Abstract
Background
Acute myeloid leukemia is a life-threatening disease associated with high mortality rates. A substantial number of patients require intensive care. This investigation analyzes risk factors predicting admission to the intensive care unit in patients with acute myeloid leukemia eligible for induction chemotherapy, the outcome of these patients, and prognostic factors predicting their survival.
Design and Methods
A total of 406 consecutive patients with de novo acute myeloid leukemia (15–89 years) were analyzed retrospectively. Markers recorded at the time of diagnosis included karyotype, fibrinogen, C-reactive protein, and Charlson comorbidity index. In patients requiring critical care, the value of the Simplified Acute Physiology Score II, the need for mechanical ventilation, and vasopressor support were recorded at the time of intensive care unit admission. The independent prognostic relevance of the parameters was tested by multivariate analysis.
Results
Sixty-two patients (15.3%) required intensive care, primarily due to respiratory failure (50.0%) or life-threatening bleeding (22.6%). Independent risk factors predicting intensive care unit admission were lower fibrinogen concentration, the presence of an infection, and comorbidity. The survival rate was 45%, with the Simplified Acute Physiology Score II being the only independent prognostic parameter (P<0.05). Survival was inferior in intensive care patients compared to patients not admitted to an intensive care unit. However, no difference between intensive care and non-intensive care patients was found concerning continuous complete remission at 6 years or survival at 6 years in patients who survived the first 30 days after diagnosis (non-intensive care patients: 28%; intensive care patients: 20%, P>0.05).
Conclusions
Ongoing infections, low fibrinogen and comorbidity are predictive for intensive care unit admission in acute myeloid leukemia. Although admission was a risk factor for survival, continuous complete remission and survival of patients alive at day 30 were similar in patients who were admitted or not admitted to an intensive care unit.
Keywords: acute myeloid leukemia, intensive care unit, outcome, infection
Introduction
Acute myeloid leukemia (AML) is a life-threatening stem cell disorder characterized by rapid and uncontrolled proliferation and accumulation of myeloblasts.1,2 The prognosis and clinical course of AML differs among patients depending on specific molecular and cytogenetic properties of the clone.1–9 Moreover, apart from disease-associated features, patient-related factors including age and pre- or co-existing diseases (comorbidity) are of prognostic importance.1,10–12 Although the rate of complete remission in AML patients is relatively high (ranging between 60 and 75%), only a small percentage of patients are permanently cured.12–14
Patients with AML usually present with severely compromised bone marrow function.1 Some patients receive intensive chemotherapy resulting in further suppression of their immune system. As a result, infectious complications are frequent and may lead to serious organ dysfunction or even (multi)organ failure requiring admission to an intensive care unit (ICU).1 High mortality rates (80–90%) have been reported in patients with AML admitted to an ICU, especially for those who need invasive mechanical ventilation.15,16 Thus, therapy in the ICU was described to be largely unsuccessful in AML.15,16 However, a recent study reported more encouraging results in AML patients admitted to the ICU.17 The current analysis was, therefore, conducted to determine the percentage of patients with de novo AML requiring ICU treatment prior to or during induction chemotherapy. Furthermore, we attempted to identify parameters predicting admission to the ICU as well as prognostic factors associated with the outcome in ICU patients.
Design and Methods
Patients’ characteristics
Between October 1994 and November 2006, a total of 406 consecutive patients with de novo AML eligible for induction chemotherapy (median age, 59 years; range, 15–89 years) were seen at the Vienna University Hospital. Diagnoses were established according to the French-American-British (FAB) cooperative study group criteria.18 White blood cell count, platelet count, age, hemoglobin, lactate dehydrogenase, C-reactive protein, and fibrinogen concentrations, the karyotype according to Southwest Oncology Group criteria,19 the presence of any infectious disease, FAB subtype, and comorbidity assessed by the Charlson comorbidity index20 at diagnosis, were recorded for all patients. During follow-up, ICU admission, date of relapse, death (if applicable), and last visit were recorded. The median follow-up was 1.1 years. Data were analyzed in a retrospective manner. In ICU patients, the Simplified Acute Physiology Score II (SAPS II)21–23 at the time of admission to the ICU, time from diagnosis of AML to ICU admission, reason for ICU admission, need for invasive mechanical ventilation and vasopressors, as well as laboratory findings at ICU admission (fibrinogen, C-reactive protein, and white blood cell count) were recorded retrospectively. The study was approved by the ethical review board of the Medical University of Vienna.
