figure S8C,F). This was accompanied by a dramatic reduction in the expression of the arterial marker Ephrinb2a (Ephb2) (Online Supplementary Figure S9A,B) and a concurrent ectopic expression of the venous marker flt4 at 30 hpf (Online Supplementary Figure S10A,B). Our observations are comparable to those made by Minehata et al. and confirm that the loss of meis1 leads to a major disruption of the segregation of angioblasts and the associated vessel lumen formation.22
Definitive hematopoiesis is severely impaired in meis1 morpholino-injected embryos
We next investigated whether definitive hematopoiesis is affected by meis1 knock-down. Definitive hematopoiesis is thought to begin at 26 hpf in cells in the subaortic mesenchyme (the zebrafish AGM).14 Current data suggest that arterial specification is required for proper hematopoietic stem cell emergence from the ventral arterial endothelium of the dorsal aorta.28,29 To determine whether hematopoietic stem cells could emerge from the trunk endothelium of meis1 MO-injected embryos, we investigated cd41low expression, using the Tg(cd41:EGFP) transgenic line, since its expression appears to be one of the first markers of definitive hematopoiesis. As expected there were no GFPlow cells present in the caudal hematopoietic tissue of meis1-depleted embryos at 72 hpf (Figure 4A–B). Consistent with these data we could not detect any thrombocytes in 3.5 dpf old embryos, (ncontrol=20 versus nMO=20) as confirmed by the absence of cd41bright-positive cells in a Tg(cd41:EGFP) transgenic line (Figure 4A–B); likewise no rag1 expression (ncontrol=24 versus nMO=22) was detectable in the thymus of meis1 MO-injected embryos at 4 dpf (Online Supplementary Figure S7E’-F’).
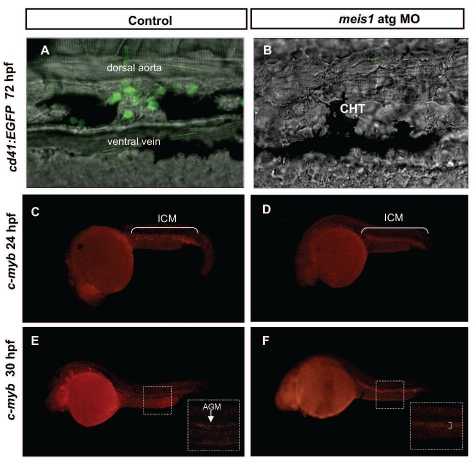
Figure 4.
Definitive hematopoiesis is severely impaired in meis1 MO-injected embryos. (A–B) At 72 hpf no cd41-positive cells were detectable in meis1 MO-injected Tg(cd41:EGFP) transgenic embryos in comparison with the control. (C–D) C-myb is normally expressed in the ICM of control MO-injected embryos (C) at 24 hpf and its expression was not affected by the loss of meis1 (D). (E–F) At 30 hpf in control embryos (E) c-myb expression was mostly confined to the AGM (arrow in white dotted inset) in contrast to meis1 MO-injected embryos (F) where c-myb was expressed throughout the aortic roof and vein (right square bracket in white dotted inset).
