Abstract
Background
Pediatric acute myeloid leukemia is a heterogeneous disease characterized by non-random genetic aberrations related to outcome. The genetic subtype is currently detected by different diagnostic procedures which differ in success rate and/or specificity.
Design and Methods
We examined the potential of gene expression profiles to classify pediatric acute myeloid leukemia. Gene expression microarray data of 237 children with acute myeloid leukemia were collected and a double-loop cross validation approach was used to generate a subtype-predictive gene expression profile in the discovery cohort (n=157) which was then tested for its true predictive value in the independent validation cohort (n=80). The classifier consisted of 75 probe sets, representing the top 15 discriminating probe sets for MLL-rearranged, t(8;21)(q22;q22), inv(16)(p13q22), t(15;17)(q21;q22) and t(7;12)(q36;p13)-positive acute myeloid leukemia.
Results
These cytogenetic subtypes represent approximately 40% of cases of pediatric acute myeloid leukemia and were predicted with 92% and 99% accuracy in the discovery and independent validation cohort, respectively. However, for NPM1, CEBPA, MLL(-PTD), FLT3(-ITD), KIT, PTPN11 and N/K-RAS gene expression signatures had limited predictive value. This may be caused by a limited frequency of these mutations and by underlying cytogenetics. This latter is exemplified by the fact that different gene expression signatures were discovered for FLT3-ITD in patients with normal cytogenetics and in those with t(15;17)(q21;q22)-positive acute myeloid leukemia, which pointed to HOXB-upregulation being specific for FLT3-ITD+ cytogenetically normal acute myeloid leukemia.
Conclusions
In conclusion, gene expression profiling correctly predicted the most prevalent cytogenetic subtypes of pediatric acute myeloid leukemia with high accuracy. In clinical practice, this gene expression signature may replace multiple diagnostic tests for approximately 40% of pediatric acute myeloid leukemia cases whereas only for the remaining cases (predicted as ‘acute myeloid leukemia-other’) are additional tests indicated. Moreover, the discriminative genes reveal new insights into the biology of acute myeloid leukemia subtypes that warrants follow-up as potential targets for new therapies.
Keywords: pediatric AML, gene expression profile, cytogenetic subtype, classification, microarray
Introduction
Pediatric acute myeloid leukemia (AML) is a heterogeneous disease that accounts for 15–20% of the acute leukemias in children1 and is classified according to the WHO classification, which is based on non-random genetic aberrations.2 Over the decades the outcome of pediatric AML has improved and current overall survival rates range from 50% to 70%.3 The most important prognostic factors in pediatric AML are response to induction therapy and the cytogenetic and molecular subtype of the disease.4,5
Gilliland et al. postulated that the pathogenesis of AML requires both type I and type II mutations.6 Type II mutations are often chromosomal rearrangements of transcription factors leading to impaired differentiation of the hematopoietic cell, such as 11q23/MLL-rearranged, t(8;21)(q22;q22)[RUNX1-RUNXT1], inv(16)(p13q22)[CBFB-MYH11], or t(15;17)(q21;q22) [PML-RARA]. Patients with t(8;21)(q22;q22), inv(16)(p13q22) and t(15;17)(q21;q22)-positive AML have a favorable prognosis in contrast to MLL-rearranged cases. Type I mutations often reflect molecular mutation hotspots in specific genes (FLT3, KIT and NRAS, KRAS, PTPN11 and NF1) involved in the proliferation of hematopoietic cells.7,8 In adult and pediatric AML FLT3-internal tandem duplications (FLT3-ITD) and KIT mutations have been correlated with an inferior outcome.9–11
In approximately 20% of pediatric AML cases no chromosomal aberrations have yet been discovered. These patients with apparent cytogenetically normal (CN) AML are currently treated as a homogeneous group with an intermediate risk factor. However, point mutations and small deletions in CEBPA and NPM1 as well as partial tandem duplications in MLL (MLL-PTD) are found in both pediatric and adult CN-AML. The frequency of these mutations is lower in children than in adults. Moreover, the prognostic impact differs between children and adults.10,12–15 These observations highlight the genetic heterogeneity within AML as well as between adults and children with AML and the need for separate studies in pediatric AML to demonstrate the value of mutations for stratification in contemporary pediatric AML treatment protocols.
A new case of AML is currently primarily identified by cytomorphology and immunophenotyping. Further characterization needed for risk-stratification includes the detection of chromosomal aberrations by conventional karyotyping and molecular cytogenetics of specific genetic lesions, for instance by fluorescence in situ hybridization (FISH) and/or reverse transcriptase (RT) polymerase chain reaction (PCR). However, it can be difficult to obtain a karyogram since this requires successful induction of in vitro cellular proliferation to obtain metaphases for analysis of chromosomal changes. In addition, FISH and RT-PCR procedures may also yield inconclusive results, for example due to poor interphase preparations (FISH), limitations in signal detection (FISH), sub-clonality (FISH and RT-PCR) and sequence variations in probe/primer-hybridizing regions (FISH and RT-PCR).
Microarray-based gene expression profiling studies showed that pediatric and adult AML can be accurately classified into cytogenetically distinct subtypes.16–20 In the Microarray Innovations-in-LEukemia (MILE) study, gene expression profiles accurately classified over 3000 cases with acute and chronic leukemia.21
We recently showed that a double-loop cross-validation classification approach yielded a highly stable and accurate classifier with high predictive value for subtypes of pediatric acute lymphoblastic leukemia in both the cross-validation cohort as well as in a totally independent cohort of pediatric ALL.22 In the current study we used this double-loop cross-validation method to determine whether gene expression signatures can predict prognostically relevant specific cytogenetic subtypes (11q23/MLL-rearranged, t(8;21)(q22;q22), inv(16)(p13q22), t(15;17)(q21;q22), t(7;12)(q36;p13) and CN-AML) as well as cases with molecular aberrations in NPM1, CEBPA, FLT3-ITD, N/K-RAS, KIT and PTPN11 in pediatric AML.
Design and Methods
Patients
Viable frozen bone marrow or peripheral blood samples from 237 children with de novo AML, 33 with relapsed and 8 with secondary AML were provided by the Dutch Childhood Oncology Group, ‘Berlin-Frankfurt-Münster’ AML Study Group, Czech Pediatric Hematology and St. Louis Hospital in Paris, France. Informed consent was obtained from patients, after Institutional Review Board approval according to national law and regulations. Leukemic cells were isolated by sucrose density centrifugation and non-leukemic cells were eliminated as previously described.23 All processed samples contained more than 80% leukemic cells, as determined morphologically using cytospins stained with May-Grünwald-Giemsa (Merck, Darmstadt, Germany). Subsequently, at least 5×106 leukemic cells were lysed in Trizol reagent (Gibco BRL, Life Technologies, Breda, the Netherlands). Genomic DNA and total RNA were isolated according to the manufacturer’s protocol, with minor modifications.24
Cytogenetics
Leukemic samples were routinely investigated for cytogenetic aberrations by standard chromosome-banding analysis, and screened, by the above mentioned study groups, for recurrent non-random genetic aberrations characteristic of AML as described by the WHO 2008 classification of myeloid neoplasms and acute leukemia,2 including MLL-rearrangements, inv(16)(p13q22), t(8;21)(q22;q22) and t(15;17)(q21;q22), using RT-PCR and/or FISH. In the case of incomplete data, the Erasmus MC group performed RT-PCR to detect inv(16)(p13q22), t(8;21)(q22;q22) and t(15;17)(q21;q22) and split-signal FISH to detect rearrangements of the MLL-gene using standardized primers and probe combinations, as previously described.25,26 In three cases, predicted as MLL-rearranged AML, screening for an MLL-rearrangement was performed with long-distance inverse (LDI) PCR as previously described.27 In addition, all patients under the age of 18 months were screened for t(7;12)(q36;p13) by FISH. The probes used were five cosmid clones covering the breakpoints in the ETV6 gene and a PAC clone (RP5-1121A15) containing the HLXB9 gene, as previously described.28
Mutation analysis
Samples were screened for hotspot mutations in NPM1, CEPBA, FLT3-ITD, NRAS, KRAS, PTPN11, KIT and MLL-PTD, as previously described.7,29–32 If positive for MLL-PTD, this was confirmed by a multiplex ligation-dependent probe amplification (MLPA) analysis (MRC Holland, Amsterdam, the Netherlands). The reaction mix for MPLA-analysis contained probes for exon 2 to 13 of MLL for MLL-PTD detection and exon 17 of MLL as an internal control. A probe in the serpinB2 gene was used as an external control.33 Data were analyzed using GeneMarker v1.5 (Softgenetics, State College, USA).
