Abstract
Background
Incubation of chronic myeloid leukemia cells in hypoxia inhibits growth and selects BCR/Abl-independent cells with stem cell properties which are refractory to imatinib-mesylate. This study aimed to characterize the relationship of this refractoriness with glucose availability in the environment.
Design and Methods
K562 or primary chronic myeloid leukemia cells were cultured at 0.1% O2, different cell densities and glucose concentrations. The stem and progenitor cell potential of these cultures at different times of incubation in relation to BCR/Ablprotein expression and sensitivity to imatinib-mesylate was explored by transferring cells to growth-permissive secondary cultures in normoxia, according to the Culture-Repopulating Ability assay methodology.
Results
Hypoxia-resistant cells maintained BCR/Ablprotein expression until glucose was no longer available in primary hypoxic cultures, where glucose availability appeared to regulate cell number and the balance between the enrichment of cells with kinetic properties typical of stem or progenitor cells. Cells surviving merely hypoxic conditions were, upon transfer to secondary cultures, immediately available for numerical expansion due to the maintained BCR/Ablprotein expression, and were consequently sensitive to imatinib-mesylate. Instead, BCR/Ablprotein–negative cells selected in primary cultures under oxygen/glucose shortage underwent a delayed numerical expansion in secondary cultures, which was completely refractory to imatinib-mesylate. Cells with the latter properties were also found in primary chronic myeloid leukemia explants.
Conclusions
Glucose shortage in hypoxia was shown to represent the condition selecting BCR/Ablprotein–negative cells refractory to imatinib-mesylate from either chronic myeloid leukemia lines or patients. These cells, exhibiting stem cell properties in vitro, are metabolically suited to home to stem cell niches in vivo and so may represent the chronic myeloid leukemia cell subset responsible for minimal residual disease.
Keywords: imatinib-mesylate, chronic myeloid leukemia, glucose, hypoxia
Introduction
We previously showed that different types of leukemias contain hypoxia-resistant cells exhibiting stem cell properties as well as less immature cells the numerical expansion of which is inhibited in hypoxia.1,2 The existence of different, metabolically characterized, functional cell subsets within leukemia cell populations reflects the organization of normal hematopoiesis, where the regulatory role of glycolysis and respiration within the stem/progenitor cell hierarchy has been long known.3–6 Such an organization is functional to the maintenance of stem cells within restricted, “metabolically-defined” bone marrow areas which we called the “hypoxic stem cell niches”.3,4 In chronic myeloid leukemia cell populations, hypoxia completely suppresses the expression of BCR/Ablprotein2,7 but not that of BCR/Abl transcript, so that hypoxia-resistant cells, while remaining genetically leukemic, are independent of BCR/Abl signaling for their maintenance in vitro and resistant to treatment with imatinib-mesylate.2 Transfer of hypoxia-selected cells to normoxia restores BCR/Ablprotein expression, enabling the cell population to expand rapidly but also rescuing sensitivity to imatinib-mesylate.
This study was undertaken to address a number of questions: 1) Is hypoxia per se capable of driving the selection of imatinib-mesylate resistant stem or progenitor chronic myeloid leukemia cells, or are additional environmental/metabolic factors required? 2) Is it possible, by modulating these factors, to resolve different hypoxia-resistant “functional phenotypes” within the immature cell compartment of chronic myeloid leukemia? 3) Is the maintenance of BCR/Ablprotein expression relevant to define these phenotypes? Identifying features of imatinib-mesylate resistant progenitors is of obvious pre-clinical interest, as their specific targeting appears to be the only means of preventing chronic myeloid leukemia relapse.
Most of the experiments reported here were carried out using the K562 BCR/Abl-positive cell line, which, like all other leukemia cell lines tested so far, resulted functionally heterogeneous when subjected to incubation in hypoxia.1,2 That this was shown for stabilized cell lines, and not just for primary cell populations which presumably progress in vivo to a polyclonal state, points to functional heterogeneity as a general property of leukemia populations. This in turn makes cell lines an optimal experimental system to address differences between leukemia stem, progenitor and bulk cells, provided an experimental model (such as incubation in hypoxia) capable of selecting each is established. Indeed, the use of cell lines ensures high numbers of genetically homogeneous cells and prevents the variability of results due to the use of cytokines to support growth of primary leukemia cells. Thus, cell lines are suitable for separate assessment of the sensitivity of individual functional phenotypes to chemotherapy, imatinib-mesylate treatment in particular.1,2 We used a two-step experimental procedure based on the time-dependent selection in hypoxic cultures, and their drug treatment therein, of cell subsets enriched with stem or progenitor cells.8 The effects of hypoxia or treatment on the stem/progenitor potential were then estimated following cell transfer to secondary normoxic cultures where the expansion of population is allowed. Hypoxia-resistant cells were evaluated on the basis of phenotypical criteria, such as BCR/Ablprotein expression,2 or functional criteria, such as the kinetics of repopulation of secondary cultures.1,8
We addressed these questions by comparing the effects of hypoxia with those of its combination with glucose shortage. K562 cells were found to comprise all main cell types sustaining the normal tissue regeneration machinery, including stem and progenitor cells. Glucose availability appeared to regulate BCR/Ablprotein expression in hypoxia as well as the balance, within hypoxia-resistant cells, between progenitor cell maintenance and stem cell selection. BCR/Ablprotein–negative stem cells which were selected under oxygen and glucose shortage resulted completely refractory to imatinib-mesylate. The most relevant results were confirmed with primary chronic myeloid leukemia cells. The possible relevance of these findings to the organization of stem/progenitor cell compartment(s) in leukemias and their behavior in vivo is discussed.
Design and Methods
Cells and culture conditions
K562, U937 and primary chronic myeloid leukemia cells were routinely cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 50 units/ml penicillin and 50 μg/mL streptomycin (all from EuroClone, Paignton, UK). Cells were incubated at 37°C in a water-saturated atmosphere containing 5% CO2 and 95% air.
U937 cells expressing p210BCR/Abl (U937/pTRE-p210BCR-Abl/c6) under the control of a Tet-On system were propagated for two weeks in the presence of 0.4 mg/mL hygromycin B and 0.5 mg/ml geneticin (both from Sigma Aldrich, St. Louis, MO, USA) for transgene and On regulator selection, respectively, and 1 μg/mL doxycycline (Sigma Aldrich) was added to the culture medium every two days to induce BCR/Abl protein expression. The U937/Tet-On/A38 clone, carrying the pTET-On regulator plasmid conferring resistance to geneticin was used as control cells.9
Primary cells from chronic myeloid leukemia patients were collected after obtaining informed consent to the use for basic in vitro research and under the approval of the Ethics Committee of Azienda Ospedaliero/Universitaria Careggi (AOUC; University Hospital) at the Division of Haematology of that institute. Mononuclear cells were obtained by centrifugation on a Ficoll-Hypaque gradient (Cedarlane Laboratories, Ontario, Canada) and cultured in the presence of Flt-3 ligand (50 ng/mL) and TPo (20 ng/mL) in LC1 and of SCF (50 ng/mL), G-CSF (100 ng/mL), and IL-6 (20 ng/mL) in LC2 (all from PeproTech, Rocky Hill, NJ, USA).