In addition to cd41low, we examined the expression of c-myb, a marker of definitive hematopoiesis,30 and ablation of which results in a complete absence of definitive hematopoiesis.31 C-myb expression is high in primitive erythroid cells of the ICM at 18 hpf and effectively down-regulated at the time when cells start to differentiate into erythrocytes.32 Thus, by 30 hpf c-myb is expressed only in hematopoietic stem cells of the AGM.33 At 24 hpf (ncontrol=13) c-myb expression was observed in the ICM of control MO-injected embryos and was not altered in meis1 MO-injected ones (nMO=10), (Figure 4C–D). Interestingly at 30 hpf we observed ectopic c-myb expression in meis1 MO-injected embryos. The c-myb in situ staining was mostly confined to the aortic roof and vein (30/32) rather than AGM (30/30) as observed in control embryos (Figure 4E–F). From these data we concluded that ectopic c-myb expression in meis1 MO-injected embryos is most likely due to the accumulation of immature erythroid cells that failed to suppress transcription of c-myb by 30 hpf. Thus, our findings strongly suggest that meis1 directly or indirectly regulates levels of c-myb in erythroid progenitors and affects their terminal differentiation. An increase in c-myb expression attributable to slower circulation was excluded because the analysis of silent heart embryos showed no alteration in c-myb expression at 30 hpf.34
Meis1 acts in association with PBX and downstream of scl to regulate hematopoiesis and vascular development
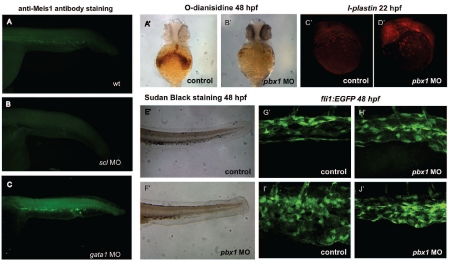
To further assess the stage at which meis1 is required and to determine its hierarchical position in the network of transcription regulation we examined the expression of meis1 in scl and gata1 MO-injected embryos (Figure 5A–C). MO knock-down of scl abolished meis1 expression (n=28) (Figure 5A–B) but gata1 depletion (n=30) had no significant effect (Figure 5A,C). From these data we infer that meis1 acts downstream of scl and independently of gata1.
Figure 5.
Meis1 acts downstream of scl and in association with Pbx1. To further examine the position of meis1 in the regulatory gene cascade we inspected the expression of meis1 in scl and gata1 MO-injected embryos (A–C). Whereas MO knock-down of scl abolished Meis1 expression (B), gata1 depletion (C) had no significant effect on Meis1 expression when compared to control (A). The numbers of mature erythrocytes, neutrophils and vascular patterning are affected in pbx1 MO-injected embryos, but the number of primitive macrophages appears to be normal (A’–J’). At 48 hpf the number of o-dianisidine-positive cells was severely reduced in pbx1 MO-injected embryos (B’) in comparison with the control (A’). Lateral view of the head and yolk sac in control (C’) and pbx1 MO-injected embryos (D’) stained with l-plastin probe shows no alteration in the number and distribution of primitive macrophages at 22 hpf.
However the number of neutrophils, as shown by Sudan black staining, was severely reduced in pbx1 MO injected embryos (F’) in comparison with the control embryos (E’). Furthermore, pbx1 MO-injected embryos had impaired vascular development with an under-developed caudal vascular plexus in comparison with control embryos (G’–J’).
The meis1 splice MO leads to a transcript that encodes a Meis1 protein that lacks the Pbx1 interaction motif but has retained its DNA binding domain. Injection of this MO induced an identical phenotype to the one observed upon injection of the meis1 atg MO. This prompted us to further examine the importance of Pbx1, one of the preferred binding partners of Meis1. We postulated that if Pbx1 is the prerequisite partner to support the function of Meis1 in hematopoiesis and arterial-venous specification then the knock-down of pbx1 by a MO would be expected to mimic the functional loss of meis1. To test this hypothesis we injected embryos at the one-cell stage with the pbx1 MO. Injected embryos were grown until 22 or 48 hpf and examined for the number and distribution of primitive macrophages or erythrocytes and neutrophils, respectively. Although the number of primitive macrophages was unaltered (Figure 5C’–D’), pbx1 MO-injected embryos showed a profound reduction in the number of erythrocytes and neutrophils (Figure 5A’,B’,E’,F’). To determine whether the loss of Pbx1 could also interrupt normal vascular patterning, we examined the vasculature in Tg(fli1:EGFP) embryos injected with pbx1 MO. As was the case for meis1 MO-treated embryos, we again observed severe disruption of vascular development (Figure 5G’–J’). In conclusion, the observed phenotypes of pbx1 MO-injected embryos were strikingly similar to those of meis1 MO-injected ones.