Microarray
The integrity of total RNA was checked using the Agilent 2100 Bio-analyzer (Agilent, Santa Clara, USA). cDNA and biotinylated cRNA were synthesized and hybridized to the Affymetrix Human Genome U133 Plus 2.0 Array (Affymetrix, Santa Clara, USA) according to the manufacturer’s guidelines. Data were acquired using ‘expresso’ (Bioconductor package ‘Affy’), and probe-set intensities were normalized using the variance stabilization normalization procedure (Bioconductor package ‘VSN’) in the statistical data analysis environment R, version 2.2.0. The original data files have been submitted to the GEO database (GSE17855).
Statistics
To find signatures for the different cytogenetic and molecular subtypes an empirical Bayes linear regression model was used to compare samples from each group to all other samples.34 This model takes advantage of the large number of probe sets to yield better estimates for the gene-specific standard error, producing more powerful tests for differential expression even if small sample sizes are involved. Moderated t-statistics P values were corrected for multiple testing using the false discovery rate (FDR) method defined by Benjamini and Hochberg.35 The top 50 most significant probe sets for each subtype were used as a starting point to construct the classifier.
The construction of the classifier
Samples were divided at random into a discovery cohort of 157 cases that were used for the double-loop cross validation approach and an independent cohort of 80 cases, which was only tested once and served as a true independent validation cohort. In all following 100 cycles of the double-loop cross-validation approach, the sample distribution of the discovery cohort reflected the distribution of cytogenetic subtypes as seen in the total cohort of 237 cases. The double-loop cross-validation method was used to build a support-vector machine-based classifier predictive for the known cytogenetic subtypes of pediatric AML. This approach avoids over-fitting of gene expression profiling data36 and has proven to yield a stable classifier with high accuracy to predict subtypes of pediatric acute lymphoblastic leukemia, as we previously showed.22 The double-loop cross-validation method was only applied to the discovery cohort of 157 cases. This consists of an inner loop containing two-thirds of cases in which the minimal number of probe sets yielding the highest prediction sensitivity is being determined (100 iterations for each selected number of probe sets) and an outer loop containing the remaining one-third of cases serving to validate the obtained results from the inner loop (also 100 iterations per list of probe sets) (Online Supplementary Figure S1).
In each of 100 runs of the inner loop, patients were randomly assigned to the inner-training (9/10) and inner-test (1/10) group (10-fold cross-validation). To start, the top 50 probe sets most discriminative for each subtype were selected by rank of P values obtained by applying an empirical Bayes linear regression model (LIMMA) to the inner-training group. These probe sets were used to construct a support vector machine-based classifier which was then tested for predictive sensitivity on the inner-test group of the remaining 1/10 of cases (100 iterations). Next, the minimum number of probe sets that optimally classified the patients in the inner loop was obtained by backwards selection starting with 250 probe sets (50 probe sets x 5 subtypes) using a global test for ranking the significance of probe sets in each iteration in order to reduce multiple testing errors, as previously described.22 The optimal number of probe sets determined in the inner loop was used to construct a classifier for which the median sensitivity was estimated via 3-fold cross-validation by applying the trained classifier to the remaining one-third of the cases of the outer loop (100 iterations; Online Supplementary Figure S1). The final gene expression classifier, trained on all 157 cases in the discovery cohort, was used to determine the prediction accuracy in the independent group of 80 cases (Online Supplementary Figure S1).
The same approach was used to select probe sets predictive for the most frequent molecular aberrations, i.e. NPM1, CEBPA, MLL-PTD, FLT3-ITD, KIT, and combined mutations in the RAS-pathway (NRAS, KRAS and PTPN11), Since NPM1, CEBPA and MLL-PTD were mutually exclusive from the other cytogenetic subgroups, these abnormalities could be simultaneously included in one model together with the known cytogenetic subgroups, for which the prediction accuracy was estimated as described above. In addition, we performed an analysis in which the most discriminative probe sets for type I mutations in FLT3-ITD, KIT and RAS-pathway were identified, after adjusting for the underlying cytogenetic aberrations.
Software
R (version 2.2.0 and version 2.5.0) and the R packages affy, vsn, e1071, globaltest, limma, multtest and marray were used to run the above-mentioned analyses.34,37–41 Hierarchical clustering analysis was performed in Genemaths XT (Applied Maths, Austin, USA).
Results
Patients’ characteristics
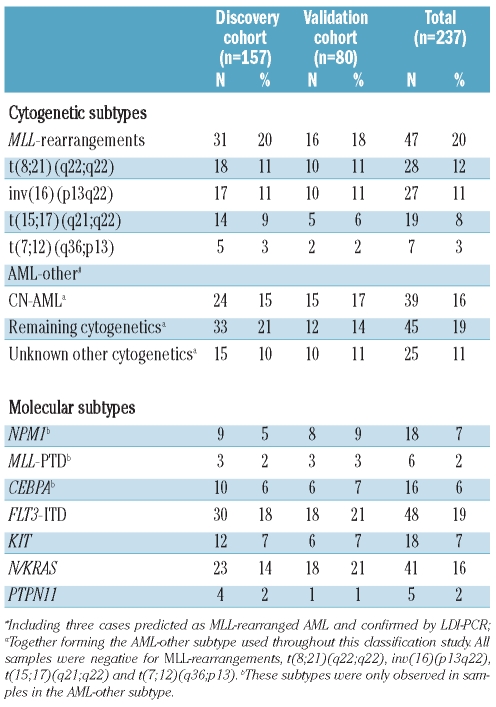
Gene expression profiles were generated from 237 newly diagnosed pediatric AML cases. Non-random cytogenetic subgroups of pediatric AML with a sufficient number of cases were included, i.e. MLL-rearranged AML, t(8;21)(q22;q22), inv(16)(p13q22), t(15;17)(q21;q22) and CN-AML. In addition, seven t(7;12)(q36;p13)-positive infant AML cases were included (Table 1). For the other cytogenetic groups, e.g. t(6;9)(p23;q34) (n=7), monosomy 7 (n=4), trisomy 8 (n=1) and complex karyotype (n=11), no significant discriminative genes were found and these cases were, therefore, combined into a single group annotated with ‘remaining cytogenetics’. No karyotype was available for 25 cases but since these cases were negative for MLL-rearrangements, t(8;21)(q22;q22), inv(16)(p13q22), t(15;17)(q21;q22) and t(7;12)(q36;p13), these cases were included in the ‘unknown other cytogenetics’ category.