Experiments were performed with exponentially growing cells plated at 3×104 or 3×105/mL and incubated in normoxia (21% O2) as above or in hypoxia (0.1% O2) in a Concept 400 anerobic incubator (Ruskinn Technology Ltd, Bridgend, UK) flushed with a water-saturated preformed gas mixture containing 5% CO2, 0.1% O2 and 94.9% N2.
Reagents
Imatinib-mesylate (STI571; Gleevec©; Glivec©; Novartis Pharma, Basel, Switzerland) was added to cultures at 0.5 μM (cell lines) or 1 μM (primary cells) at the indicated times.
Measurement of cell viability and apoptosis
Viable cells were counted in a hemocytometer by trypan blue exclusion. Apoptotic cells were detected by using a FACSCanto flow cytometer (Becton & Dickinson, Franklin Lakes, NJ, USA) after staining with FITC-conjugated annexin V and propidium iodide (PI) using the Annexin V-fluos staining kit (Roche Diagnostics, Basel, Switzerland) and following the manufacturer’s instructions. The combined percentages of annexin-V+/PI-, annexin-V+/PI+ and annexin-V-/PI+ were referred to as “percentage of dead cells”.
The Culture-Repopulating Ability (CRA) assay
This assay estimates the culture-repopulating power of normal8,10–13 or leukemic1,2,7 hematopoietic cells undergoing a selection treatment (e.g. hypoxia) in a primary liquid culture (LC1) by means of their wash and transfer in fresh medium to non-selective conditions (e.g. normoxia) in a secondary culture (LC2) and following a further incubation therein. The yield of Culture-Repopulating Ability assay with respect to LC2 repopulation provides an estimate of the content of culture-repopulating cells within the hypoxia-resistant cell subset rescued at the end of LC1. The Culture-Repopulating Ability assay is a non-clonogenic assay capable of revealing in vitro different types of normal hematopoietic stem or progenitor cells endowed with marrow-repopulating ability in vivo,8 as well as leukemic stem or progenitor cells.1 The Culture-Repopulating Ability assay, in contrast to clonogenic assays such as the LTC-IC assay, is not biased by procedures affecting the maintenance of clonogenic cells within the cell population to be tested.14
Measurement of glucose concentration in culture medium
Medium samples were harvested at the indicated times and stored frozen at −20 °C until analysis was performed by the glucose hexokinase method using the ADVIA 2400 Chemistry System (Siemens, Camberley, Surrey, UK).
Protein separation and detection
Cells (5×106) were washed twice with ice-cold phosphate buffered saline containing Na3VO4 100 μM and solubilized by incubating for 10 min at 95°C in Laemmli buffer (Tris/HCl 62.5 mM, pH 6.8, 10% glycerol, 0.005% blue bromophenol, 2% SDS). Lysates were clarified by centrifugation (20,000 g, 10 min, RT) and protein concentration in supernatants was determined by the BCA method. Aliquots (30 μg/sample) were boiled for 10 min in the presence of 100 mM 2-mercaptoethanol, subjected to SDS-PAGE in 7.6% polyacrylamide minigels and then transferred onto polyvinylidene fluoride membranes (Millipore Corporate, Billerica, MA, USA) by electroblotting.
Membranes were blocked in Odyssey Blocking Buffer (OBB)/PBS (1:1) for 1 h at RT and incubated in a 1:1,000 dilution of antibody in phosphate buffered saline 0.1% Tween (T-PBS)/OBB (1:1) overnight at 4°C. Primary (all rabbit) antibodies were: anti-phospho(tyr177)BCR, anti-phospho(Tyr245)c-Abl, anti-c-Abl (all from Cell Signaling Technology, Danvers, MA, USA), anti-ERK1/2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-vinculin (Sigma Aldrich, St. Louis, MO, USA). Secondary anti-IgG antibodies were IRDye®800CW- or IRDye®680-conjugated (LI-COR® Biosciences, Lincoln, NE, USA). Antibody-coated protein bands were visualized by the Odyssey Infrared Imaging System Densitometry (LI-COR®) and images analyzed by Adobe Photoshop to measure the mean intensity value of the area selected for each band. A background measurement was also taken. BCR/Abl band intensity values were normalized (to obtain the relative densitometric intensity) with respect to those of ERK1/2.
Statistical analysis
All experiments were performed in triplicate or higher numbers of repeats. Statistical significance was evaluated by Student’s t-test for paired samples. P values less than 0.05 (two-sided) were considered statistically significant.
Results
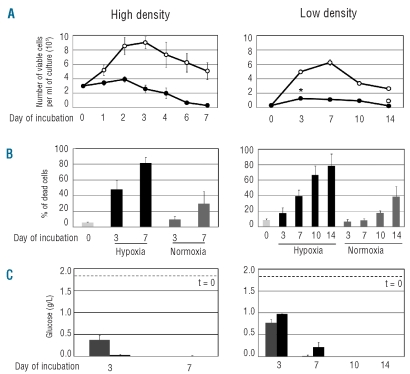
Figure 1A shows the time-course of the total number of viable cells in K562 cultures established at different cell densities (3×105 or 3×104 cells/mL) and incubated for seven or 14 days, respectively, in normoxia (21% O2) or hypoxia (0.1% O2). In cultures at the higher time 0 cell density, cell number increased in normoxia about 3-fold over the first three days of incubation, to decrease thereafter as an effect of culture crowding. In hypoxia, this increase was suppressed and cell number markedly decreased after day 2, to reach values 1-log lower than that of time 0 at day 7. At low cell density, cell number in normoxia underwent an approximately 20-fold increase, to peak at day 7 of incubation. In hypoxia, cell number increased approximately 5-fold over the first week of incubation, remained at those levels until day 10, and markedly decreased thereafter, to reach values lower than that of time 0 at day 14. Thus, growth was markedly reduced in hypoxia cells, but their kinetics were influenced by cell density, so that low-density cultures were able to significantly expand in hypoxia with respect to time 0. There was no significant difference in cell cycle distribution at either time 0 cell density or incubation atmosphere or incubation time (data not shown). Figure 1B shows that in hypoxia the percentage of dead cells reached markedly higher values than those in normoxia, with a faster kinetics at high cell density. Glucose concentration in culture medium was measured to determine whether the above results were paralleled by different kinetics of glucose consumption (Figure 1C). At high cell density, this concentration approached zero in hypoxia at day 3. At low cell density, glucose consumption was markedly less rapid in both normoxia and hypoxia, as glucose concentration approached zero at day 7 in normoxia and reached zero at day 10 in hypoxia. Taken together, the results shown in Figure 1 suggest that the decay of viable cell number and the increase of the percentage of dead cells might be influenced by glucose consumption from culture medium.