Treatment of acute myeloid leukemia
Induction chemotherapy was performed according to the DAV protocol24 in all patients except those with AML FAB M3. In the case of persistence of blast cells after the first induction cycle, patients received a second cycle of induction chemotherapy (in patients aged <60 years: MiDAC13; in patients aged >60 years another cycle of DAV). In 37 patients, a third induction cycle (primarily FLAG25) was administered. Patients with AML M3 were treated according to the AIDA protocol.26
Supportive therapy
Routine supportive therapy was administered according to institutional guidelines: red cell concentrates and platelet concentrates were given to maintain the hemoglobin level above 8.0 g/dL and the platelet count greater than 10×109/L. Patients received prophylactic gastrointestinal decontamination (ciprofloxacin and fluconazole during induction chemotherapy). Granulocyte colony-stimulating factor was not administered routinely. In the case of neutropenic fever associated with a severe infection or a known history of a severe infection during a preceding cycle of chemotherapy, granulocyte colony-stimulating factor (30×106 U/day s.c. until neutrophil recovery) was given together with antibiotic and antifungal therapy. Admission to the ICU was granted to all patients with de novo AML prior to or during induction chemotherapy. There were no specific ICU admission criteria. The decision to admit a patient to the ICU was taken by the senior hematologist and the senior intensivist.
Statistical analysis
The prognostic value of parameters at diagnosis, such as white blood cell count, age, lactate dehydrogenase, C-reactive protein and fibrinogen concentrations, karyotype, infections, FAB subtype, and Charlson comorbidity index as well as critical parameters at ICU admission including white blood cell count, fibrinogen and C-reactive protein concentrations, SAPS II, invasive mechanical ventilation, time from diagnosis to ICU admission, and the cause of admission, were analyzed by Cox regression for survival and logistic regression for ICU admission and ICU outcome. All parameters were first tested in univariate analyses and factors significant at the P=0.05 level were then tested simultaneously in a multivariate analysis. Survival was defined as the time from admission to death from any cause. Patients still at risk or lost from follow-up were censored. Continuous complete remission was defined as the time from achievement of complete hematologic remission to a relapse. Patients who died from non-leukemia associated disorders, those lost from follow-up, or still at risk were censored. Survival of patients alive at day 30 was defined as the survival of patients from day 30 after diagnosis until death. Day 30 was chosen because the majority of patients who undergo induction chemotherapy show hematologic reconstitution within this period. Patients still at risk or lost from follow-up were censored. The product limit method of Kaplan and Meier was used to analyze the probability of overall survival, continuous complete remission, and survival of patients alive at day 30. Differences were considered to be statistically significant when the P value was less than 0.05.
As a screening procedure patients’ characteristics and baseline measurements of clinical parameters were compared with respect to ICU admission by univariate methods. Metric variables were tested by the Mann-Whitney test, dichotomous variables by Fisher’s exact probability test, and categorical variables with more than two categories by χ2 tests.
Results
Intensive care unit admission rate
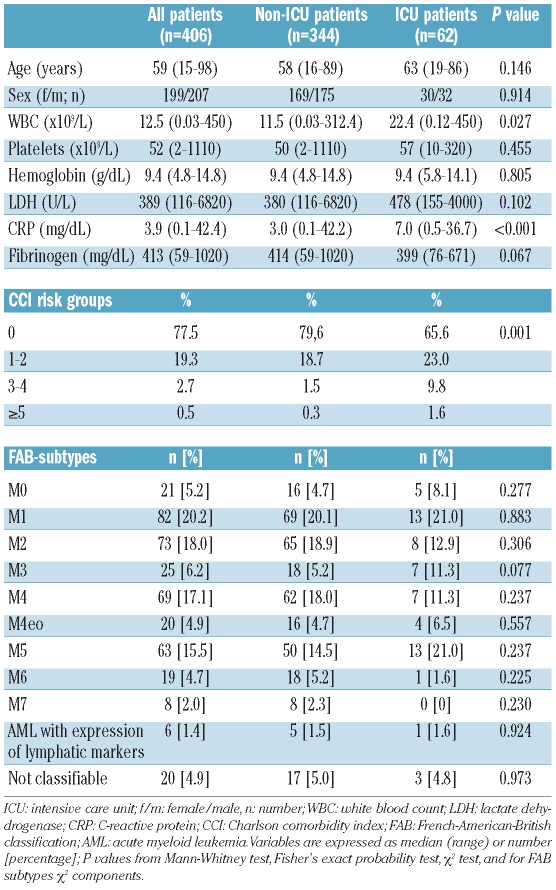
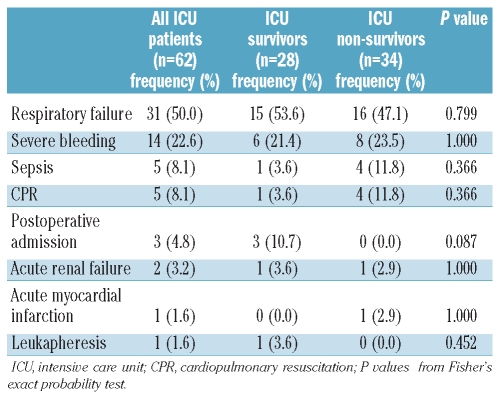
Of a total of 406 consecutive patients with de novo AML, 62 (15.3%) required admission to the ICU. The patients’ characteristics are shown in Table 1. Twenty-five patients were admitted to the ICU prior to the initiation of induction chemotherapy, and 37 were admitted during induction chemotherapy. Patients were admitted to the ICU at a median of 13 days (range, 0–97 days) after diagnosis. The median SAPS II at admission to the ICU was 64 (range, 30–107). The primary reasons for ICU admission were respiratory failure (n=31; 50%) and life-threatening bleeding (n=14; 23%) (Table 2).