Table 1.
Cytogenetic and molecular characteristics of the pediatric acute myeloid leukemia patients in this study.
Definition of subgroups
Using an empirical Bayes linear regression model many discriminative probe sets, with high statistical significance (P<1.0−08), were found for MLL-rearranged, t(8;21)(q22;q22), inv(16)(p13q22), t(15;17)(q21;q22) and t(7;12)(q36;p13)-positive AML (Online Supplementary Table S2). In contrast, only a limited number of discriminative probe sets were found to be significant at lower P value (P<1.0−04) for CN-AML, cases with remaining genetic aberrations or unknown other cytogenetics (Online Supplementary Table S2). Hereafter, this mixed group is referred to as the ‘AML-other’ group. Based on an equal distribution of these groups, the overall cohort was divided into a discovery cohort (n=157) to construct the classifier and an independent validation cohort (n=80).
Probe set selection for classifier estimated with the discovery cohort
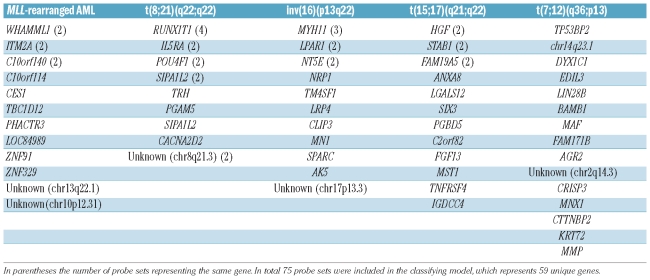
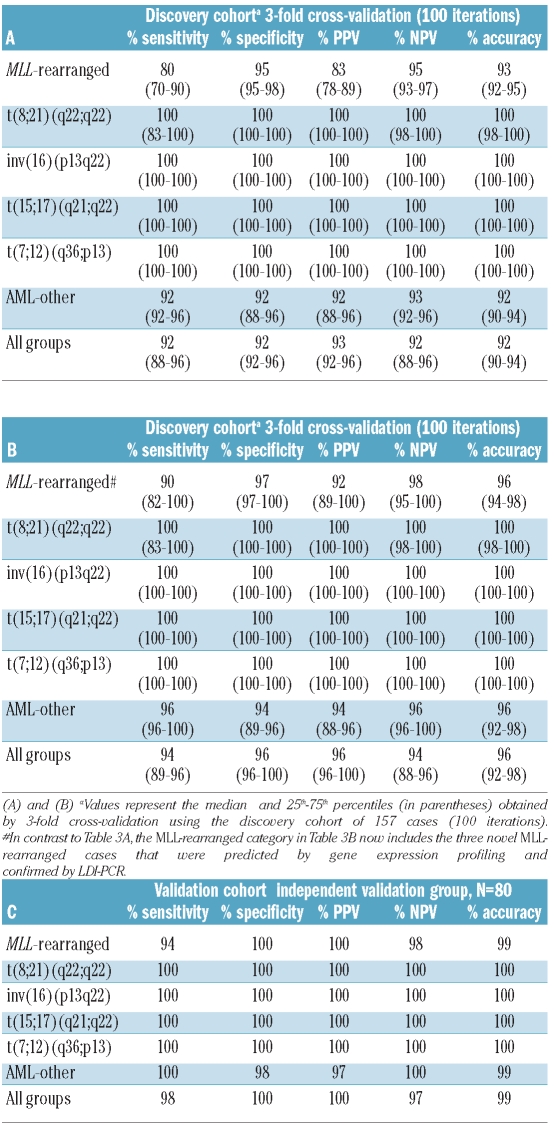
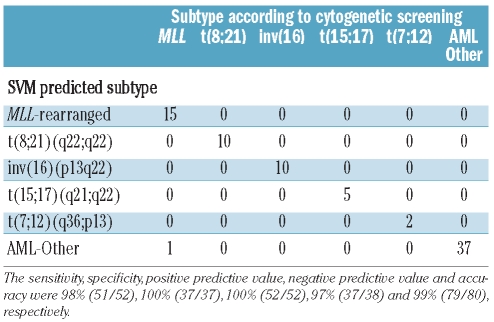
The classifier was constructed by selecting the most statistically significantly discriminative probe sets for each of the five cytogenetic subtypes: MLL-rearranged, t(8;21)(q22;q22), inv(16)(p13q22), t(15;17)(q21;q22) and t(7;12)(q36;p13)-positive AML. A double-loop cross validation approach was used that also included a backward selection procedure to keep the number of probe sets needed for most accurate classification to a minimum in order to avoid over-fitting of data, as previously described.22 In the inner-loop the minimum number needed for the highest predictive sensitivity of 100% was determined to be 75 probe sets (Table 2, Online Supplementary Table S3), i.e. 15 probe sets per cytogenetic subtype, whereas randomly selected probe sets only yielded a median sensitivity of 60% (Online Supplementary Figure S2). Some of these probe sets represented the same gene, e.g. four probe sets presented RUNX1T1 for the t(8;21)(q22;q22) subtype (Table 2). The constructed classifier took into account the expression levels of all 75 probe sets, including the 60 probe sets that were not selected for a particular subtype. The classifier built with these 75 probe sets yielded a median accuracy of 92% in the outer loop. Notably, all inv(16)(p13q22), t(15;17)(q21;q22) and t(7;12)(q36;p13)-positive cases were correctly predicted in each of the 100 iterations (100% sensitivity, specificity, positive predictive value and negative predictive value) (Table 3A).
Table 2.
Overview of discriminative genes used in the classification of pediatric acute myeloid leukemia.
Table 3.
Diagnostic test values for the classification of pediatric acute myeloid leukemia by a gene expression signature consisting of 75 probe sets.
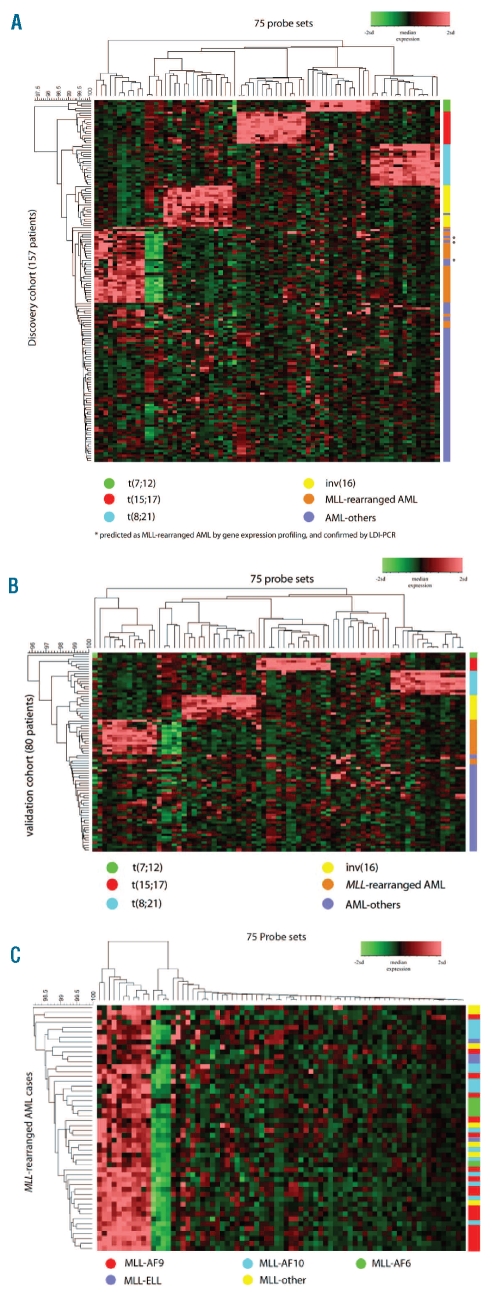
Hierarchical clustering showed that the cytogenetic subtypes formed distinct clusters according to the gene expression signature using these 75 probe sets (Figure 1A, Online Supplementary Table S3). Only three AML-other patients were misclassified as having MLL-rearranged AML. In these cases, MLL involvement could not be confirmed with FISH, but LDI-PCR revealed that all three samples did indeed harbor an MLL-rearrangement (Online Supplementary Table S4). These three samples were, therefore, included as true positive MLL-rearranged cases in the construction of the final classifier in the discovery cohort. As expected, including these cases as MLL-rearranged AML improved the diagnostic values of the 3-fold cross validation in the outer loop (Table 3B).