Figure 1.
Effects of hypoxia on cell number and viability and glucose concentration in cultures established at different time 0 cell densities. Exponentially growing K562 cells from routine cultures were replated in fresh medium at 3x105 (left graphs) or 3x104 (right graphs) cells/mL and incubated in normoxia (21% O2) or hypoxia (0.1% O2) for the indicated times. (A) Time-course of the number of viable cells, as determined by trypan blue exclusion; ○: normoxia; ●: hypoxia. Values are means ± S.E.M. of data from 6 independent experiments; the differences between time 0 and day 3 (*; P<0.005) and between day 10 and day 14 (°; P<0.01) were statistically significant. (B) Time-course of the percentage of dead cells, as determined by flow-cytometry of cells stained with propidium iodide (PI) and fluo-resceinated anti-annexin V (aV) antibody. Histograms represent the total percentage of apoptotic plus dead cells (sum of the percentages of aV-positive/PI-negative, aV-negative/PI-positive, double-positive cells). Light gray: time 0; dark gray: normoxia; black: hypoxia. Values are means ± S.E.M. of data from 4 independent experiments. (C) Time-course of glucose concentration in culture medium. Gray: normoxia; black: hypoxia. Dashed lines: time 0 concentration. Values are means ± S.E.M. of data from 3 independent experiments.
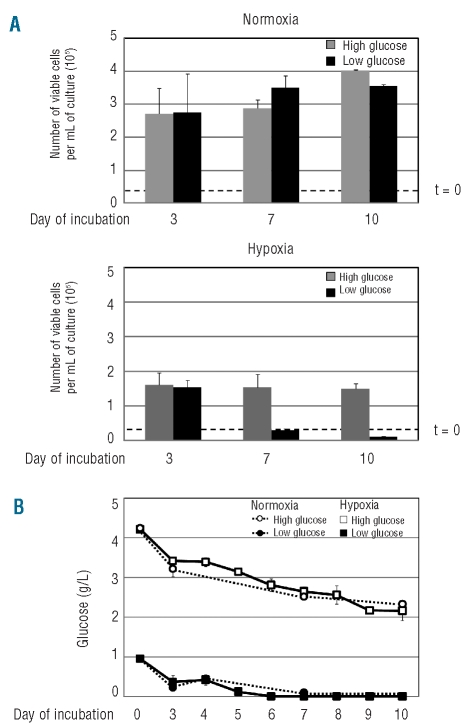
Cultures established at low time 0 cell density, where, as shown above, hypoxia-driven changes occur more smoothly, were used to determine the effects of different time 0 glucose concentrations, double or half of that routinely used (2 g/L). In normoxia, there were no significant differences in the number of viable cells throughout incubation due to glucose concentration (Figure 2A, upper graph). In hypoxia, cell number increased until day 3 irrespective of glucose concentration (Figure 2A, lower graph) but later at high glucose concentration, cell number followed the kinetics of Figure 1A (right), whereas at low glucose concentrations this fell below the time 0 cell number. Figure 2B shows that at low time 0 glucose, either in hypoxia or normoxia, glucose concentration in culture medium decreased following a kinetics very similar to that of routine cultures (Figure 1C, right). At high glucose concentrations, either in hypoxia or normoxia, glucose concentration also decreased in the course of incubation, but did not go below 2 g/L, i.e. the value of routine cultures at time 0. Thus, the results of Figure 2 confirmed that, while hypoxia per se inhibited cell growth significantly, it was actually the onset of glucose shortage in hypoxia which reduced the number of viable cells with respect to time 0. Such a reduction represents the key for the selection of the relatively small subset of cells resistant to oxygen/glucose shortage, the properties of which are described below.
Figure 2.
Effects of different time 0 glucose concentrations on the time-courses of cell number and glucose concentration in normoxic or hypoxic cultures. K562 cells were plated at low density (3x104 cells/mL) in medium containing glucose concentrations double (4.5 g/L; gray; open symbols) or half (1 g/L; black; solid symbols) of that routinely used and incubated in normoxia (A, upper graph; B, dashed lines) or hypoxia (A, lower graph; B, solid lines) for the indicated times. (A) Time-courses of the total number of viable cells (dashed lines: time 0 values). (B) Time-courses of glucose concentration in culture medium. Values are means ± S.E.M. of data from 3 independent experiments.
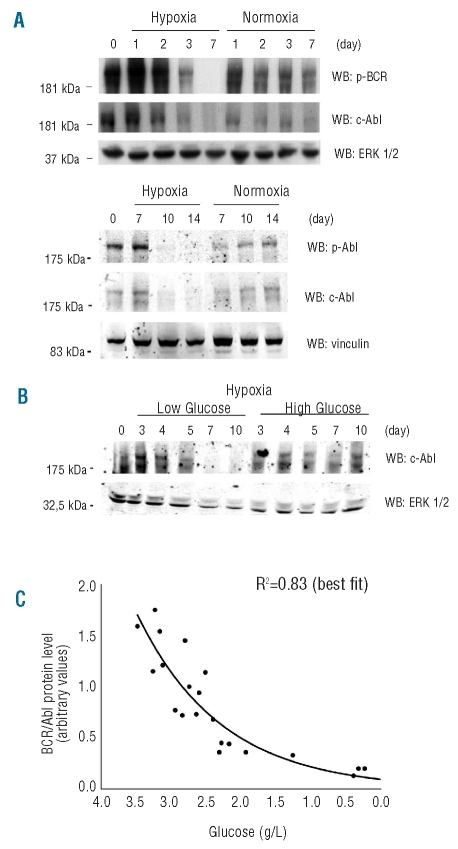
As BCR/Abl is a powerful agent of growth promotion and apoptosis inhibition, we determined the effects of time 0 cell density or glucose concentration on the expression of BCR/Ablprotein in hypoxia versus normoxia (Figure 3). BCR/Ablprotein expression and phosphorylation were transiently up-regulated in hypoxia. Later, in cultures incubated in standard medium (glucose 2 g/L), BCR/Ablprotein was markedly reduced and then suppressed in hypoxia, but not normoxia, from day 3 at high cell density (Figure 3A, upper panel) and beyond day 7 at low density (lower panel). These results, when evaluated together with those of Figure 1C, suggested that BCR/Ablprotein suppression followed glucose exhaustion in culture medium. The use of different time 0 glucose concentrations in hypoxic low cell density cultures (Figure 3B) showed that at low glucose concentrations BCR/Ablprotein was suppressed earlier (beyond day 5) than in the hypoxic samples of Figure 3A, lower panel, while at high glucose concentration it was still markedly expressed at day 10. We concluded that the kinetics of BCR/Ablprotein suppression in hypoxia reflected that of glucose concentration in culture medium. Such a conclusion is emphasized in Figure 3C, where the densitometric values of BCR/Ablprotein bands of Figure 3A/B and of similar blots from replicate independent experiments (data not shown) were plotted according to glucose concentration in the corresponding culture. It is worth pointing out here that the comparisons of Figure 3A with Figure 1B and of Figure 3B with Figure 2A (lower graph) show that BCR/Ablprotein suppression in hypoxia was paralleled by a marked increase in cell death (from about 40% to 80%) or drop in viable cells, respectively. Thus, the data reported so far led to the conclusion that in hypoxia cell bulk maintained BCR/Ablprotein expression and remained viable until glucose was no longer available in the culture medium.
