Abstract
Background
Treatment of central nervous system relapse in adult acute lymphoblastic leukemia is a challenge and outcome is poor. Liposomal cytarabine has a prolonged half-life and, given intrathecally, has produced high response rates in patients with central nervous system relapse of non-Hodgkin's lymphoma. The aim of this study was to evaluate the efficacy and tolerability of liposomal cytarabine in central nervous system relapse of acute lymphoblastic leukemia or Burkitt's lymphoma/leukemia.
Design and Methods
Liposomal cytarabine (50 mg) was given intrathecally together with systemic or intrathecal dexamethasone once every 2 weeks in a phase II European trial. The primary end-point, cytological response in the cerebrospinal fluid after one or two cycles, was evaluated at the time of next treatment.
Results
Nineteen heavily pretreated patients (median age, 53 years; range 24–76 years) were evaluable: 14 with acute lymphoblastic leukemia and 5 with Burkitt’s lymphoma/leukemia). Complete cytological remission as best response after two cycles of liposomal cytarabine was confirmed in 74% of the patients: 86% of those with acute lymphoblastic leukemia and 40% of those with Burkitt’s lymphoma/leukemia). Nine of the 14 patients who achieved complete remission relapsed after a median of 7 months. The median overall survival was 11 months. Adverse events were observed in 89% of the patients (57% of cycles). Grade III–IV events with potential correlation to liposomal cytarabine occurred in 32% of the patients. The most frequent adverse event was headache. One patient developed severe neurological complications with loss of vision and a conus syndrome.
Conclusions
Overall, liposomal cytarabine showed excellent antileukemic activity. Toxicity was acceptable but appeared to increase with the number of cycles. Future evaluation in prophylaxis is of interest
Keywords: liposomal cytarabine, acute lymphoblastic leukemia, aggressive lymphoma, CNS relapse, adults
Introduction
In acute lymphoblastic leukemia (ALL), the central nervous system (CNS) may be involved at initial diagnosis and also at relapse. The CNS is considered a sanctuary site because of the limited penetration of cytostatic drugs across the blood-brain barrier into the cerebrospinal fluid and the brain parenchyma. With current treatment regimens, the CNS relapse rate has been reduced to below 5% by a combination of systemic therapy with CNS active drugs such as high-dose cytarabine and high-dose methotrexate, intrathecal therapy and additional CNS irradiation in some trials.1,2 CNS relapse in ALL may occur in isolation or in combination with bone marrow relapse. It can be associated with characteristic symptoms such as headache, nausea, cranial nerve palsies and neurological dysfunction, but may also be detected during a routine lumbar puncture. CNS relapse is generally confirmed by the demonstration of more than five blast cells per microliter of cerebrospinal fluid.
Prospective studies on the treatment of CNS relapse in adult ALL are not available. Complete remissions in the CNS have been achieved with various treatments including intrathecal therapy with or without additional systemic chemotherapy in 32–94% of the patients.3–7 However, despite the generally good response of CNS relapse, the survival of affected adults is extremely poor: the median overall survival is only 6 months and the probability of long-term survival just 6–8%.3–7 Cure is almost exclusively restricted to patients who undergo allogeneic stem cell transplantation (SCT). The standard therapy of CNS relapse is based on two or three weekly intrathecal injections of methotrexate or a triple combination with methotrexate, cytarabine and steroids until blast clearance, followed by one or two more cycles of treatment. Frequent lumbar punctures are required and have a significant negative impact on the quality of life of the patients. Cumulative neurotoxicity such as leukoencephalopathy is an important issue in patients with CNS relapse when various CNS active treatments are combined.8,9 One of the major aims is, therefore, to achieve complete remission in the CNS, i.e. clearance of blasts from the cerebrospinal fluid, with as little cumulative toxicity as possible and to prepare the patients rapidly for SCT.
Liposomal cytarabine is a sustained-release formulation of cytarabine, which is encapsulated in spherical multivesicular particles. After administration, free cytarabine is detectable for up to 14 days. The prolonged activity allows for even distribution of the drug in the whole lumbar space and cerebral ventricles.10,11 Previous studies have shown that, when compared with methotrexate (12 mg) or cytarabine (50 mg twice a week), liposomal cytarabine has a similar safety profile and a similar or better effectiveness in the treatment of neoplastic or lymphomatous meningitis, respectively.10,12 Based on the promising efficacy reported from these studies, the European Working Group for Adult ALL (EWALL) initiated a prospective trial with the aim of demonstrating the feasibility and efficacy of liposomal cytarabine in adults with ALL. The primary end-point was the CNS response rate after one or two cycles. To our knowledge, this is the first prospective trial addressing this question.