Figure 1.
Hierarchical clustering of the cytogenetic subtypes of pediatric AML by gene expression profiling. (A) Hierarchical clustering of 157 patients in the discovery cohort by gene expression signature derived from 75 classifying probe sets (Online Supplementary Table S2). (B) Validation of the gene expression pattern in 80 patients of the independent validation cohort (C) MLL-rearranged AML cases do not separate into distinct clusters based on similarity in expression pattern related to the translocation partner using the 75 classifying probe sets.
Independent validation of the classifier
The true accuracy of the classifier was tested in the independent validation cohort of 80 patients. The true sensitivity, specificity, positive predictive value, negative predictive value and accuracy in this validation cohort was 98%, 100%, 100%, 97% and 99%, respectively (Table 3C). Only one MLL-rearranged AML case was misclassified as AML-other (Table 4). Hierarchical cluster analysis also demonstrated the discriminative value of the selected probe sets in the independent validation cohort (Figure 1B). At this point, only patients at initial diagnosis of AML had been included. Next, we addressed whether the classifier was also suitable for predicting the subtype of 33 relapsed and eight secondary AML cases. All nine MLL-rearranged cases (3 secondary and 6 relapsed AML cases), all five t(8;21)(q22;q22) relapsed cases and all 27 other relapsed and secondary AML cases were correctly predicted by our classifier (Online Supplementary Table S5).
Table 4.
Prediction of the classifier on the independent validation cohort.
Comparison with other gene expression profiles in pediatric and adult acute myeloid leukemia
Ross et al. demonstrated that children with AML could be classified using gene expression profiles generated by Affymetrix U133A microarrays containing 22,283 probe sets.17 An overall accuracy of 93% was achieved using 150 probe sets to classify MLL-rearranged AML, t(8;21)(q22;q22), inv(16)(p13q22), t(15;17)(q21;q22) and acute megakaryoblastic leukemias (M7). In that study, t(7;12)(q36;p13)-positive AML cases were not included. The 150 probe sets of Ross et al. were used to construct a classifier in our discovery cohort which was then applied to the independent validation cohort, exactly as done for testing our own 75 probe set-based classifier. Five out of 80 patients were misclassified, yielding an overall predictive accuracy of 94% for Ross’ set compared to 99% for the 75 probe sets selected in this study (Online Supplementary Table S6). The misclassified cases included two MLL-rearranged and two t(7;12)(q36;p13)-positive cases which were assigned to the AML-other category and one AML-other case which was predicted as an MLL-rearranged case. Since only 40 out of our 75 probe sets were present on the U133A microarray used by Ross et al., the reciprocal comparison of our list of selected probe sets on Ross’ dataset was not informative.
Valk et al. described 16 different subgroups in adult AML using 2,856 probe sets present on Affymetrix U133A microarrays.18 A classifier built with these 2,856 probe sets resulted in an overall accuracy of 94% when applied to our independent pediatric validation cohort (Online Supplementary Table S6). Three MLL-rearranged cases and one t(7;12)(q36;p13)-positive case were misclassified as AML-others. One AML-other patient was misclassified as having MLL-rearranged AML.
Potential type II molecular aberrations: NPM1, CEBPA and MLL-PTD
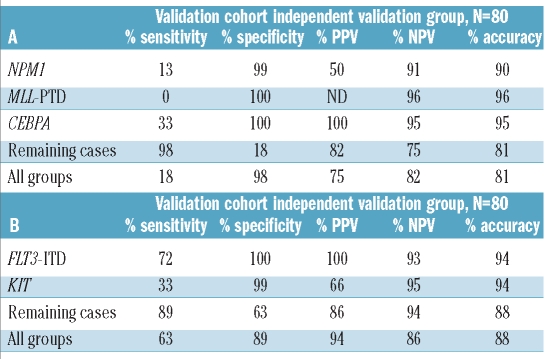
Mutations in NPM1 and CEBPA and partial tandem duplications in MLL (MLL-PTD) might be considered as various type II mutations and were only observed in samples belonging to the AML-other group (Online Supplementary Table S7). Moreover, these molecular abnormalities were mutually exclusive reflecting heterogeneity among the AML-other cases. For CEBPA, 852 probe sets were found to be statistically discriminative between mutated and germ-line cases whereas only 12 probe sets were found to be discriminative for MLL-PTD at the same cut-off value of P<0.05 (FDR-corrected; Online Supplementary Table S8). Three-fold cross validation with the top 15 most discriminative probe sets for mutations in NPM1, CEBPA and MLL (i.e. MLL-PTD) revealed a median sensitivity and accuracy in the outer loop of 43% and 92%, respectively, and sensitivity, specificity, positive predictive value, negative predictive value and accuracy of 18%, 98%, 75%, 82% and 81%, respectively, in the independent validation cohort (Table 5A, Online Supplementary Table S9). In the independent validation cohort 7/8 cases with an NPM1 mutation, 4/6 cases with a CEBPA mutation and all three MLL-PTD cases were misclassified. Moreover, when adding these three molecular subtypes to the previously used five cytogenetic subtypes, the accuracy of 99% based on the five cytogenetic subtypes dropped to 78% in the validation cohort (Online Supplementary Table S10). All misclassified cases were assigned to the AML-other category.
Table 5.
Diagnostic test values for the prediction of mutations in NPM1, CEBP· and MLL-PTD for the independent validation cohort by the gene expression signature consisting of 45 probe sets (A) and for the prediction of type I molecular subtypes for the independent validation cohort by the gene expression signature consisting of 30 probe sets (B).
Type I mutations: FLT3-ITD, KIT, N/K-RAS and PTPN11
Internal tandem duplication in FLT3 (FLT3-ITD), mutations in KIT and mutations in genes involved in the RAS-pathway [NRAS (n=34), KRAS (n=7) and PTPN11 (n=5)] were observed in 44% of all cases. In contrast to FLT3-ITD and KIT aberrations, no discriminative probe sets were found for N/K-RAS and only a limited number for PTPN11 (Online Supplementary Table S8). Combining the aberrations in the RAS-pathway into one group still did not identify discriminative probe sets. We, therefore, only included FLT3-ITD and KIT into a classification model for the prediction of type I mutations. However, the 30 most discriminative probe sets for these subtypes resulted in a classifier with limited predictive value. The highest predictive values were found for FLT3-ITD, with a positive predictive value and negative predictive value of 100% and 93%, respectively (Table 5B, Online Supplementary Table S11). Inclusion of the top 15 discriminative probe sets for aberrations in the RAS-pathway (although with P>0.05) did not result in prediction of this subtype (Online Supplementary Table S12).