Figure 3.
Effects of time 0 cell density or glucose concentration on the time-course of BCR/Ablprotein expression in normoxia or hypoxia. (A, B) K562 cells were plated at 3x105 (A, higher panel) or 3x104 (A, lower panel; B) cells/mL in medium containing standard or low/high (B) glucose concentrations (2, 1 or 4.5 g/L, respectively) and incubated in hypoxia or normoxia (A) or in hypoxia (B) for the indicated times. Total cell lysates were subjected to SDS-PAGE and electroblotting, and proteins revealed using the indicated antibodies. Equalization of protein loading was verified by anti-ERK1/2 or anti-vinculin immunoblotting. For each panel, one representative experiment out of 3 with similar outcome is shown. (C) Relationship between glucose concentration in culture medium and BCR/Ablprotein expression in hypoxic cultures of A and B and of 2 additional, independent experiments. The densitometric values of BCR/Ablprotein bands in cell lysates were plotted in function of glucose concentration in the corresponding culture, irrespective of cell or glucose concentration at time 0 and of incubation time. Only values corresponding to cultures where glucose concentration was reliably detectable (>0.2 g/L) were considered. Values were normalized for the densitometric values of bands used to check equalization of protein loading. The interpolating curve was obtained by best fit and the relative R2 value is reported.
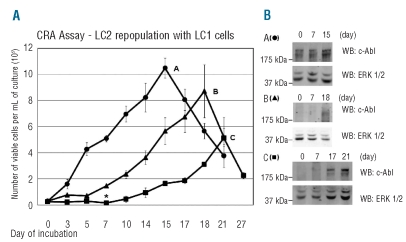
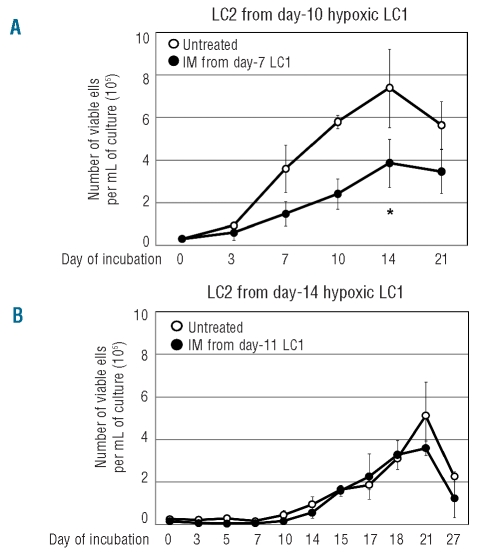
The maintenance of stem/progenitor cells in hypoxia was then tested by the Culture-Repopulating Ability (CRA) assay in cultures established at low cell density (and standard glucose concentration), where hypoxia-driven changes occur more slowly and it is, therefore, easier to select critical incubation times in relation to BCR/Ablprotein suppression and glucose consumption. Cells were recovered from hypoxic primary cultures (LC1) at days 7, 10 or 14 and transferred into growth-permissive normoxic secondary cultures (LC2) to define therein the time-course of the number of viable cells (Figure 4A). LC2 established with cells recovered from LC1 at day 7, when glucose was still present in culture medium and BCR/Ablprotein was expressed, were repopulated rapidly, to peak at day 15 (time-course A). A much less rapid expansion of LC2 was obtained with cells recovered from LC1 at day 10 (time-course B), upon glucose exhaustion and BCR/Ablprotein suppression. In LC2 established with cells recovered from LC1 at day 14 (time-course C), long after glucose exhaustion, when cell number had decreased markedly and a small cell subset had been selected, culture repopulation started after a one-week lag, to reach its peak at day 21, at a value about half of that of repopulation determined by cells rescued from LC1 at day 7. Thus, three different cell subsets containing culture-repopulating cells were time-dependently obtained from hypoxic cultures. Figure 4B shows that eventually LC2 repopulation was always sustained by cells expressing BCR/Ablprotein.
Figure 4.
Culture-Repopulating Ability (CRA) assay of cells incubated in hypoxia. (A) Time-courses of the number of viable cells in non-selective, normoxic secondary cultures (LC2), as an indicator of the progenitor/stem cell content (CRA) of selective, hypoxic primary cultures (LC1). K562 cells were plated at 3x104 cells/mL in LC1 supplemented with standard glucose concentration and incubated for 7 (●; A), 10 (▴; B) or 14 (▪; C) days in hypoxia. Cells were then replated at 3x104 cells/mL in LC2, to be incubated in normoxia for the indicated times. Values are means ± S.E.M. of data from 4 independent experiments. The difference at day 7 between time-courses B and C was statistically significant (*; P<0.05). (B) BCR/Ablprotein expression in LC2. Cells were lysed at the indicated times, total lysates subjected to SDS-PAGE and electroblotting and proteins revealed by using an anti-c-Abl antibody. Equalization of protein loading was verified by anti-ERK1/2 immunoblotting. For each panel, one representative experiment out of 3 with similar outcome is shown.
The Culture-Repopulating Ability of cell subsets identified in Figure 4 was tested for its sensitivity to imatinib-mesylate. In the experiments shown in Figure 5A, LC2 were established with cells rescued at day 10 from hypoxic LC1 treated or not with imatinib-mesylate from day 7 to day 10, bracketing the time-window of BCR/Ablprotein suppression (from full BCR/Ablprotein expression until its disappearance) shown in Figure 3A (lower panel). LC2 repopulation was significantly reduced by imatinib-mesylate treatment of LC1 (shifting approximately from time-course A to time-course B of Figure 4A), in keeping with the fact that treatment was applied when BCR/Ablprotein was still fully expressed. The slower growth kinetics of LC2 repopulation exhibited by imatinib-mesylate treated LC1 cells was not due to increased death or altered cycle distribution of cells in LC2 (parameters monitored through day 21 of LC2; data not shown). We concluded that culture-repopulating cells rescued from day 10 LC1 were in part resistant to imatinib-mesylate and that imatinib-mesylate reduced growth fraction (i.e. the number of culture-repopulating cells contained within the cell subset transferred to LC2) apparently by killing culture-repopulating cells which still expressed BCR/Abl protein. In the experiments shown in Figure 5B, cells were transferred to LC2 from day 14 hypoxic LC1 treated or not with imatinib-mesylate from day 11 to day 14, i.e. following complete BCR/Ablprotein suppression and the selection of cells driving time-course C in Figure 4A. In this case, the time-course of LC2 repopulation was completely unaffected by imatinib-mesylate treatment of LC1. This indicates that culture-repopulating cells contained in the cell subset selected under oxygen and glucose shortage in day-11 LC1 were refractory to imatinib-mesylate.