Table 1.
Patients’ characteristics at diagnosis.
Table 2.
Reason for ICU admission.
Factors predicting admission to the intensive care unit
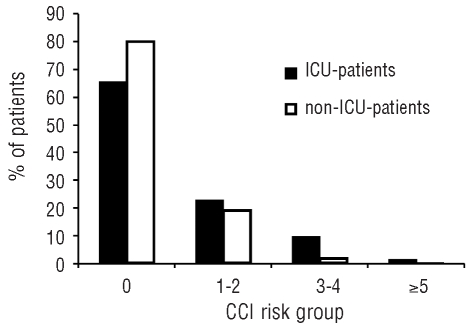
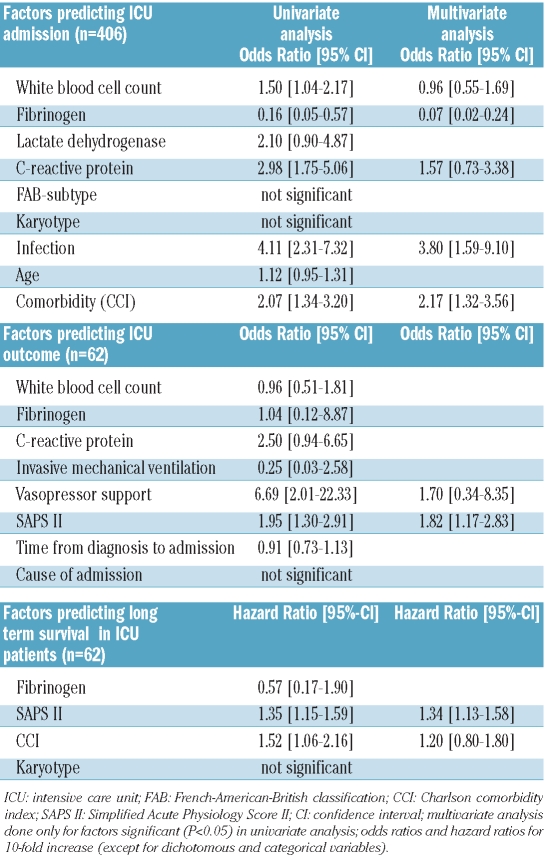
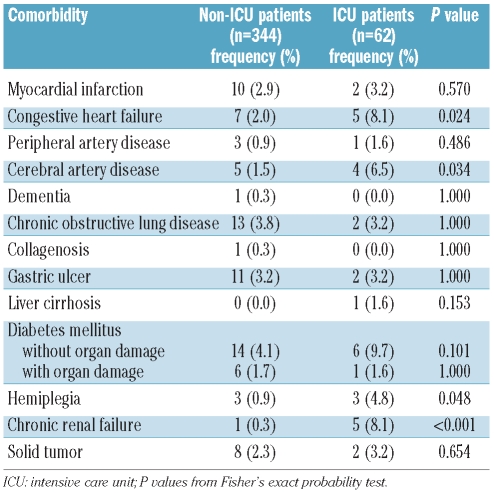
A number of parameters recorded at the time of diagnosis were analyzed with respect to their predictive value regarding the need for an admission to an ICU. In univariate analysis, white blood cell count, C-reactive protein, the presence of an infectious disease, fibrinogen, and Charlson comorbidity index were found to be predictive parameters indicating a high probability of an ICU admission. In contrast, age, karyotype, FAB subtype, and lactate dehydrogenase levels were not of predictive value. Parameters that were independently predictive for an ICU admission were fibrinogen, presence of an infection, and Charlson comorbidity index (Figure 1). We found that the risk of an ICU admission increased continuously as the levels of fibrinogen decreased. No significant differences were detected when analyzing our data excluding patients with AML M3. Table 3 shows a summary of the univariate and multivariate analyses. When analyzing specific comorbidities, marked differences were found between ICU and non-ICU patients, i.e. congestive heart failure, peripheral artery disease, diabetes mellitus, hemiplegia, and chronic renal failure (Table 4).
Figure 1.
Admission to the intensive care unit (ICU). Percentages of ICU patients and non-ICU patients according to Charlson comorbidity index (CCI) risk group.
Table 3.
Possible predictive factors for ICU admission, ICU outcome, and long-term survival in ICU patients.