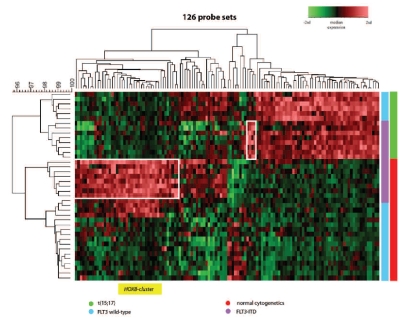
Although a large number of discriminative genes were found for FLT3-ITD (Online Supplementary Table S8), many probe sets were similar to those found for t(15;17)(q21;q22) instead of being specific for FLT3-ITD. This is in line with the fact that FLT3-ITD is often found in t(15;17)(q21;q22)-positive cases. To correct for these cytogenetic effects, we applied the Bayes linear regression model while adjusting for cytogenetic subtype. In this multivariate analysis, unique gene expression signatures specific for FLT3-ITD-positive cases were found that differ between t(15;17)(q21;q22)-positive and CN-AML cases (Figure 2, Online Supplementary Table S13). Specifically, the genes of the HOXB cluster were over-expressed in all patients with a FLT3-ITD-positive CN-AML and not in FLT3-ITD-negative CN-AML or t(15;17)(q21;q22) patients (Figure 2, Online Supplementary Figure S3). The same multivariate approach for KIT and RAS-pathway mutations did not result in cytogenetic subtype-specific gene expression signatures.
Figure 2.
Hierarchical clustering of FLT3-ITD positive cases in t(15;17)(q21;q22) and CN-AML. Hierarchical clustering for FLT3-ITD in t(15;17)(q21;q22) and CN-AML based on 126 probe sets selected by multivariate analysis including molecular and cytogenetic subtypes (Online Supplementary Table S12). Highlighted boxes represent probe sets for FLT3-ITD and the specific cytogenetic subtype. The HOXB cluster probe sets are represented in yellow.
When adding the 15 probe sets discriminative for t(15;17)(q21;q22)/FLT3-ITD and the nine most discriminative probe sets [the 6 other probe sets showed overlap with the t(15;17)(q21;q22) subgroup] for CN-AML/FLT3-ITD from the multivariate analysis to our classifier, we still could not accurately predict all FLT3-ITD cases. Although numbers were small in these subgroups, the accuracy in the independent validation cohort dropped to 86% due to misclassification of cases, especially CN-AML/FLT3-ITD cases (Online Supplementary Table S14).
Discussion
Cytogenetic aberrations have prognostic value in pediatric AML and, hence, genetic subtypes are used for risk stratification in most current pediatric AML treatment protocols and are part of the current WHO classification of myeloid neoplasms and acute leukemia.2 In the present study we explored the possibility of microarray-based gene expression profiling to identify the cytogenetic and molecular subtypes in pediatric AML. A gene expression signature of 75 probe sets predicted the most important non-random cytogenetic aberrations in an independent pediatric AML cohort with 99% accuracy and a positive and negative predictive value of 100% and 97%, respectively. In addition, unique gene-expression signatures were found for FLT3-ITD AML which differed between cytogenetic subtypes.
Gene expression profiling has been shown to predict the major cytogenetic subgroups in both pediatric and adult AML.17–19 The sensitivity and specificity of such signatures should be addressed in an independent and representative cohort, since microarray data analysis can easily result in over-interpretation of data.42 Recently, the MILE study group, using an independent validation cohort of 1,152 cases, robustly showed that gene expression profiles can be used to classify different types of (mainly adult) myelodysplastic syndrome and chronic and acute leukemia cases into known cytogenetic subtypes.21 This study mainly included adult cases (whose disease may differ in etiology from that of children) and did not address the prediction of molecular abnormalities (e.g. FLT3-ITD and RAS mutations) by gene expression profiles. In the present study we specifically addressed the value of gene expression profiles for prediction of and classification based on cytogenetic and molecular subtypes of children at initial diagnosis of AML. We previously showed the predictive value of a double-loop cross-validation approach to select classifying probe sets and validated these using an independent validation cohort in pediatric ALL,22 and therefore used the same unbiased approach in this childhood AML study.
In the present study we identified a gene expression signature of 75 probe sets, representing 15 probe sets for each subgroup, to predict MLL-rearranged, t(8;21)(q22;q22), inv(16)(p13q22), t(15;17)(q21;q22) and t(7;12)(q36;p13) positive AML. When applied to our independent validation cohort the sensitivity, specificity, and negative and positive predictive values of this signature were 98%, 100%, 100% and 97%, respectively. The prediction of MLL-rearranged AML, in particular, was better with the newly selected probe sets than with the previously used probe sets compiled by Ross et al.17 and Valk et al.18 Since 35 probe sets were not present on the Affymetrix U133A microarrays used in both of the former studies, these new probe sets are perhaps decisive for correct prediction of cytogenetic subgroups in pediatric AML.
The 75 probe sets harbored probe sets for genes involved in the specific translocations, e.g. the probe sets for RUNX1T1/ETO were highly discriminative for t(8;21)(q22;q22), those for MYH11 for inv(16)(p13q22) and the probe set for HLXB9 for t(7;12)(q36;p13). The high expression of these probe sets is probably related to specific hybridization to the fusion transcript, as suggested by Kohlmann et al.43 Remarkably, six out of 15 probe sets discriminative for MLL-rearranged AML (Table 2) were located in non-protein coding regions of the genome. Four of these probe sets were located in a relative small (<40 Kb) region on chromosome 10. This is of interest, since nowadays these regions cannot be considered as junk DNA, but might be involved in the regulation of other genes, such as miRNA.44
In parallel to the present study we found that expression of one of the discriminative genes for MLL-rearranged AML, i.e. brain and reproductive organ-expressed gene (BRE), was highly associated with a favorable prognosis in cases with t(9;11)(p22;q23). Functional studies with BRE did not reveal that the proliferation, apoptosis or sensitivity towards drugs was altered upon re-expression in AML cells, suggesting that BRE itself has no anti-proliferative function.45 Besides BRE, other new prognostic genes have recently been identified using gene expression profiling. An example is the angiogenic factor VEGFC, for which a high level of expression was associated with an unfavorable clinical outcome in both childhood and adult AML.46 According to gene expression profiling, the EVI1 gene had no prognostic impact in children with AML, in contrast to the situation in adults, emphasizing the need for separate analysis of pediatric and adult AML.47 In adult AML gene expression profiling showed that high expression of ERG and MN1 was related to outcome, although their role in malignant transformation remains unknown.48,49 More recently a unique gene expression signature was identified for the prognostically relevant mutation in IDH2, which may help to unravel the role of IDH2 in the biology of AML.50 Thus, gene expression profiling identifies new genes linked to subtypes and/or prognosis of AML and may provide important information about the biology of disease when further functional studies have been performed. This knowledge is needed for the rational development and optimization of treatment protocols; moreover, affected genes and pathways may serve as targets for new therapies (‘targeted therapy’).