Figure 5.
Effects of imatinib-mesylate (IM) on CRA of cells incubated in hypoxia. Time-courses of the number of viable cells in normoxic LC2 established with cells treated or not with IM 0.5 μM during incubation in hypoxic LC1. LC1 established with 3x104 K562 cells/mL and standard glucose concentration were treated with IM (●) or not (○) from day 7 to day 10 (A) or from day 11 to day 14 (B) and cells then replated at 3x104 cells/mL in LC2 to be incubated in normoxia for the indicated times. Values are means ± S.E.M. of data from 3 independent experiments. In (A), the difference at day 14 between IM-treated and control cultures was statistically significant (*; P<0.05).
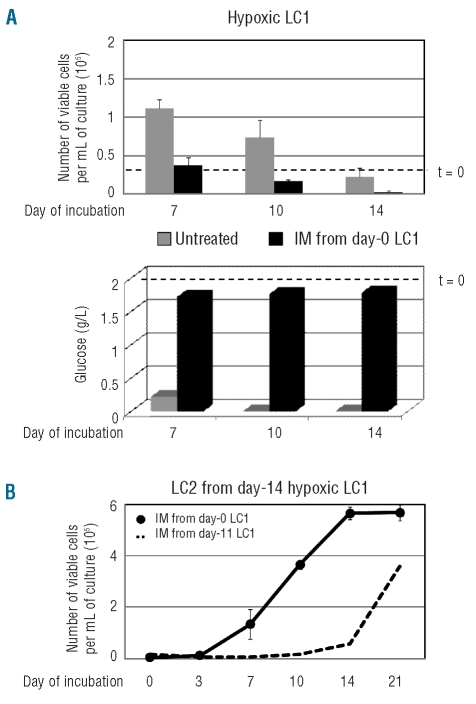
To further characterize the effects of imatinib-mesylate in our system, hypoxic low cell density LC1 were treated with imatinib-mesylate from time 0. Imatinib-mesylate suppressed the cell population expansion occurring in hypoxia and then determined a marked reduction of the number of viable cells with respect to time 0 (Figure 6A, upper graph), which was paralleled by the block of glucose consumption from culture medium (lower graph). The Culture-Repopulating Ability assay of imatinib-mesylate treated LC1 cells transferred to LC2 at day 14 (Figure 6B) showed that LC2 repopulation followed the time-course B in Figure 4A, i.e. was much faster than that of time-course C (dashed line), despite the fact that the latter is also obtained by transfer of LC1 cells at day 14. Such an outcome was most likely due to the fact that the maintenance of relatively high glucose concentrations in culture medium throughout incubation prevented the selection of culture-repopulating cells responsible for time-course C of LC2 repopulation.
Figure 6.
Effects of IM administered to cells before their incubation in hypoxia. (A) Time-courses of the number of viable cells (upper graph) or glucose concentration in culture medium (lower graph) in hypoxic LC1 established with 3x104 K562 cells/mL and standard glucose concentration, incubated in the absence (gray) or the presence (black) of IM 0.5 μM from time 0. Values are means ± S.E.M. of data from 3 independent experiments (upper graph) or from a single, representative experiment (lower graph). Dashed lines correspond to time 0 values. (B) Time-course of the number of viable cells in normoxic LC2 (CRA) established with 3x104 K562 cells/mL rescued at day 14 from the above LC1 treated with IM from time 0 (●). Values are means ± S.E.M. of data from 3 independent experiments. Dashed line corresponds to the repopulation kinetics of LC2 established with cells rescued at day 14 from hypoxic LC1 treated with IM from day 11 to day 14 of incubation (see Figure 5B).
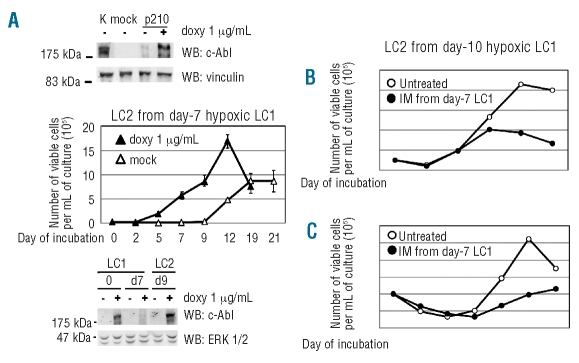
Figure 4A shows that rapid LC2 repopulation (time-course A) was paralleled by the availability of BCR/Abl-dependent signaling at time 0 of LC2, suggesting that the earlier the BCR/Ablprotein expression following incubation in LC1, the faster the LC2 repopulation. The relationship of BCR/Ablprotein expression to LC2 repopulation was confirmed by comparing control U937 cells to U937 cells induced to express ectopical BCR/Abl (Figure 7A, top panel). Control U937 cells recovered from hypoxic LC1 repopulated LC2 after a lag-phase; with BCR/Abl-expressing U937 cells, this lag-phase was suppressed (Figure 7A, graph). The induced expression of BCR/Ablprotein in U937 cells at critical time-points of LC1 and LC2 is shown in Figure 7A, bottom panel.
Figure 7.
CRA assay of hypoxia-incubated U937 cells expressing or not BCR/Abl or primary CML cells treated or not with IM. (A) Ectopical induction of BCR/Abl in U937 cells (top panel) and BCR/Ablprotein expression in LC1 and LC2 (bottom panel). Total cell lysates were subjected to SDS-PAGE and electroblotting, and proteins revealed by using anti-c-Abl antibody. Equalization of protein loading was verified by anti-vinculin or anti-ERK1/2 immunoblotting. One representative experiment out of 3 with similar outcome is shown. Time-courses of the number of viable cells in normoxic LC2 (CRA) established with U937 cells induced to express BCR/Abl or mock-infected, rescued from hypoxic LC1 (graph). LC1 established at high cell density (3x105 cells/mL) were incubated for 7 days in hypoxia and then cells replated at 3x104 cells/mL in LC2, to be incubated in normoxia for the indicated times; ▵: mock-infected, BCR/Abl-negative cells; ▴: doxycyclin-induced, BCR/Abl-positive cells. Values are means ±S.E.M. of data from 6 independent experiments. (B, C) Time-courses of the number of viable cells in normoxic LC2 (CRA) established with bone marrow cells explanted from blast-crisis (B) or chronic-phase (C) CML patients and rescued from hypoxic LC1 treated (●) or not (○) with IM 1.0 μM from day 7 to day 10 of LC1. LC1 were established at 3x105 cells/mL and LC2 at 105 cells/mL.