Design and Methods
We report the results of a prospective non-randomized multi-center and multinational phase II trial. The study was started in April 2004. The study protocol is registered (NCT00199108) and was approved by the ethics committee of the University of Frankfurt and other responsible ethics committees and was performed in accordance with the declaration of Helsinki. Written informed consent was obtained from all patients.
Entry criteria
Patients with ALL or very aggressive non-Hodgkin’s lymphoma (Burkitt/Burkitt-like or B-lymphoblastic non-Hodgkin’s lymphoma) with CNS relapse, isolated or combined with bone marrow relapse, were eligible. Inclusion criteria were age 18 years or more, Karnofsky index 60% or more, absence of uncontrolled infection, recovered from previous grade III/IV toxicities (with the exception of hematologic toxicity) and absence of severe heart, lung, liver or kidney dysfunction. CNS relapse was confirmed either by positive cerebrospinal fluid cytology, defined as cerebrospinal fluid cell counts greater than 5/μL within 10 days prior to inclusion, or by characteristic signs and symptoms, or magnetic resonance imaging (MRI) or computed tomography (CT) scans indicating the presence of meningeal or brain involvement. One prior intrathecal treatment of the current CNS relapse was allowed, but the cerebrospinal fluid sample taken before the first administration of liposomal cytarabine had to confirm the CNS involvement.
Study therapy
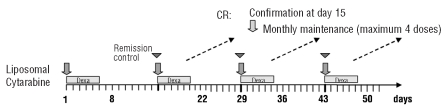
In all patients, a cerebrospinal fluid sample was taken before administration of liposomal cytarabine. The study drug was administered immediately thereafter via lumbar puncture on day 1 and continued with one administration every 14 days for a maximum of five additional induction cycles. After demonstration that the cerebrospinal fluid was clear of blasts at two time-points within 14 days, monthly injections were scheduled for maintenance (Figure 1). The dose of liposomal cytarabine was 50 mg per injection. Liposomal cytarabine was provided by Mundipharma GmbH, Limburg, Germany. In patients with combined CNS and bone marrow relapse, the choice of systemic therapy was left to the discretion of the physician. However, during induction the use of CNS-active drugs such as high-dose cytarabine, methotrexate or thiotepa had to be withheld.
Figure 1.
Treatment schedule.
In the initial version of the protocol, prophylaxis of arachnoiditis was provided in the form of oral dexamethasone (4 mg bid) for 5 days. On August 10, 2006 the study was amended and the prophylaxis changed to intrathecal dexamethasone (4 mg).
Assessments
The cell count and morphology of the cerebrospinal fluid were evaluated as part of the pre-treatment analysis. All patients also underwent physical and neurological examination. MRI/CT scans or other staging investigations were performed as clinically indicated. Bone marrow aspiration was part of the initial study procedure and repeated at restaging if clinically indicated. Restaging assessment of CNS involvement was performed on day 14 (day 28 during maintenance) of each cycle. This assessment included a lumbar puncture with cerebrospinal fluid analysis, physical and neurological examination in all patients and additional investigations e.g. MRI/CT scans in patients with brain involvement.
Response criteria
Complete CNS cytological response was defined as conversion from a positive to at least one negative cerebrospinal fluid cytology. In patients with initial positive MRI/CT scans, complete response was defined as complete resolution of all tumor manifestations in the MRI/CT scans and no progression of neurological symptoms. Partial response was defined as regression of the tumor manifestations to less than 50%.
Statistical analysis
The major end-point for efficacy evaluation was best CNS response, defined as any achievement of CNS cytological response after one or two cycles of liposomal cytarabine. Toxicity was analyzed as the proportion of patients or cycles with adverse events according to Common Toxicity Criteria. Furthermore, adverse events with a possible or probable correlation to liposomal cytarabine, as assessed by the investigators, were analyzed separately. Responses and correlations with the characteristics of the patients, their diseases or treatments were analyzed with the χ2-test. Time to neurological disease progression in patients with a CNS cytological response was defined as the time between the first day of study treatment and the day the patient was diagnosed as having neurological progression or the date of the last follow-up. Overall survival was calculated from the date of the first administration of the study drug to death or to the date of the last follow-up. Survival was analyzed using the Kaplan-Meier method. The statistical analysis was performed in the GMALL study center with the SAS programme (SAS-PC, Version 8; SAS Institute, Cary, NC, USA). For all analyses, P values of 0.05 or less were considered statistically significant.