Discussion
In this study we demonstrate that zebrafish meis1 is necessary for both primitive and definitive hematopoiesis as well as for proper arterial-venous specification. Primitive hematopoiesis consists of anterior myelopoiesis and posterior ICM erythropoiesis, two events that occur independently.12 Our loss-of-function analysis indicates that, although anterior myeloid development can occur unhampered, posterior erythroid differentiation requires meis1 and its absence results in a severe reduction of the number of mature erythrocytes. To understand the mechanisms underlying the observed defects in erythropoiesis we analyzed the expression of several early and late lineage-specific hematopoietic markers. Gata1 promotes erythroid cell fate in zebrafish and is required for their specification and differentiation.35,36 Whereas in control embryos gata1 expression in the ICM slowly decreased after 30 hpf, as blood cells matured and entered the circulation, it persisted at high levels in meis1 MO-injected embryos. Furthermore, by 30 hpf a large fraction of meis1 MO-injected embryos had about a 70% increase in the number of alas2-positive cells in the ICM and a complete lack of mature o-dianisidine-positive erythrocytes, when compared with control and silent heart embryos, respectively. The increase in number of gata1- and alas2-positive cells was not generated by either excessive cell proliferation or decreased apoptosis of progenitor blood cells, suggesting an alteration in the kinetics of hemocytes entering the circulation. In addition, the accumulated erythroid cells failed to achieve terminal differentiation, as shown by the lack of o-dianisidine staining, and persistence of high levels of gata1 expression in the ICM, even at 48 hpf. Erythroid progenitors also abundantly express the proto-oncogene c-myb, which is typically down-regulated upon differentiation.32 In control embryos at 30 hpf c-myb expression was confined to the hematopoietic stem cells of the AGM, whilst in meis1 MO-injected ones it persisted at high levels in erythroid progenitor cells, preventing their further maturation. The increase in c-myb was not related to the slower circulation in meis1 MO-injected embryos because the same was not observed in silent heart embryos.
Notably, Pillay et al. also recently reported that MO meis1 knock-down leads to a severe decrease in the number of mature erythrocytes.37 They proposed that meis1 acts upstream of gata1 and suggested that this was caused by a modest reduction in the level of the gata1 transcript.37 We obtained several lines of evidence for a possible alternative model in which meis1 is required for terminal erythroid differentiation during primitive hematopoiesis in a gata1-independent manner. First, we observed in meis1 MO-injected Tg(gata1:EGFP) transgenic embryos that immature erythroid cells accumulated in the ICM for at least 48 hpf. Second, this observation was supported by the results of the gata1, alas2 and c-myb gene expression data. Finally, we showed that meis1 acts downstream of scl and independently of gata1. Further experimental studies may be required to elucidate the cause of the discrepant results between the two studies on the roles of meis1 in erythroid specification.
Pbx1 is a major DNA-binding partner of Meis1 and pbx1 and meis1 knock-out mice share several common features.38 Definitive myeloerythroid lineages are present in pbx1−/− and meis1−/− mice, but both the number of colony-forming cells and the repopulating capacity of fetal liver cells are severely reduced.38 Furthermore pbx1−/− mice have a severe reduction in the number of megakaryocytes while meis1−/− mutant mice have a complete loss of the megakaryocytic lineage. Early lethality has, however, prevented more detailed studies of either transcription factor in the murine model. Here we demonstrated that the phenotype of pbx1 MO knock-down in zebrafish is reminiscent of the one observed in meis1 MO-injected fish, indicating that Pbx1 is the preferred binding partner of Meis1 in hematopoiesis and vascular patterning.