The selection in hypoxia of imatinib-mesylate resistant progenitors was confirmed using primary cells explanted from chronic myeloid leukemia patients (Figure 7B). Hypoxic LC1 were treated or not with imatinib-mesylate from day 7 to day 10 and then cells transferred to normoxic LC2. LC2 repopulation, which was sustained in any case by BCR/abl-positive cells (data not shown), was significantly reduced, but not abolished, by imatinib-mesylate treatment of LC1. These results indicate that hypoxia-resistant culture-repopulating cells contained in primary chronic myeloid leukemia explants were in part imatinib-mesylate insensitive.
Discussion
In this study, incubation in hypoxia of leukemia cells resulted in a time-dependent reduction of the number of viable cells and the consequent enrichment of different subsets of immature hypoxia-resistant cells exhibiting different metabolic, phenotypical and expansion properties. This represents in our opinion a relevant step into the functional characterization of regeneration hierarchy of chronic myeloid leukemia, especially in view of the lack of models to monitor the reversion from the “normal” BCR/Abl-dependent to a BCR/Abl-independent phenotype and viceversa.15 Glucose shortage in hypoxia emerged as the crucial environmental condition for the selection of cells exhibiting stem properties and complete refractoriness to imatinib-mesylate.
Hypoxia always reduced viable cell number in culture significantly with respect to normoxia. Nevertheless, cultures at low cell density were capable of expanding significantly in hypoxia with respect to time 0, in keeping with the capacity of normal hematopoietic and leukemic cells to cycle in hypoxia.1,8,11 Thus, the effects of hypoxia on the size of the chronic myeloid leukemia cell population appeared to be regulated by additional environmental conditions. Glucose availability turned out to be the crucial condition, and culture expansion in hypoxia was possible until glucose was no longer available in culture medium. This is in keeping with the increased glucose consumption in hypoxia (the “Pasteur effect”) and the fact that glucose shortage determines cell death. BCR/Ablprotein expression was also maintained in hypoxia until glucose was no longer available in culture medium, suggesting that culture expansion in hypoxia was sustained by BCR/Ablprotein expression and the drop of viability following glucose shortage was most likely determined via BCR/Ablprotein suppression.
None of this happens in a minority of “metabolically adapted” cells, including stem cells, as we discussed elsewhere16 (the “survival” metabolic profile of stem cells), so that the onset of glucose shortage in hypoxia determined the selection of a minority of BCR/Ablprotein-negative cells. That BCR/Ablprotein was suppressed completely in these cells was indicated by their complete refractoriness to imatinib-mesylate, which strongly supports the data obtained by Western blotting, in keeping with our previous findings.2 BCR/Abl transcript, on the other hand, was only partially reduced in hypoxia, to 16–20% (data not shown), values in keeping with those observed following imatinib-mesylate treatment.17 Such a discrepancy between BCR/Abl transcript and protein suppression in hypoxia indicates that suppression is operated at both the post-translational and transcriptional levels, the latter being insufficient alone to determine complete suppression. This discrepancy, which has already been reported for chronic myeloid leukemia patients,18 also clearly explains the imatinib-mesylate resistance of chronic myeloid leukemia progenitors shown to be BCR/Abl-positive by FISH or polymerase chain reaction,17,19 which indeed we believe to be, by and large, transcript-positive/protein-negative.
By measuring the ability of hypoxia-incubated LC1 cells to repopulate the growth-permissive normoxic LC2 of culture-repopulating cells assays, three different hypoxia-resistant cell subsets were identified (Figure 4): BCR/Ablprotein-positive, capable of immediate clonal expansion upon transfer to LC2 (time-course A); BCR/Ablprotein-negative, unsuitable for prompt growth resumption (time-course B) but capable of an LC2 repopulation of size comparable to that of time-course A; BCR/Ablprotein-negative, capable of delayed and reduced LC2 repopulation only and rescued from LC1 following prolonged glucose shortage and selection of a small minority of cells (time-course C). Time-course C of LC2 repopulation is compatible with the selection in LC1 of stem-type culture-repopulating cells.1–4 The difference between time-courses A and B further emphasizes the role of BCR/Ablprotein expression in determining a rapid LC2 repopulation, which also emerged from the shift from time-courses C to A determined by ectopic BCR/Abl expression in U937 cells. Moreover, the time-course C of LC2 repopulation obtained with wild-type, BCR/Abl-negative U937 cells supports our previous conclusion that all leukemia cell lines contain a hypoxia-selectable subset of stem-type culture-repopulating cells.1,2
Taken together, the kinetic data reported here indicate that chronic myeloid leukemia cell population dynamics is sustained by the combined action of cell subsets characterized by different levels of metabolic adaptation, so that stem-type culture-repopulating cells capable of withstanding hypoxia and glucose shortage, when allowed to expand (normoxia), are progressively diluted with progenitor-type culture-repopulating cells which then take advantage of the rescue of BCR/Ablprotein expression to boost population expansion and the consequent rescue of culture-repopulating ability. We believe the lag-phase of time-course C of LC2 repopulation reflects the time necessary for stem-type culture-repopulating cells to generate BCR/Ablprotein-negative progenitor-type culture-repopulating cells. Thus this study, well in keeping with very recent findings,20 provides kinetic support to the current view of chronic myeloid leukemia as a stem cell-derived but progenitor cell-driven disease.20,21 The results also point to BCR/Ablprotein suppression, rather than quiescence,22,23 as the feature determining leukemia stem cell refractoriness to imatinib-mesylate (see below), a conclusion well in keeping with the fact that cycling in hypoxia is a property of normal and leukemic stem cells,1,8,24 most probably directed to self-renewal.11 It is worth pointing out, however, that the longer cell selection under oxygen and glucose shortage, the higher the percentage of quiescent culture-repopulating cells, as indicated by experiments in which LC1 treatment with 5-fluoro-uracil or tomudex from day 14 to day 17 did not markedly alter LC2 repopulation (data not shown).