Table 4.
Comorbidities in ICU and non-ICU patients.
Intensive care unit survival and prognostic factors
The ICU survival rate was 45%. Fifteen of the 25 patients (60%) admitted to the ICU died before induction chemotherapy could be initiated, mostly within the first 24 h after admission to the ICU. Factors significantly associated with ICU outcome were the SAPS II as well as the need for vasopressor support. In contrast, mechanical ventilation, cause of ICU admission, time from diagnosis to ICU admission, fibrinogen level, C-reactive protein concentration, and white blood cell count at the time of ICU admission were not of predictive value. In multivariate analysis, only the SAPS II was independently associated with ICU survival (Table 3). ICU survivors presented with a median SAPS II of 49 (range, 30–77), which was significantly lower than that of non-survivors who had a median SAPS II of 73 (range, 31–107; P<0.05). To analyze whether the improvement of supportive care could have influenced the outcome of our patients, we compared the survival of patients admitted between 1994 and 2000 (n=34) with those who were admitted between 2000 and 2006 (n=28). As assessed by the log rank test, no difference could be detected between these two groups of patients (P=0.6).
Long-term survival of intensive care unit patients
Univariate analysis of prognostic factors indicative of long-term survival revealed that high SAPS II at ICU admission, as well as a higher Charlson comorbidity index were associated with an adverse survival outcome (Table 3). In contrast, the karyotype was not of prognostic significance. In multivariate analysis only SAPS II remained an independent prognostic variable predicting survival.
Comparison of patients admitted or not to the intensive care unit
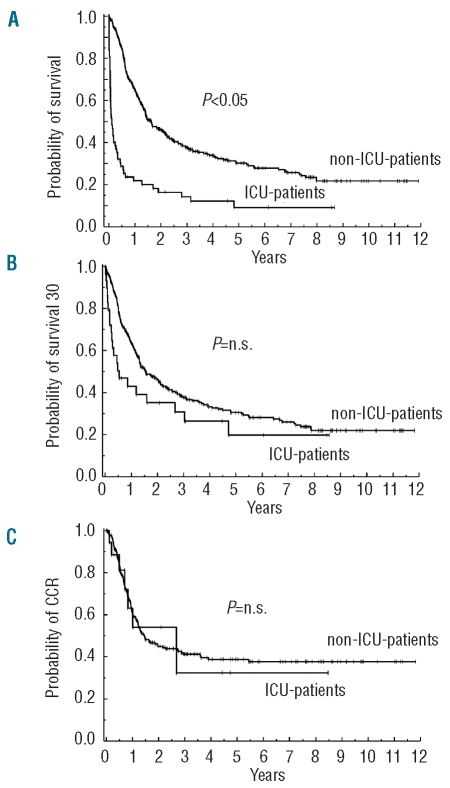
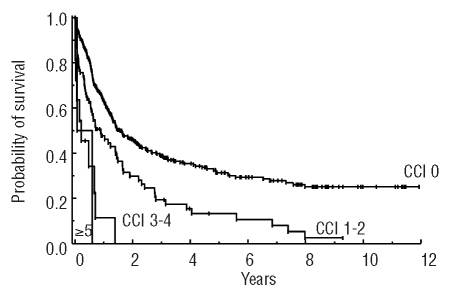
Patients not admitted to the ICU (n=344) had a better survival than those admitted to the ICU (n=62) (non-ICU patients: median survival, 19.6 months; survival rate at 8 years: 21%; ICU patients: median survival, 1.3 months; survival rate at 8 years: 9%; P<0.05) (Figure 2). ICU admission was an independent adverse prognostic factor with respect to survival. Additional factors prognostic of survival were karyotype, age, Charlson comorbidity index (Figure 3), as well as white blood cell count and lactate dehydrogenase concentration at diagnosis, whereas FAB subtype, C-reactive protein level, and the presence of an infectious disease at diagnosis showed no predictive value. In contrast to survival, no significant differences were found between ICU patients and non-ICU patients when comparing the continuous complete remission and early-phase survival (Figure 2). At 6 years, the continuous complete remission rate was 38% among non-ICU-patients and 33% among ICU patients (P≥0.05). Similarly, the 6-year survival rate of those patients who had survived the first 30 days was 28% among non-ICU patients and 20% among ICU patients (P>0.05). ICU admission was not of prognostic significance with regard to continuous complete remission or survival of patients alive at day 30. Eight non-ICU patients and 18 ICU patients died within the first 30 days after diagnosis.
Figure 2.
Kaplan-Meier estimates of survival (A), survival from day 30 after diagnosis (B), and continuous complete remission (C) in non-intensive care unit patients (non-ICU patients) and intensive care unit patients (ICU patients) with AML.
Figure 3.
Kaplan-Maier estimates of survival according to Charlson comorbidity index (CCI) risk group.