Unlike primitive erythrocytes, primitive macrophages appear before circulation commences and are spatially uncorrelated to blood vessels. Although embryos in which meis1 was knocked-down have a normal number and distribution of primitive macrophages at 24 hpf, it appears that they are almost absent from the posterior blood island of the embryo from 28 hpf onwards. The posterior blood island is also an early site of multilineage hematopoiesis13 wherein a distinct population of cells, erythromyeloid progenitors, gives rise to cells of erythroid and myeloid lineages. Lack of myeloid cells in the posterior blood island from 28 hpf onwards strongly suggests that erythromyeloid progenitor cell-dependent definitive hematopoiesis is abolished in meis1-depleted embryos. Just like macrophages, neutrophils had a distribution pattern different from that of wild-type embryos, all being confined to superficial locations. These data imply that meis1 is dispensable for specification and differentiation of pre-macrophages and neutrophils from cephalic mesoderm (which is in accordance with our Meis1 immunohistochemistry findings) but is essential for erythromyeloid progenitor-dependent definitive hematopoiesis.
The intimate association and close lineage relationship of endothelial and early blood cells,39 suggest that the former also regulate hematopoietic stem cell development from their inception through to their occupation of the pronephros and thymus. Indeed, the vasculature must be properly formed and patterned in order to support normal hematopoietic stem cell emergence and differentiation.40,41 The results of our studies of the large blood vessels and intersegmental vessels in Tg(fli1:EGFP) meis1 MO-injected embryos consistently show that fli1 was detected in the endothelial cells of dorsal aorta, posterior caudal vein but also in intersegmental vessels. Notwithstanding that fli1 transcript levels were comparable between MO-injected embryos and controls we detected a defect in vascular tube formation, with dorsal aorta and posterior caudal vein forming a single tube. A shift in arteriovenous identity, already seen by others,22 in meis1 MO-injected embryos could explain the observed alteration in arteriovenous segregation. In addition, it was previously observed that meis1 MO-injected embryos have a defect in sprouting of endothelial cells and the formation of intersegmental vessels.22 The results of our study, which used higher resolution imaging by confocal microscopy of Tg(fli1:EGFP) embryos, suggest otherwise and show the presence of intersegmental vessels. In contrast to vegfa MO-injected embryos,42 meis1 MO-injected embryos still formed inter-segmental vessels, an observation that is compatible with the notion that meis1 expression is most likely independent of vegfa and notch signaling. Meis1 MO-injected embryos do exhibit a heart loop defect,22 with a lower beating rate but we consider it unlikely that this contributes to the atypical vascular tube formation and the lack of proper remodeling of the posterior caudal vein. Studies of the silent heart mutant zebrafish, which develop without a beating heart, reveal that primary vascular patterning is intact and not reliant on blood flow.43,44 However, the second wave of definitive hematopoiesis, which occurs through hematopoietic stem cell generation in the AGM, is dependent on blood flow and proper vasculature development. Thus the relative paucity of cd41low cells in meis1 MO-injected embryos is most likely explained by a strongly reduced specification of hematopoietic stem cells due to the shift in arteriovenous identity and decreased circulation.
In conclusion, our data strongly suggest that meis1 knock-down has a profound effect on primitive erythropoiesis and erythromyeloid progenitor cells and also severely disrupts the proper development of the vasculature, which results in a severe impairment of the second wave of definitive hematopoiesis. This is associated with the absence of mature lymphoid, myeloid progeny and thrombocytes. Hence meis1 function is essential in early and later events of hematopoiesis, but further studies are required to determine the precise position of Meis1 in the regulatory networks that control hematopoiesis.
Footnotes
Funding: AC and DLS are supported by Wellcome Trust grants number 082597/Z/07/Z and numbers WT 077037/Z/05/Z, and WT 077047/Z/05/Z, respectively. JSC is a Marie-Curie training fellow and supported by NetSim (number 215820) and WHO is funded by a grant from the National Institute for Health Research in England to NHS Blood and Transplant (RP-PG-0310-1002).