Three AML-other cases that were negative by MLL-split signal FISH were initially thought to be misclassified as MLL-rearranged AML. However, more detailed analysis of the MLL-gene using LDI-PCR confirmed that the MLL-gene was indeed rearranged, indicating the higher sensitivity of our gene expression signature than the routine diagnostic FISH procedure in the detection of MLL-rearranged cases. Cryptic MLL-rearrangements can also be detected using single nucleotide polymorphism (SNP)-array platforms e.g. t(6;11)(q27;q23).51,52 These finding illustrate the high potential of advanced methods, such as gene expression profiles, LDI-PCR and SNP-arrays, for detecting rearrangements of the MLL gene in clinical AML samples.
The cytogenetic subgroups that could be correctly predicted in our study represented approximately 40% of the pediatric AML cases (Online Supplementary Table S1). The remaining patients in this study had CN-AML or cytogenetic aberrations other than the five cytogenetic subgroups tested in this study. Recently the subgroup with CN-AML has been further characterized by recurrent molecular aberrations in NPM1, CEBPA and MLL (-PTD). The heterogeneity of pediatric AML is further illustrated by molecular aberrations detected in different cytogenetic subgroups, i.e. FLT3-ITD, KIT and mutations in the RAS-pathway. Our results show a less accurate prediction of these molecular aberrations by gene expression signatures compared to signatures predictive for cytogenetic subtypes, as was also observed in adult AML.19,53 Only FLT3-ITD could be predicted with a high negative predictive value (93%) and positive predictive value (100%) in the independent validation cohort, but showed lower sensitivity, as described in adult AML cases with FLT3-ITD.54 The low predictive value for the molecular subtypes including mutations in NPM1, MLL-PTD, KIT and PTPN11 can be explained by the limited number of discriminative probe sets found for each of these aberrations (Online Supplementary Table S8 versus Online Supplementary Table S2). This may be because of both limited sample size for each molecular subtype and underlying cytogenetic lesions that have a differential effect on gene expression signatures. A limited sample size itself does not, per se, hamper the accuracy of classification if the number of statistically significant probe sets and the fold-change in expression levels of discriminative probe sets is relatively high (exemplified by the high accuracy at predicting t(7;12)(q36;p13)-positive cases despite only five cases being included in the discovery cohort, Online Supplementary Table S2). However, in combination with heterogeneity in underlying cytogenetic abnormalities (or other genetic lesions) the number of highly discriminative probe sets becomes limited when sample size is also limited.
Since some of these mutations are not mutually exclusive or are restricted to distinct cytogenetic subtypes, we also selected discriminative probe sets for FLT3-ITD using a multivariate approach including cytogenetic subtype. For the other molecular subtypes probe sets could not be identified in a multivariate setting, presumably because of the limited frequency of occurrence of these mutations. Moreover, overlapping signaling pathways between subgroups, no effect of the mutation on transcript level, or different mutations per gene can make it difficult to predict these molecular aberrations correctly by gene expression profiles.15,55
Depending on the cytogenetic background, specific genetic aberrations may play different roles in the leukemogenesis of pediatric AML. Interestingly, the genes of the HOXB cluster were over-expressed in all patients with a FLT3-ITD positive CN-AML, but not in those with a FLT3-ITD-positive t(15;17)(q21;q22), which is in concordance with differences in prognostic relevance between these two subgroups. In adult AML, some of these HOXB genes were also identified as discriminating genes for patients with a FLT3-ITD.19 In pediatric AML, HOXB up-regulation has been correlated with NPM1 mutations in CN-AML.56 Here we show that HOXB over-expression is not restricted to NPM1-mutated cases, but is also found in all patients with FLT3-ITD-positive CN-AML.
In conclusion, a specific gene expression signature existing of 75 probe sets could accurately identify five cytogenetic subgroups in pediatric AML. Molecular aberrations were hard to predict, which could be due to the low frequency of some of these aberrations and/or gene expression signatures being affected by the underlying cytogenetic abnormality. It remains to be determined whether underlying but yet unknown genetic aberrations in the remaining cases of AML will result in distinct gene expression patterns that can be used for classification. Classification by gene expression profiling may reduce the number of cases for which multiple diagnostic procedures (cytomorphology, FISH, RT-PCR, karyotyping) are performed by at least 40%. In order to use gene expression signatures as a new diagnostic tool, prospective studies are needed that determine the feasibility of obtaining sufficient high-quality RNA for successful gene expression profiling in clinical practice. Importantly, gene expression profiles may give more insight into the biology and the pathophysiology of the different subtypes of AML which may then point to new ways to treat these patients more effectively.
Footnotes
Funding: BVB was funded by the ‘Netherlands Organisation for Scientific Research’ (NWO). RXM was partially funded by the Dutch Cancer Society (grant EMCR 2005-3662) and the Center of Medical Systems Biology (CMSB) established by the Netherlands Genomics Initiative/Netherlands Organisation for Scientific Research (NGI/NWO). HBB was partly funded by the Dutch Cancer Society (grant EMCR 2003-2871). This research was sponsored by a grant from the Quality of Life Foundation, the Netherlands (R.P, MLDB). JT was funded by the Czech Ministry of Education (grant COST-OC09051).
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Downing JR, Shannon KM. Acute leukemia: a pediatric perspective. Cancer Cell. 2002;2(6):437–45. doi: 10.1016/s1535-6108(02)00211-8. [DOI] [PubMed] [Google Scholar]
- 2.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–51. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 3.Kaspers GJ, Zwaan CM. Pediatric acute myeloid leukemia: towards high-quality cure of all patients. Haematologica. 2007;92(11):1519–32. doi: 10.3324/haematol.11203. [DOI] [PubMed] [Google Scholar]
- 4.Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood. 1998;92(7):2322–33. [PubMed] [Google Scholar]
- 5.Raimondi SC, Chang MN, Ravindranath Y, Behm FG, Gresik MV, Steuber CP, et al. Chromosomal abnormalities in 478 children with acute myeloid leukemia: clinical characteristics and treatment outcome in a cooperative pediatric oncology group study-POG 8821. Blood. 1999;94(11):3707–16. [PubMed] [Google Scholar]