The core finding of this study is that the different hypoxia-resistant chronic myeloid leukemia cell subsets described above were refractory or sensitive to imatinib-mesylate. Imatinib-mesylate treatment of hypoxic LC1 when BCR/Ablprotein was still expressed in cell bulk determined the shift of LC2 repopulation from time-course A to time-course B, but not time-course C (Figure 5). This led to the conclusion that: 1) imatinib-mesylate pressure enriched the overall BCR/Ablprotein-negative culture-repopulating cells, including both progenitor-type culture-repopulating cells and stem-type culture-repopulating cells; and 2) the latter were enriched only under oxygen and glucose shortage, the selection power of which was higher than that of imatinib-mesylate itself. Such a conclusion was confirmed by the results shown in Figure 6, in which imatinib-mesylate treatment from time 0 suppressed BCR/Ablprotein-positive cells and the expansion of cell population, thereby inhibiting the consequent glucose consumption. Thus, glucose availability resulted in the maintenance of progenitor-type culture-repopulating cells, preventing the selection of stem-type culture-repopulating cells which occurs instead under oxygen and glucose shortage, but not in the presence of imatinib-mesylate. These experiments also indicated that at time 0, i.e. even before their adaptation to hypoxia, the K562 population contains stem-type culture-repopulating cells capable per se of surviving independently of BCR/Ablprotein expression and thereby suitable to escape imatinib-mesylate induced apoptosis irrespective of time of drug addition. The detection of such cells clearly explains the antiproliferative, but not suppressive, effects of imatinib-mesylate on leukemia stem cells,22 as well as of “second generation” BCR/Abl inhibitors, despite their enhanced action on the bulk of the chronic myeloid leukemia population.25,26
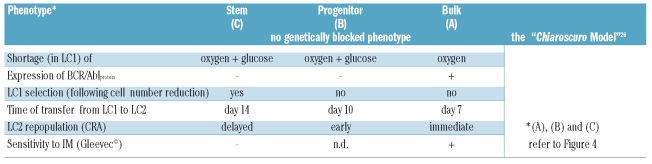
The emergence, even within cloned and supposedly homogeneous BCR/Abl-positive cell lines, of a BCR/Ablprotein-negative phenotype determining the refractoriness of stem or progenitor cells to imatinib-mesylate appears to be an adaptive feature which is flexibly and reversibly enforced in a subset of cells under metabolic pressure in tissue environment. This scenario adapts well to the Chiaroscuro model of reversible transition between the stem and progenitor cell phenotypes proposed for normal hematopoiesis27 (Table 1). In this light, our results do not contrast with the finding of equal or higher BCR/Abl expression in primary chronic myeloid leukemia stem cells when compared to progenitors,28 as the Chiaroscuro flexibility based on BCR/Ablprotein expression would emerge only within the context of oxygen/glucose shortage chronic myeloid leukemia cells physiologically encounter in vivo. All this impacts on the distribution of chronic myeloid leukemia cells within the bone marrow environment. We hypothesized that cells are distributed in vivo along a gradient of metabolic adaptation3,4 so that: (i) stem cells would be selectively maintained within recessed hypoxic/ischemic “niches” which would, therefore, represent the tissue reservoir of cells responsible for minimal residual disease;6 (ii) progenitor generation would be driven within hypoxic areas of bone marrow; (iii) expansion of cell population would occur in relatively better oxygenated tissue areas.
Table 1.
Selection of immature leukemia cell subsets under oxygen/glucose shortage and the Chiaroscuro model.
Finally, the experimental system presented here represents a proof of concept for a separate screening on stem or progenitor cells of the effects of anti-leukemic drugs, or of modulators of signaling or gene expression. The Culture-Repopulating Ability assay is indeed suitable for a rapid yet accurate quantification of these effects, provided stem or progenitor cells are properly enriched, for instance by incubation under oxygen/glucose shortage. On the other hand, the use of a stabilized cell line as target population ensures consistently high cell numbers for each stem or progenitor cell-enriched subset and prevents the variability of results due to the use of cytokines to support growth of primary leukemia cells. Information obtained in such a way would be of value as a preliminary pre-clinical screening, given the consistency of data gathered with cell lines with those obtained for primary cells.
Acknowledgments
We thank Dr. S. Mercurio, Clinical Biochemistry Laboratory, Azienda Ospedaliera-Universitaria di Careggi (AOUC), Firenze, for the measure of glucose concentration in culture media. U937/p210BCR-Abl/c6 cells were kindly provided by Dr. W. Seifarth, Medizinische Universität, Mannheim. Primary CML cells were kindly provided by the Division of Haematology of Università di Firenze/AOUC. PDS is MC member of the project (Action TD0901) of the COoperation in Science and Technology agency (European Science Foundation; EU-RTD Framework Programme; European Commission – Council of the European Union).
Footnotes
Funding: this work was supported by grants from Istituto Toscano Tumori (ITT), Associazione Italiana per la Ricerca sul Cancro (AIRC; contract # IG5220), Associazione Italiana per la lotta contro le Leucemie e i Linfomi (AIL, Sezione di Prato), Fondazione Cassa di Risparmio di Volterra. VB was supported by Federazione Italiana per la Ricerca sul Cancro (FIRC).
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Giuntoli S, Rovida E, Gozzini A, Barbetti V, Cipolleschi MG, Olivotto M, Dello Sbarba P. Severe hypoxia defines heterogeneity and selects highly immature progenitors within clonal erythroleukemia cells. Stem Cells. 2007;25(5):1119–25. doi: 10.1634/stemcells.2006-0637. [DOI] [PubMed] [Google Scholar]
- 2.Giuntoli S, Rovida E, Barbetti V, Cipolleschi MG, Olivotto M, Dello Sbarba P. Hypoxia suppresses BCR/Abl and selects Imatinib-insensitive progenitors within clonal CML population. Leukemia. 2006;20(7):1291–3. doi: 10.1038/sj.leu.2404224. [DOI] [PubMed] [Google Scholar]
- 3.Dello Sbarba P, Cipolleschi MG, Olivotto M. Hemopoietic progenitor cells are sensitive to the cytostatic effect of pyruvate. Exp Hematol. 1987;15(2):137–42. [PubMed] [Google Scholar]
- 4.Cipolleschi MG, Dello Sbarba P, Olivotto M. The role of hypoxia in the maintenance of hematopoietic stem cells. Blood. 1993;82(7):2031–7. [PubMed] [Google Scholar]