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Moskow JJ, Bullrich F, Huebner K, Daar IO, Buchberg AM. Meis1, a PBX1-related homeobox gene involved in myeloid leukemia in BXH- 2 mice. Mol Cell Biol. 1995;15(10):5434–43. doi: 10.1128/mcb.15.10.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong P, Iwasaki M, Somervaille TCP, So CWE, Cleary ML. Meis1 is an essential and rate-limiting regulator of MLL leukemia stem cell potential. Gen Dev. 2007;21(21):2762–74. doi: 10.1101/gad.1602107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mercader N, Leonardo E, Azpiazu N, Serrano A, Morata G, Martinez-A C, et al. Conserved regulation of proximodistal limb axis development by Meis1/Hth. Nature. 1999;402(7285):425–9. doi: 10.1038/46580. [DOI] [PubMed] [Google Scholar]
- 4.Argiropoulos B, Yung E, Humphries RK. Unraveling the crucial roles of Meis1 in leukemogenesis and normal hematopoiesis. Genes Dev. 2007;21(22):2845–9. doi: 10.1101/gad.1619407. [DOI] [PubMed] [Google Scholar]
- 5.Pineault N, Helgason CD, Lawrence HJ, Humphries RK. Differential expression of Hox, Meis1, and Pbx1 genes in primitive cells throughout murine hematopoietic ontogeny. Exper Hemat. 2002;30(1):49–57. doi: 10.1016/s0301-472x(01)00757-3. [DOI] [PubMed] [Google Scholar]
- 6.Imamura T, Morimoto A, Takanashi M, Hibi S, Sugimoto T, Ishii E, et al. Frequent co-expression of HoxA9 and Meis1 genes in infant acute lymphoblastic leukaemia with MLL rearrangement. Br J Haemat. 2002;119(1):119–21. doi: 10.1046/j.1365-2141.2002.03803.x. [DOI] [PubMed] [Google Scholar]
- 7.Watkins NA, Gusnanto A, de Bono B, De S, Miranda-Saavedra D, Hardie DL, et al. A HaemAtlas: characterising gene expression in differentiated human blood cells. Blood. 2009;113(19):4479–80. doi: 10.1182/blood-2008-06-162958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hisa T, Spence SE, Rachel RA, Fujita M, Nakamura T, Ward JM, et al. Hematopoietic, angiogenic and eye defects in Meis1 mutant animals. EMBO J. 2004;23(2):450–9. doi: 10.1038/sj.emboj.7600038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azcoitia V, Aracil M, Martínez AC, Torres M. The homeodomain protein Meis1 is essential for definitive hematopoiesis and vascular patterning in the mouse embryo. Dev Biol. 2005;280(2):307–20. doi: 10.1016/j.ydbio.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Carradice D, Lieschke GJ. Zebrafish in hematology: sushi or science? Blood. 2008;111(7):3331–42. doi: 10.1182/blood-2007-10-052761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cumano A, Godin I. Ontogeny of the hematopoietic system. Annu Rev Immunol. 2007;25(1):745–85. doi: 10.1146/annurev.immunol.25.022106.141538. [DOI] [PubMed] [Google Scholar]
- 12.De Jong JLO, Zon LI. Use of the zebrafish system to study primitive and definitive hematopoiesis. Annu Rev Genet. 2005;39 (1):481–501. doi: 10.1146/annurev.genet.39.073003.095931. [DOI] [PubMed] [Google Scholar]
- 13.Bertrand JY, Kim AD, Violette EP, Stachura DL, Cisson JL, Traver D. Definitive hematopoiesis initiates through a committed erythromyeloid progenitor in the zebrafish embryo. Development. 2007;134 (23):4147–56. doi: 10.1242/dev.012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kissa K, Murayama E, Zapata A, Cortes A, Perret E, Machu C, et al. Live imaging of emerging hematopoietic stem cells and early thymus colonization. Blood. 2008;111(3):1147–56. doi: 10.1182/blood-2007-07-099499. [DOI] [PubMed] [Google Scholar]