- 6.Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100(5):1532–42. doi: 10.1182/blood-2002-02-0492. [DOI] [PubMed] [Google Scholar]
- 7.Balgobind BV, Van Vlierberghe P, van den Ouweland AM, Beverloo HB, Terlouw-Kromosoeto JN, van Wering ER, et al. Leukemia-associated NF1 inactivation in patients with pediatric T-ALL and AML lacking evidence for neurofibromatosis. Blood. 2008;111(8):4322–8. doi: 10.1182/blood-2007-06-095075. [DOI] [PubMed] [Google Scholar]
- 8.Renneville A, Roumier C, Biggio V, Nibourel O, Boissel N, Fenaux P, et al. Cooperating gene mutations in acute myeloid leukemia: a review of the literature. Leukemia. 2008;22(5):915–31. doi: 10.1038/leu.2008.19. [DOI] [PubMed] [Google Scholar]
- 9.Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98(6):1752–9. doi: 10.1182/blood.v98.6.1752. [DOI] [PubMed] [Google Scholar]
- 10.Zwaan CM, Meshinchi S, Radich JP, Veerman AJ, Huismans DR, Munske L, et al. FLT3 internal tandem duplication in 234 children with acute myeloid leukemia: prognostic significance and relation to cellular drug resistance. Blood. 2003;102(7):2387–94. doi: 10.1182/blood-2002-12-3627. [DOI] [PubMed] [Google Scholar]
- 11.Paschka P, Marcucci G, Ruppert AS, Mrozek K, Chen H, Kittles RA, et al. Adverse prognostic significance of KIT mutations in adult acute myeloid leukemia with inv(16) and t(8;21): a Cancer and Leukemia Group B Study. J Clin Oncol. 2006;24(24):3904–11. doi: 10.1200/JCO.2006.06.9500. [DOI] [PubMed] [Google Scholar]
- 12.Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005;352(3):254–66. doi: 10.1056/NEJMoa041974. [DOI] [PubMed] [Google Scholar]
- 13.Frohling S, Schlenk RF, Stolze I, Bihlmayr J, Benner A, Kreitmeier S, et al. CEBPA mutations in younger adults with acute myeloid leukemia and normal cytogenetics: prognostic relevance and analysis of cooperating mutations. J Clin Oncol. 2004;22(4):624–33. doi: 10.1200/JCO.2004.06.060. [DOI] [PubMed] [Google Scholar]
- 14.Basecke J, Whelan JT, Griesinger F, Bertrand FE. The MLL partial tandem duplication in acute myeloid leukaemia. Br J Haematol. 2006;135(4):438–49. doi: 10.1111/j.1365-2141.2006.06301.x. [DOI] [PubMed] [Google Scholar]
- 15.Wouters BJ, Lowenberg B, Erpelinck-Verschueren CA, van Putten WL, Valk PJ, Delwel R. Double CEBPA mutations, but not single CEBPA mutations, define a subgroup of acute myeloid leukemia with a distinctive gene expression profile that is uniquely associated with a favorable outcome. Blood. 2009;113(13):3088–91. doi: 10.1182/blood-2008-09-179895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bullinger L, Dohner K, Bair E, Frohling S, Schlenk RF, Tibshirani R, et al. Use of gene-expression profiling to identify prognostic subclasses in adult acute myeloid leukemia. N Engl J Med. 2004;350(16):1605–16. doi: 10.1056/NEJMoa031046. [DOI] [PubMed] [Google Scholar]
- 17.Ross ME, Mahfouz R, Onciu M, Liu HC, Zhou X, Song G, et al. Gene expression profiling of pediatric acute myelogenous leukemia. Blood. 2004;104(12):3679–87. doi: 10.1182/blood-2004-03-1154. [DOI] [PubMed] [Google Scholar]
- 18.Valk PJ, Verhaak RG, Beijen MA, Erpelinck CA, Barjesteh van Waalwijk van Doorn-Khosrovani S, Boer JM, et al. Prognostically useful gene-expression profiles in acute myeloid leukemia. N Engl J Med. 2004;350 (16):1617–28. doi: 10.1056/NEJMoa040465. [DOI] [PubMed] [Google Scholar]
- 19.Verhaak RG, Wouters BJ, Erpelinck CA, Abbas S, Beverloo HB, Lugthart S, et al. Prediction of molecular subtypes in acute myeloid leukemia based on gene expression profiling. Haematologica. 2009;94(1):131–4. doi: 10.3324/haematol.13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersson A, Ritz C, Lindgren D, Eden P, Lassen C, Heldrup J, et al. Microarray-based classification of a consecutive series of 121 childhood acute leukemias: prediction of leukemic and genetic subtype as well as of minimal residual disease status. Leukemia. 2007;21(6):1198–203. doi: 10.1038/sj.leu.2404688. [DOI] [PubMed] [Google Scholar]
- 21.Haferlach T, Kohlmann A, Wieczorek L, Basso G, Kronnie GT, Bene MC, et al. Clinical utility of microarray-based gene expression profiling in the diagnosis and subclassification of leukemia: report from the International Microarray Innovations in Leukemia Study Group. J Clin Oncol. 2010;28(15):2529–37. doi: 10.1200/JCO.2009.23.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Den Boer ML, van Slegtenhorst M, De Menezes RX, Cheok MH, Buijs-Gladdines JG, Peters ST, et al. A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: a genome-wide classification study. Lancet Oncol. 2009;10(2):125–34. doi: 10.1016/S1470-2045(08)70339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Den Boer ML, Harms DO, Pieters R, Kazemier KM, Gobel U, Korholz D, et al. Patient stratification based on pred-nisolone-vincristine-asparaginase resistance profiles in children with acute lymphoblastic leukemia. J Clin Oncol. 2003;21(17):3262–8. doi: 10.1200/JCO.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 24.Van Vlierberghe P, van Grotel M, Tchinda J, Lee C, Beverloo HB, van der Spek PJ, et al. The recurrent SET-NUP214 fusion as a new HOXA activation mechanism in pediatric T-cell acute lymphoblastic leukemia. Blood. 2008;111(9):4668–80. doi: 10.1182/blood-2007-09-111872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Dongen JJ, Macintyre EA, Gabert JA, Delabesse E, Rossi V, Saglio G, et al. Standardized RT-PCR analysis of fusion gene transcripts from chromosome aberrations in acute leukemia for detection of minimal residual disease. Report of the BIOMED-1 Concerted Action: investigation of minimal residual disease in acute leukemia. Leukemia. 1999;13(12):1901–28. doi: 10.1038/sj.leu.2401592. [DOI] [PubMed] [Google Scholar]
- 26.von Bergh A, Emanuel B, van Zelderen-Bhola S, Smetsers T, van Soest R, Stul M, et al. A DNA probe combination for improved detection of MLL/11q23 breakpoints by double-color interphase-FISH in acute leukemias. Genes Chromosomes Cancer. 2000;28(1):14–22. doi: 10.1002/(sici)1098-2264(200005)28:1<14::aid-gcc2>3.3.co;2-o. [DOI] [PubMed] [Google Scholar]
- 27.Meyer C, Schneider B, Reichel M, Angermueller S, Strehl S, Schnittger S, et al. Diagnostic tool for the identification of MLL rearrangements including unknown partner genes. Proc Natl Acad Sci USA. 2005;102(2):449–54. doi: 10.1073/pnas.0406994102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Bergh AR, van Drunen E, van Wering ER, van Zutven LJ, Hainmann I, Lonnerholm G, et al. High incidence of t(7;12)(q36;p13) in infant AML but not in infant ALL, with a dismal outcome and ectopic expression of HLXB9. Genes Chromosomes Cancer. 2006;45(8):731–9. doi: 10.1002/gcc.20335. [DOI] [PubMed] [Google Scholar]
- 29.Barjesteh van Waalwijk van Doorn-Khosrovani S, Erpelinck C, Meijer J, van Oosterhoud S, van Putten WL, Valk PJ, et al. Biallelic mutations in the CEBPA gene and low CEBPA expression levels as prognostic markers in intermediate-risk AML. Hematol J. 2003;4(1):31–40. doi: 10.1038/sj.thj.6200216. [DOI] [PubMed] [Google Scholar]