Discussion
In the present investigation, every seventh patient eligible for induction chemotherapy had to be admitted to the ICU prior to or during induction chemotherapy. A recently published paper by Attalah et al.27 revealed that 28% of patients with AML undergoing induction chemotherapy were admitted to the ICU (range, 12% to 44%, depending on the induction chemotherapy regimen). This difference between studies might be explained by the different treatment protocols used. Attalah et al. employed several different induction regimens with different intensities and thus different toxicities. In our cohort, all patients, except those with FAB M3 (who received the AIDA protocol) were treated with the DAV protocol. However, both studies show that a considerable number of patients with AML develop serious complications leading to admission to the ICU.
Identifying risk factors for clinical deterioration leading to ICU admission is of particular interest when treating patients with AML. Multivariate analysis revealed that infection, lower fibrinogen levels and comorbidity at diagnosis were independent prognostic factors for ICU admission. Indeed, more than half of our patients were admitted to the ICU due to infectious complications, such as septicemia, and respiratory failure known to be mostly of infectious origin.28,29–31 Interestingly, other disease- and patient-related factors including age, FAB subtype, and the karyotype, all known to be correlated with long-term survival, were not associated with ICU admission. Our findings underline the importance of tight adherence to guidelines concerning the prevention and treatment of infectious complications in patients with hematologic malignancies.32,33 To reduce the risk of ICU admission high-risk patients should, therefore, receive early prophylaxis with antibiotics and antifungal agents. In patients with AML, decreased fibrinogen levels are frequently associated with disseminated intravascular coagulation, which is known to be associated with an increased risk of severe bleeding.34–36 Life-threatening bleeding was the second major reason for ICU admission in our cohort of AML patients, and was found to be associated with a poor outcome. Our observations are in line with previously published data showing that bleeding is one of the primary causes of an ICU admission in critically ill patients with hematologic malignancies,37–41 and particularly in patients with AML.17,29 With this in mind, it is tempting to speculate whether a strategy of early replacement with fresh-frozen plasma or fibrinogen concentrates with the aim of preserving high normal values would reduce the risk of life-threatening bleedings. The third independent prognostic factor for ICU admission was the presence of comorbid conditions, despite the exclusion of ‘unfit’ patients not eligible for induction chemotherapy. To the best of our knowledge, this is the first study demonstrating that comorbidity in AML is associated with a high risk of ICU admission, which may have clinical implications. It seems important, especially in older patients, to screen for comorbid conditions in order to define the patients’ overall risk in AML, including the risk of being admitted to the ICU which is per se an adverse prognostic factor.
The ICU survival rate in our cohort of patients was 45%, which is higher than that reported previously for AML patients admitted to an ICU.15,16 However, more recently published data show that a considerably higher percentage of AML patients admitted to an ICU can survive. In particular, Rabbat et al.17 reported an ICU survival rate of 66%. Compared to these data the survival rate in our patients seems to be inferior. However, patients in our cohort were more severely ill at the time of ICU admission, as reflected by a higher mean SAPS II (64 in our patients versus 55 in the report by Rabbat et al.). Moreover, invasive mechanical ventilation, the strongest predictor of ICU mortality in patients with hematologic malignancies,37,38,42,43 had to be used in a higher percentage of our patients (68%) than in those of the study by Rabbat et al.17 (47%). The comparison between the two cohorts is further complicated by the fact that in our cohort only patients with de novo AML before, during or after induction therapy were analyzed, whereas Rabbat et al.17 also described the ICU course of AML patients during consolidation chemotherapy, after stem cell transplantation, and in relapse.
In our analysis the only independent prognostic factor for ICU survival was the SAPS II, as assessed by multivariate analysis. This is in line with several reports showing that the ICU survival of patients with hematologic malignancies might not depend on disease-related parameters, but rather on the severity of the acute illness.17,38,39,41,43–46 Long-term survival of patients with hematologic malignancies has been described to be independent of the severity of disease at ICU admission but rather associated with disease-related parameters.17,38,39,44
Our analysis, albeit retrospective in nature, is one of the first to investigate a consecutive homogeneous cohort of patients with a distinct hematologic disease (AML) in detail, starting from the day of diagnosis and focusing on a possible independent effect of an ICU admission on outcome. So far, the survival of ICU patients has not been compared with that of non-ICU patients in AML. With regards to the survival of the whole cohort of AML patients eligible for induction chemotherapy, ICU admission was an independent adverse prognostic factor. However, no significant differences were found between ICU patients and non-ICU patients when comparing continuous complete remission rates. Moreover, ICU admission was not of prognostic significance with regards to long-term survival when the analysis was limited to patients who had survived at least 30 days. Thus, ICU patients surviving the initial phase of the disease have the same long-term survival as non-ICU patients, which is remarkable and of clinical importance. In fact, based on this result, we recommend that full ICU support is provided for critically ill patients with AML during the initial phase of their disease. On the other hand, it should also be stated that the current data reflect the experience of a single, specialized center and, therefore, might not be applicable to other centers, since the ICU outcome of hematologic patients has been described to be “dependent” on the number of patients treated in a center.47
In conclusion, ICU admission is a frequent complication in patients with de novo AML eligible for induction chemotherapy. Patients admitted to the ICU have a markedly reduced short-term survival, which seems to be mainly determined by the severity of the acute illness, and not by AML-specific parameters. The long-term outcome of patients surviving the first 30 days after diagnosis appears to be similar when comparing ICU and non-ICU patients. We strongly recommend admission to the ICU for patients with AML undergoing induction chemotherapy whenever necessary.