- 15.Hutchinson S, Trede NS. Stem-cell trafficking at vascular borders. Blood. 2008;111 (3):975–6. [Google Scholar]
- 16.Jin H, Xu J, Wen Z. Migratory path of definitive hematopoietic stem/progenitor cells during zebrafish development. Blood. 2007;109(12):5208–14. doi: 10.1182/blood-2007-01-069005. [DOI] [PubMed] [Google Scholar]
- 17.Murayama E, Kissa K, Zapata A, Mordelet E, Briolat V, Lin Hui-Feng, et al. Tracing hematopoietic precursor migration to successive hematopoietic organs during zebrafish development. Immunity. 2006;25(6):963–75. doi: 10.1016/j.immuni.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 18.Detrich R, H W, Kieran MW, Chan FY, Barone LM, Yee K, Rundstadler JA, et al. Intraembryonic hematopoietic cell migration during vertebrate development. PNAS. 1995;92(23):10713–7. doi: 10.1073/pnas.92.23.10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Welten MCM, Haan SBd, van den Boogert N, Noordermeer JN, Lamers GEM, Spaink HP, et al. ZebraFISH: fluorescent in situ hybridization protocol and three-dimensional imaging of gene expression patterns. Zebrafish. 2006;3(4):465–74. doi: 10.1089/zeb.2006.3.465. [DOI] [PubMed] [Google Scholar]
- 20.Sheehan H, Storey G. An improved method of staining leukocyte granules with Sudan black B. J Pathol Bacteriol. 1947;59(1–2):336. doi: 10.1002/path.1700590142. [DOI] [PubMed] [Google Scholar]
- 21.Cvejic A, Hall C, Bak-Maier M, Flores MV, Crosier P, Redd MJ, et al. Analysis of WASp function during the wound inflammatory response - live-imaging studies in zebrafish larvae. J Cell Sci. 2008;121(19):3196–206. doi: 10.1242/jcs.032235. [DOI] [PubMed] [Google Scholar]
- 22.Minehata K-i, Kawahara A, Suzuki T. meis1 regulates the development of endothelial cells in zebrafish. Biochem Biophys Res Comm. 2008;374(4):647–52. doi: 10.1016/j.bbrc.2008.07.075. [DOI] [PubMed] [Google Scholar]
- 23.Waskiewicz AJ, Rikhof HA, Hernandez RE, Moens CB. Zebrafish Meis functions to stabilize Pbx proteins and regulate hindbrain patterning. Development. 2001;128(21):4139–51. doi: 10.1242/dev.128.21.4139. [DOI] [PubMed] [Google Scholar]
- 24.Bessa J, Tavares MJ, Santos J, Kikuta H, Laplante M, Becker TS, et al. meis1 regulates cyclin D1 and c-myc expression, and controls the proliferation of the multipotent cells in the early developing zebrafish eye. Development. 2008;135(5):799–803. doi: 10.1242/dev.011932. [DOI] [PubMed] [Google Scholar]
- 25.North TE, Goessling W, Peeters M, Li PCC, Lord AM, Weber GJ, et al. Hematopoietic stem cell development is dependent on blood flow. Cell. 2009;137(4):736–48. doi: 10.1016/j.cell.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herbomel P, Thisse B, Thisse C. Ontogeny and behaviour of early macrophages in the zebrafish embryo. Development. 1999;126 (17):3735–45. doi: 10.1242/dev.126.17.3735. [DOI] [PubMed] [Google Scholar]
- 27.Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248 (2):307–18. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- 28.Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464 (7285):112–5. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- 29.Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DYR, Traver D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464(7285):108–11. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davidson AJ, Zon LI. The ‘definitive’ (and ‘primitive’) guide to zebrafish hematopoiesis. Oncogene. 2004;23(43):7233–46. doi: 10.1038/sj.onc.1207943. [DOI] [PubMed] [Google Scholar]