- 30.Kiyoi H, Naoe T, Yokota S, Nakao M, Minami S, Kuriyama K, et al. Internal tandem duplication of FLT3 associated with leukocytosis in acute promyelocytic leukemia. Leukemia Study Group of the Ministry of Health and Welfare (Kohseisho) Leukemia. 1997;11(9):1447–52. doi: 10.1038/sj.leu.2400756. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto Y, Kiyoi H, Nakano Y, Suzuki R, Kodera Y, Miyawaki S, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97(8):2434–9. doi: 10.1182/blood.v97.8.2434. [DOI] [PubMed] [Google Scholar]
- 32.Caligiuri MA, Strout MP, Schichman SA, Mrozek K, Arthur DC, Herzig GP, et al. Partial tandem duplication of ALL1 as a recurrent molecular defect in acute myeloid leukemia with trisomy 11. Cancer Res. 1996;56(6):1418–25. [PubMed] [Google Scholar]
- 33.Balgobind BV, Hollink IH, Reinhardt D, van Wering ER, de Graaf SS, Baruchel A, et al. Low frequency of MLL-partial tandem duplications in paediatric acute myeloid leukaemia using MLPA as a novel DNA screenings technique. Eur J Cancer. 2010;46(10):1892–9. doi: 10.1016/j.ejca.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 34.Smyth G. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3(1):1. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 35.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc B Met. 1995;57(1):289–300. [Google Scholar]
- 36.Wessels LF, Reinders MJ, Hart AA, Veenman CJ, Dai H, He YD, et al. A protocol for building and evaluating predictors of disease state based on microarray data. Bioinformatics. 2005;21(19):3755–62. doi: 10.1093/bioinformatics/bti429. [DOI] [PubMed] [Google Scholar]
- 37.Huber W, von Heydebreck A, Sultmann H, Poustka A, Vingron M. Variance stabilization applied to microarray data calibration and to the quantification of differential expression. Bioinformatics. 2002;18 (Suppl 1):S96–104. doi: 10.1093/bioinformatics/18.suppl_1.s96. [DOI] [PubMed] [Google Scholar]
- 38.Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31(4):e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dimitriadou E, Hornik K, Leisch F, Meyer D, Weingessel A. In: e1071: Misc Functions of the Department of Statistics (e1071) Wien TU, editor. 2007. [Google Scholar]
- 40.Pollard KS, Ge Y, Taylor S, Dudoit S. multtest: Resampling-based multiple hypothesis testing. R package version 1.16.1 2005 [Google Scholar]
- 41.Yang YH. marray: Exploratory analysis for two-color spotted microarray data. 2007. [Google Scholar]
- 42.Michiels S, Koscielny S, Hill C. Prediction of cancer outcome with microarrays: a multiple random validation strategy. Lancet. 2005;365(9458):488–92. doi: 10.1016/S0140-6736(05)17866-0. [DOI] [PubMed] [Google Scholar]
- 43.Kohlmann A, Schoch C, Schnittger S, Dugas M, Hiddemann W, Kern W, et al. Molecular characterization of acute leukemias by use of microarray technology. Genes Chromosomes Cancer. 2003;37(4):396–405. doi: 10.1002/gcc.10225. [DOI] [PubMed] [Google Scholar]
- 44.Schotte D, Chau JC, Sylvester G, Liu G, Chen C, van der Velden VH, et al. Identification of new microRNA genes and aberrant microRNA profiles in childhood acute lymphoblastic leukemia. Leukemia. 2009;23(2):313–22. doi: 10.1038/leu.2008.286. [DOI] [PubMed] [Google Scholar]
- 45.Balgobind BV, Zwaan CM, Arentsen-Peters STCJM, Reinhardt D, Creutzig U, de Haas V, et al. High BRE expression in pediatric MLL-rearranged AML is associated with favorable outcome. Leukemia. 2010;24(12):2048–55. doi: 10.1038/leu.2010.211. [DOI] [PubMed] [Google Scholar]
- 46.de Jonge HJ, Valk PJ, Veeger NJ, Ter Elst A, den Boer ML, Cloos J, et al. High VEGFC expression is associated with unique gene expression profiles and predicts adverse prognosis in pediatric and adult acute myeloid leukemia. Blood. 2010;116(10):1747–54. doi: 10.1182/blood-2010-03-270991. [DOI] [PubMed] [Google Scholar]
- 47.Balgobind BV, Lugthart S, Hollink IH, Arentsen-Peters ST, van Wering ER, de Graaf SS, et al. EVI1 overexpression in distinct subtypes of pediatric acute myeloid leukemia. Leukemia. 2010;24(5):942–9. doi: 10.1038/leu.2010.47. [DOI] [PubMed] [Google Scholar]
- 48.Metzeler KH, Dufour A, Benthaus T, Hummel M, Sauerland MC, Heinecke A, et al. ERG expression is an independent prognostic factor and allows refined risk stratification in cytogenetically normal acute myeloid leukemia: a comprehensive analysis of ERG, MN1, and BAALC transcript levels using oligonucleotide microarrays. J Clin Oncol. 2009;27(30):5031–8. doi: 10.1200/JCO.2008.20.5328. [DOI] [PubMed] [Google Scholar]
- 49.Langer C, Marcucci G, Holland KB, Radmacher MD, Maharry K, Paschka P, et al. Prognostic importance of MN1 transcript levels, and biologic insights from MN1-associated gene and microRNA expression signatures in cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. J Clin Oncol. 2009;27(19):3198–204. doi: 10.1200/JCO.2008.20.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marcucci G, Maharry K, Wu YZ, Radmacher MD, Mrozek K, Margeson D, et al. IDH1 and IDH2 gene mutations identify novel molecular subsets within de novo cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. J Clin Oncol. 2010;28(14):2348–55. doi: 10.1200/JCO.2009.27.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Radtke I, Mullighan CG, Ishii M, Su X, Cheng J, Ma J, et al. Genomic analysis reveals few genetic alterations in pediatric acute myeloid leukemia. Proc Natl Acad Sci USA. 2009;106(31):12944–9. doi: 10.1073/pnas.0903142106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bullinger L, Kronke J, Schon C, Radtke I, Urlbauer K, Botzenhardt U, et al. Identification of acquired copy number alterations and uniparental disomies in cytogenetically normal acute myeloid leukemia using high-resolution single-nucleotide polymorphism analysis. Leukemia. 2010;24(2):438–49. doi: 10.1038/leu.2009.263. [DOI] [PubMed] [Google Scholar]
- 53.Kohlmann A, Bullinger L, Thiede C, Schaich M, Schnittger S, Dohner K, et al. Gene expression profiling in AML with normal karyotype can predict mutations for molecular markers and allows novel insights into perturbed biological pathways. Leukemia. 2010;24(6):1216–20. doi: 10.1038/leu.2010.73. [DOI] [PubMed] [Google Scholar]
- 54.Bullinger L, Dohner K, Kranz R, Stirner C, Frohling S, Scholl C, et al. An FLT3 gene-expression signature predicts clinical outcome in normal karyotype AML. Blood. 2008;111(9):4490–5. doi: 10.1182/blood-2007-09-115055. [DOI] [PubMed] [Google Scholar]
- 55.Loriaux MM, Levine RL, Tyner JW, Frohling S, Scholl C, Stoffregen EP, et al. High-throughput sequence analysis of the tyro-sine kinome in acute myeloid leukemia. Blood. 2008;111(9):4788–96. doi: 10.1182/blood-2007-07-101394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mullighan CG, Kennedy A, Zhou X, Radtke I, Phillips LA, Shurtleff SA, et al. Pediatric acute myeloid leukemia with NPM1 mutations is characterized by a gene expression profile with dysregulated HOX gene expression distinct from MLL-rearranged leukemias. Leukemia. 2007;21 (9):2000–9. doi: 10.1038/sj.leu.2404808. [DOI] [PubMed] [Google Scholar]