Acknowledgments
The authors would like to thank all physicians, the nursing staff, and the administration team for their support in the treatment of critically ill patients with AML in our department.
Footnotes
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Stone RM, O’Donnell MR, Sekeres MA. Acute myeloid leukemia. Hematology Am Soc Hematol Educ Program. 2004:98–117. doi: 10.1182/asheducation-2004.1.98. [DOI] [PubMed] [Google Scholar]
- 2.Vellenga E, Griffin JD. The biology of acute myeloblastic leukemia. Semin Oncol. 1987;14(4):365–71. [PubMed] [Google Scholar]
- 3.Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood. 1998;92(7):2322–33. [PubMed] [Google Scholar]
- 4.Grimwade D, Walker H, Harrison G, Oliver F, Chatters S, Harrison CJ, et al. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98(5):1312–20. doi: 10.1182/blood.v98.5.1312. [DOI] [PubMed] [Google Scholar]
- 5.Strout MP, Marcucci G, Caligiuri MA, Bloomfield CD. Core-binding factor (CBF) and MLL-associated primary acute myeloid leukemia: biology and clinical implications. Ann Hematol. 1999;78(6):251–64. doi: 10.1007/s002770050511. [DOI] [PubMed] [Google Scholar]
- 6.Baer MR, Bloomfield CD. Multidrug resistance in acute myeloid leukemia. J Natl Cancer Inst. 1991;83(10):663–5. doi: 10.1093/jnci/83.10.663. [DOI] [PubMed] [Google Scholar]
- 7.Kornblau SM, Tibes R, Qiu YH, Chen W, Kantarjian HM, Andreeff M, et al. Functional proteomic profiling of AML predicts response and survival. Blood. 2009;113(1):154–64. doi: 10.1182/blood-2007-10-119438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garzon R, Volinia S, Liu CG, Fernandez-Cymering C, Palumbo T, Pichiorri F, et al. MicroRNA signatures associated with cytogenetics and prognosis in acute myeloid leukemia. Blood. 2008;111(6):3183–9. doi: 10.1182/blood-2007-07-098749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van de Loosdrecht AA. The dendritic cell: the piano player in orchestrating the immune response in leukemia. Leuk Lymphoma. 2007;48(2):217–8. doi: 10.1080/10428190601126628. [DOI] [PubMed] [Google Scholar]
- 10.Dombret H, Raffoux E, Gardin C. New insights in the management of elderly patients with acute myeloid leukemia. Curr Opin Oncol. 2009;21(6):589–93. doi: 10.1097/CCO.0b013e3283313e10. [DOI] [PubMed] [Google Scholar]
- 11.Dombret H, Raffoux E, Gardin C. Acute myeloid leukemia in the elderly. Semin Oncol. 2008;35(4):430–8. doi: 10.1053/j.seminoncol.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Estey EH. Prognostic factors in acute myelogenous leukemia. Leukemia. 2001;15(4):670–2. doi: 10.1038/sj.leu.2402057. [DOI] [PubMed] [Google Scholar]
- 13.Hiddemann W, Kreutzmann H, Straif K, Ludwig WD, Mertelsmann R, Donhuijsen-Ant R, et al. High-dose cytosine arabinoside and mitoxantrone: a highly effective regimen in refractory acute myeloid leukemia. Blood. 1987;69(3):744–9. [PubMed] [Google Scholar]
- 14.Estey EH. Therapeutic options for acute myelogenous leukemia. Cancer. 2001;92 (5):1059–73. doi: 10.1002/1097-0142(20010901)92:5<1059::aid-cncr1421>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 15.Rabe C, Mey U, Paashaus M, Musch A, Tasci S, Glasmacher A, et al. Outcome of patients with acute myeloid leukemia and pulmonary infiltrates requiring invasive mechanical ventilation - a retrospective analysis. J Crit Care. 2004;19(1):29–35. doi: 10.1016/j.jcrc.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Tremblay LN, Hyland RH, Schouten BD, Hanly PJ. Survival of acute myelogenous leukemia patients requiring intubation/ventilatory support. Clin Invest Med. 1995;18(1):19–24. [PubMed] [Google Scholar]
- 17.Rabbat A, Chaoui D, Montani D, Legrand O, Lefebvre A, Rio B, et al. Prognosis of patients with acute myeloid leukaemia admitted to intensive care. Br J Haematol. 2005;129(3):350–7. doi: 10.1111/j.1365-2141.2005.05459.x. [DOI] [PubMed] [Google Scholar]