- 5.Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci USA. 2007;104(13):5431–6. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eliasson P, Jönsson JI. The hematopoietic stem cell niche: low in oxygen but a nice place to be. J Cell Physiol. 2010;222(1):17–22. doi: 10.1002/jcp.21908. [DOI] [PubMed] [Google Scholar]
- 7.Desplat V, Faucher JL, Mahon FX, Dello Sbarba P, Praloran V, Ivanovic Z. Hypoxia modifies proliferation and differentiation of CD34(+) CML cells. Stem Cells. 2002;20(4):347–54. doi: 10.1634/stemcells.20-4-347. [DOI] [PubMed] [Google Scholar]
- 8.Cipolleschi MG, Rovida E, Ivanovic Z, Praloran V, Olivotto M, Dello Sbarba P. The expansion of murine bone marrow cells preincubated in hypoxia as an in vitro indicator of their marrow-repopulating ability. Leukemia. 2000;14(4):735–9. doi: 10.1038/sj.leu.2401744. [DOI] [PubMed] [Google Scholar]
- 9.Håkansson P, Lassen C, Olofsson T, Baldetorp B, Karlsson A, Gullberg U, et al. Establishment and phenotypic characterization of human U937 cells with inducible P210 BCR/ABL expression reveals upregulation of CEACAM1 (CD66a) Leukemia. 2004;18(3):538–47. doi: 10.1038/sj.leu.2403255. [DOI] [PubMed] [Google Scholar]
- 10.Ivanović Z, Dello Sbarba P, Trimoreau F, Faucher JL, Praloran V. Primitive human HPCs are better maintained and expanded in vitro at 1 percent oxygen than at 20 percent. Transfusion. 2000;40(12):1482–8. doi: 10.1046/j.1537-2995.2000.40121482.x. [DOI] [PubMed] [Google Scholar]
- 11.Ivanović Z, Belloc F, Faucher JL, Cipolleschi MG, Praloran V, Dello Sbarba P. Hypoxia maintains and interleukin-3 reduces the pre-colony-forming cell potential of dividing CD34(+) murine bone marrow cells. Exp Hematol. 2002;30(1):67–73. doi: 10.1016/s0301-472x(01)00765-2. [DOI] [PubMed] [Google Scholar]
- 12.Ivanovic Z, Hermitte F, Brunet de la Grange P, Dazey B, Belloc F, Lacombe F, et al. Simultaneous maintenance of human cord blood SCID-repopulating cells and expansion of committed progenitors at low O2 concentration (3%) Stem Cells. 2004;22(5):716–24. doi: 10.1634/stemcells.22-5-716. [DOI] [PubMed] [Google Scholar]
- 13.Kovacević-Filipović M, Petakov M, Hermitte F, Debeissat C, Krstić A, Jovcić G, et al. Interleukin-6 (IL-6) and low O(2) concentration (1%) synergize to improve the maintenance of hematopoietic stem cells (pre-CFC) J Cell Physiol. 2007;212(1):68–75. doi: 10.1002/jcp.21003. [DOI] [PubMed] [Google Scholar]
- 14.Heaney NB, Pellicano F, Zhang B, Crawford L, Chu S, Kazmi SM, et al. Bortezomib induces apoptosis in primitive chronic myeloid leukemia cells including LTC-IC and NOD/SCID repopulating cells. Blood. 2010;115(11):2241–50. doi: 10.1182/blood-2008-06-164582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren R. Mechanisms of BCR-ABL in the pathogenesis of chronic myelogenous leukaemia. Nat Rev Cancer. 2005;5(3):172–83. doi: 10.1038/nrc1567. [DOI] [PubMed] [Google Scholar]
- 16.Olivotto M, Dello Sbarba P. Environmental restrictions within tumor ecosystems select for a convergent, hypoxia-resistant phenotype of cancer stem cells. Cell Cycle. 2008;7(2):176–87. doi: 10.4161/cc.7.2.5315. [DOI] [PubMed] [Google Scholar]
- 17.Abe A, Minami Y, Hayakawa F, Kitamura K, Nomura Y, Murata M, et al. Retention but significant reduction of BCR-ABL transcript in hematopoietic stem cells in chronic myelogenous leukemia after imatinib therapy. Int J Hematol. 2008;88(5):471–5. doi: 10.1007/s12185-008-0221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donato NJ, Wu JY, Stapley J, Lin H, Arlinghaus R, Aggarwal BB, et al. Imatinib mesylate resistance through BCR-ABL independence in chronic myelogenous leukemia. Cancer Res. 2004;64(2):672–7. doi: 10.1158/0008-5472.can-03-1484. erratum in: Cancer Res 2004;64(6):2306. [DOI] [PubMed] [Google Scholar]
- 19.Bhatia R, Holtz M, Niu N, Gray R, Snyder DS, Sawyers CL, et al. Persistence of malignant hematopoietic progenitors in chronic myelogenous leukemia patients in complete cytogenetic remission following imatinib mesylate treatment. Blood. 2003;101(12):4701–7. doi: 10.1182/blood-2002-09-2780. [DOI] [PubMed] [Google Scholar]
- 20.Schemionek M, Elling C, Steidl U, Baumer N, Hamilton A, Spieker T, et al. BCR-ABL enhances differentiation of long-term repopulating hematopoietic stem cells. Blood. 2010;115(16):3185–95. doi: 10.1182/blood-2009-04-215376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Savona M, Talpaz M. Getting to the stem of chronic myeloid leukaemia. Nat Rev Cancer. 2008;8(5):341–50. doi: 10.1038/nrc2368. [DOI] [PubMed] [Google Scholar]
- 22.Graham SM, Jørgensen HG, Allan E, Pearson C, Alcorn MJ, Richmond L, et al. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99(1):319–25. doi: 10.1182/blood.v99.1.319. [DOI] [PubMed] [Google Scholar]
- 23.Goldman J, Gordon M. Why do chronic myelogenous leukemia stem cells survive allogeneic stem cell transplantation or imatinib: does it really matter? Leuk Lymphoma. 2006;47(1):1–7. doi: 10.1080/10428190500407996. [DOI] [PubMed] [Google Scholar]
- 24.Ivanović Z, Bartolozzi B, Bernabei PA, Cipolleschi MG, Rovida E, Milenković P, et al. Incubation of murine bone marrow cells in hypoxia ensures the maintenance of marrow-repopulating ability together with the expansion of committed progenitors. Br J Haematol. 2000;108(2):424–9. doi: 10.1046/j.1365-2141.2000.01842.x. [DOI] [PubMed] [Google Scholar]
- 25.Copland M, Hamilton A, Elrick LJ, Baird JW, Allan EK, Jordanides N, et al. Dasatinib (BMS-354825) targets an earlier progenitor population than imatinib in primary CML but does not eliminate the quiescent fraction. Blood. 2006;107(11):4532–9. doi: 10.1182/blood-2005-07-2947. [DOI] [PubMed] [Google Scholar]
- 26.Konig H, Holtz M, Modi H, Manley P, Holyoake TL, Forman SJ, et al. Enhanced BCR-ABL kinase inhibition does not result in increased inhibition of downstream signaling pathways or increased growth suppression in CML progenitors. Leukemia. 2008;22(4):748–55. doi: 10.1038/sj.leu.2405086. [DOI] [PubMed] [Google Scholar]
- 27.Quesenberry PJ, Colvin GA, Lambert JF. The chiaroscuro stem cell: a unified stem cell theory. Blood. 2002;100(13):4266–71. doi: 10.1182/blood-2002-04-1246. [DOI] [PubMed] [Google Scholar]
- 28.Jiang X, Zhao Y, Smith C, Gasparetto M, Turhan A, Eaves A, et al. Chronic myeloid leukemia stem cells possess multiple unique features of resistance to BCR-ABL targeted therapies. Leukemia. 2007;21(5):926–35. doi: 10.1038/sj.leu.2404609. [DOI] [PubMed] [Google Scholar]