- 31.Mucenski ML, McLain K, Kier AB, Swerdlow SH, Schreiner CM, Miller TA, et al. A functional c-myb gene is required for normal murine fetal hepatic hematopoiesis. Cell. 1991;65(4):677–89. doi: 10.1016/0092-8674(91)90099-k. [DOI] [PubMed] [Google Scholar]
- 32.Bartunek P, Kralova J, Blendinger G, Dvorak M, Zenke M. GATA-1 and c-myb crosstalk during red blood cell differentiation through GATA-1 binding sites in the c-myb promoter. Oncogene. 2003;22(13):1927–35. doi: 10.1038/sj.onc.1206281. [DOI] [PubMed] [Google Scholar]
- 33.Hsia N, Zon LI. Transcriptional regulation of hematopoietic stem cell development in zebrafish. Exp Hematol. 2005;33(9):1007–14. doi: 10.1016/j.exphem.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 34.Jin H, Sood R, Xu J, Zhen F, English MA, Liu PP, et al. Definitive hematopoietic stem/progenitor cells manifest distinct differentiation output in the zebrafish VDA and PBI. Development. 2009;136(4):647–54. doi: 10.1242/dev.029637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galloway JL, Wingert RA, Thisse C, Thisse B, Zon LI. Loss of Gata1 but not Gata2 converts erythropoiesis to myelopoiesis in zebrafish embryos. Dev Cell. 2005;8(1):109–16. doi: 10.1016/j.devcel.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Lyons SE, Lawson ND, Lei L, Bennett PE, Weinstein BM, Liu PP. A nonsense mutation in zebrafish gata1 causes the bloodless phenotype in vlad tepes. PNAS. 2002;99(8):5454–9. doi: 10.1073/pnas.082695299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pillay LM, Forrester AM, Erickson T, Berman JN, Waskiewicz AJ. The Hox cofactors Meis1 and Pbx act upstream of gata1 to regulate primitive hematopoiesis. Dev Biol. 2010;340(2):306–17. doi: 10.1016/j.ydbio.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 38.DiMartino JF, Selleri L, Traver D, Firpo MT, Rhee J, Warnke R, et al. The Hox cofactor and proto-oncogene Pbx1 is required for maintenance of definitive hematopoiesis in the fetal liver. Blood. 2001;98(3):618–26. doi: 10.1182/blood.v98.3.618. [DOI] [PubMed] [Google Scholar]
- 39.Eilken HM, Nishikawa S-I, Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457(7231):896–901. doi: 10.1038/nature07760. [DOI] [PubMed] [Google Scholar]
- 40.Gering M, Patient R. Hedgehog signaling is required for adult blood stem cell formation in zebrafish embryos. Dev Cell. 2005;8(3):389–400. doi: 10.1016/j.devcel.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 41.Thompson MA, Ransom DG, Pratt SJ, MacLennan H, Kieran MW, Detrich HWI, et al. The cloche and spadetail genes differentially affect hematopoiesis and vasculogenesis. Dev Biol. 1998;197(2):248–69. doi: 10.1006/dbio.1998.8887. [DOI] [PubMed] [Google Scholar]
- 42.Herbert SP, Huisken J, Kim TN, Feldman ME, Houseman BT, Wang RA, et al. Arterial-venous segregation by selective cell sprouting: an alternative mode of blood vessel formation. Science. 2009;326 (5950):294–8. doi: 10.1126/science.1178577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Isogai S, Lawson ND, Torrealday S, Horiguchi M, Weinstein BM. Angiogenic network formation in the developing vertebrate trunk. Development. 2003;130(21):5281–90. doi: 10.1242/dev.00733. [DOI] [PubMed] [Google Scholar]
- 44.Herpers R, van de Kamp E, Duckers HJ, Schulte-Merker S. Redundant roles for Sox7 and Sox18 in arteriovenous specification in zebrafish. Circ Res. 2008;102(1):12–5. doi: 10.1161/CIRCRESAHA.107.166066. [DOI] [PubMed] [Google Scholar]