- 18.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol. 1976;33(4):451–8. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 19.Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96 (13):4075–83. [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–63. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 22.Schellongowski P, Benesch M, Lang T, Traunmüller F, Zauner C, Laczika K, et al. Comparison of three severity scores for critically ill cancer patients. Intensive Care Med. 2004;30(3):430–6. doi: 10.1007/s00134-003-2043-1. [DOI] [PubMed] [Google Scholar]
- 23.Cornet AD, Issa AI, van de Loosdrecht AA, Ossenkoppele GJ, Strack van Schijndel RJ, Groeneveld AB, et al. Sequential organ failure predicts mortality of patients with a haematological malignancy needing intensive care. Eur J Haematol. 2005;74(6):511–6. doi: 10.1111/j.1600-0609.2005.00418.x. [DOI] [PubMed] [Google Scholar]
- 24.Bishop JF, Lowenthal RM, Joshua D, Matthews JP, Todd D, Cobcroft R, et al. Etoposide in acute nonlymphocytic leukemia. Australian Leukemia Study Group. Blood. 1990;75(1):27–32. [PubMed] [Google Scholar]
- 25.Estey E, Thall P, Andreeff M, Beran M, Kantarjian H, O’Brien S, et al. Use of granulocyte colony-stimulating factor before, during, and after fludarabine plus cytarabine induction therapy of newly diagnosed acute myelogenous leukemia or myelodysplastic syndromes: comparison with fludarabine plus cytarabine without granulocyte colony-stimulating factor. J Clin Oncol. 1994;12(4):671–8. doi: 10.1200/JCO.1994.12.4.671. [DOI] [PubMed] [Google Scholar]
- 26.Avvisati G, Lo Coco F, Diverio D, Falda M, Ferrara F, Lazzarino M, et al. AIDA (all-trans retinoic acid + idarubicin) in newly diagnosed acute promyelocytic leukemia: a Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto (GIMEMA) pilot study. Blood. 1996;88(4):1390–8. [PubMed] [Google Scholar]
- 27.Atallah E, Cortes J, O’Brien S, Pierce S, Rios MB, Estey E, et al. Establishment of baseline toxicity expectations with standard frontline chemotherapy in acute myelogenous leukemia. Blood. 2007;110(10):3547–51. doi: 10.1182/blood-2007-06-095844. [DOI] [PubMed] [Google Scholar]
- 28.Hämäläinen S, Kuittinen T, Matinlauri I, Nousiainen T, Koivula I, Jantunen E. Neutropenic fever and severe sepsis in adult acute myeloid leukemia (AML) patients receiving intensive chemotherapy: causes and consequences. Leuk Lymphoma. 2008;49(3):495–501. doi: 10.1080/10428190701809172. [DOI] [PubMed] [Google Scholar]
- 29.Chaoui D, Legrand O, Roche N, Cornet M, Lefebvre A, Peffault de Latour R, et al. Incidence and prognostic value of respiratory events in acute leukemia. Leukemia. 2004;18(4):670–5. doi: 10.1038/sj.leu.2403270. [DOI] [PubMed] [Google Scholar]
- 30.Rabbat A, Chaoui D, Lefebvre A, Roche N, Legrand O, Lorut C, et al. Is BAL useful in patients with acute myeloid leukemia admitted in ICU for severe respiratory complications? Leukemia. 2008;22(7):1361–7. doi: 10.1038/leu.2008.100. [DOI] [PubMed] [Google Scholar]
- 31.Azoulay E, Mokart D, Rabbat A, Pene F, Kouatchet A, Bruneel F, et al. Diagnostic bronchoscopy in hematology and oncology patients with acute respiratory failure: prospective multicenter data. Crit Care Med. 2008;36(1):100–7. doi: 10.1097/01.CCM.0000295590.33145.C4. [DOI] [PubMed] [Google Scholar]
- 32.Buchheidt D, Böhme A, Cornely OA, Fätkenheuer G, Fuhr HG, Heussel G, et al. Diagnosis and treatment of documented infections in neutropenic patients--recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO) Ann Hematol. 2003;82 (Suppl 2):S127–32. doi: 10.1007/s00277-003-0766-2. [DOI] [PubMed] [Google Scholar]
- 33.Maschmeyer G, Beinert T, Buchheidt D, Cornely OA, Einsele H, Heinz W, et al. Diagnosis and antimicrobial therapy of lung infiltrates in febrile neutropenic patients: Guidelines of the infectious diseases working party of the German Society of Haematology and Oncology. Eur J Cancer. 2009;45(14):2462–72. doi: 10.1016/j.ejca.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Weltermann A, Pabinger I, Geissler K, Jäger U, Gisslinger H, Knöbl P, et al. Hypofibrinogenemia in non-M3 acute myeloid leukemia. Incidence, clinical and laboratory characteristics and prognosis. Leukemia. 1998;12(8):1182–6. doi: 10.1038/sj.leu.2401101. [DOI] [PubMed] [Google Scholar]
- 35.Uchiumi H, Matsushima T, Yamane A, Doki N, Irisawa H, Saitoh T, et al. Prevalence and clinical characteristics of acute myeloid leukemia associated with disseminated intravascular coagulation. Int J Hematol. 2007;86(2):137–42. doi: 10.1532/IJH97.06173. [DOI] [PubMed] [Google Scholar]
- 36.Levi M. Disseminated intravascular coagulation. Crit Care Med. 2007;35(9):2191–5. doi: 10.1097/01.ccm.0000281468.94108.4b. [DOI] [PubMed] [Google Scholar]
- 37.Benoit DD, Vandewoude KH, Decruyenaere JM, Hoste EA, Colardyn FA. Outcome and early prognostic indicators in patients with a hematologic malignancy admitted to the intensive care unit for a life-threatening complication. Crit Care Med. 2003;31(1):104–12. doi: 10.1097/00003246-200301000-00017. [DOI] [PubMed] [Google Scholar]
- 38.Massion PB, Dive AM, Doyen C, Bulpa P, Jamart J, Bosly A, et al. Prognosis of hematologic malignancies does not predict intensive care unit mortality. Crit Care Med. 2002;30(10):2260–70. doi: 10.1097/00003246-200210000-00014. [DOI] [PubMed] [Google Scholar]
- 39.Staudinger T, Stoiser B, Müllner M, Locker GJ, Laczika K, Knapp S, et al. Outcome and prognostic factors in critically ill cancer patients admitted to the intensive care unit. Crit Care Med. 2000;28(5):1322–8. doi: 10.1097/00003246-200005000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Azoulay E, Recher C, Alberti C, Soufir L, Leleu G, Le Gall JR, et al. Changing use of intensive care for hematological patients: the example of multiple myeloma. Intensive Care Med. 1999;25(25):1395–401. doi: 10.1007/s001340051087. [DOI] [PubMed] [Google Scholar]
- 41.Kress JP, Christenson J, Pohlman AS, Linkin DR, Hall JB. Outcomes of critically ill cancer patients in a university hospital setting. Am J Respir Crit Care Med. 1999;160(6):1957–61. doi: 10.1164/ajrccm.160.6.9812055. [DOI] [PubMed] [Google Scholar]
- 42.Depuydt PO, Benoit DD, Vandewoude KH, Decruyenaere JM, Colardyn FA. Outcome in noninvasively and invasively ventilated hematologic patients with acute respiratory failure. Chest. 2004;126(4):1299–306. doi: 10.1378/chest.126.4.1299. [DOI] [PubMed] [Google Scholar]
- 43.Azoulay E, Alberti C, Bornstain C, Leleu G, Moreau D, Recher C, et al. Improved survival in cancer patients requiring mechanical ventilatory support: impact of noninvasive mechanical ventilatory support. Crit Care Med. 2001;29(3):519–25. doi: 10.1097/00003246-200103000-00009. [DOI] [PubMed] [Google Scholar]
- 44.Kroschinsky F, Weise M, Illmer T, Haenel M, Bornhaeuser M, Hoeffken G, et al. Outcome and prognostic features of intensive care unit treatment in patients with hematological malignancies. Intensive Care Med. 2002;28(9):1294–300. doi: 10.1007/s00134-002-1420-5. [DOI] [PubMed] [Google Scholar]
- 45.Pene F, Percheron S, Lemiale V, Viallon V, Claessens YE, Marqué S, et al. Temporal changes in management and outcome of septic shock in patients with malignancies in the intensive care unit. Crit Care Med. 2008;36(3):690–6. doi: 10.1097/CCM.0B013E318165314B. [DOI] [PubMed] [Google Scholar]
- 46.Lecuyer L, Chevret S, Thiery G, Darmon M, Schlemmer B, Azoulay E. The ICU trial. A new admission policy for cancer patients requiring mechanical ventilation. Crit Care Med. 2007;35(3):808–14. doi: 10.1097/01.CCM.0000256846.27192.7A. [DOI] [PubMed] [Google Scholar]
- 47.Lecuyer L, Chevret S, Guidet B, Aegerter P, Martel P, Schlemmer B, et al. Case-volume and mortality in hematological patients with acute respiratory failure. Eur Respir J. 2008;32(3):748–54. doi: 10.1183/09031936.00142907. [DOI] [PubMed] [Google Scholar]