Results
Patients' characteristics
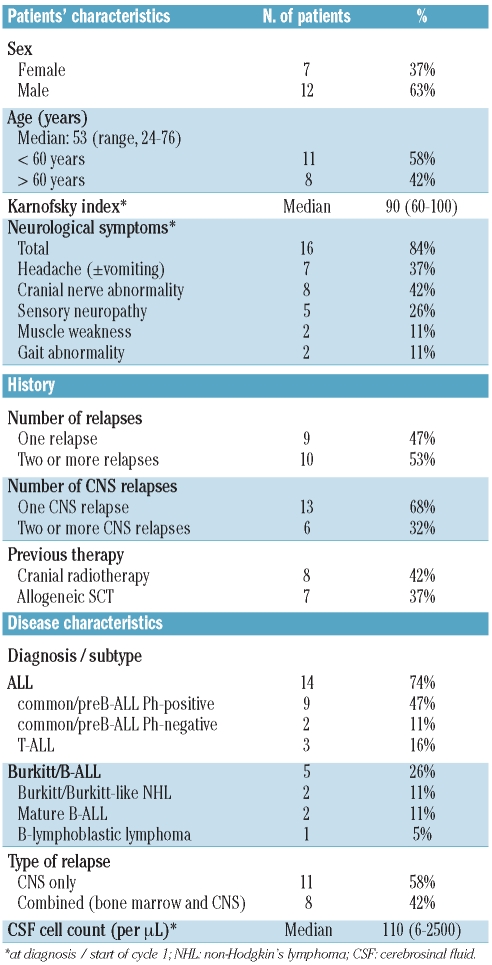
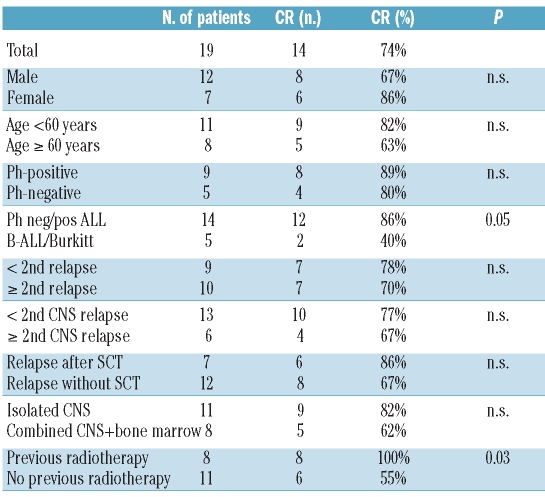
Twenty-two adult patients from five countries (Germany 11, France 4, Italy 3, Spain 3, Austria 1) with CNS relapse of ALL or very aggressive non-Hodgkin's lymphoma were enrolled in 15 centers. Two patients were not eligible because they did not meet the inclusion criteria (1 patient had had prior CNS relapse within the preceding month, 1 patient had diffuse large B-cell lymphoma). One patient was already in complete remission after one administration of pre-study triple intrathecal therapy on the day of the first administration of liposomal cytarabine. The median age of the 19 eligible patients was 53 years (range, 24–76 years). Half of the patients (47%) had Philadelphia chromosome-positive ALL and five patients (26%) had relapse of mature B-cell ALL, Burkitt’s lymphoma or B-lymphoblastic lymphoma; 53% had advanced disease with at least one prior relapse (range, 1–4) and 32% at least one prior CNS relapse (range, 1–3). Most of the patients were heavily pretreated, including some who had relapsed after SCT (Table 1). Eighteen patients had positive cerebral spinal fluid cytology, whereas one patient did not have blasts in the cerebrospinal fluid but showed signs of neoplastic meningitis plus a characteristic lesion in the CT scan. Sixteen patients (84%) had at least one clinical sign or symptom of neoplastic meningitis on diagnosis, most frequently headache (37%) and cranial nerve abnormalities (42%).
Table 1.
Patients’ baseline characteristics.
Administration of therapy
A median of four cycles (range, 1–8) of liposomal cytarabine were administered. Two patients (both with Burkitt's lymphoma) received only one cycle because of immediate neurological disease progression, while the other 17 patients were given two or more cycles. Parallel systemic therapy was administered during cycles 1 and 2 to four patients. In five patients with Philadelphia chromosome-positive ALL, treatment with tyrosine-kinase inhibitors (3 dasatinib, 2 imatinib) was continued. In six patients, treatment with liposomal cytarabine was stopped after four to seven cycles, following achievement of cerebrospinal fluid cytological response, partly due to adverse events (5 during maintenance, 1 during induction therapy). Steroid prophylaxis of arachnoiditis was given to all patients: 15 patients were given only oral steroids (79%), one patient was given only intrathecal steroids and three patients received both formulations.
Response
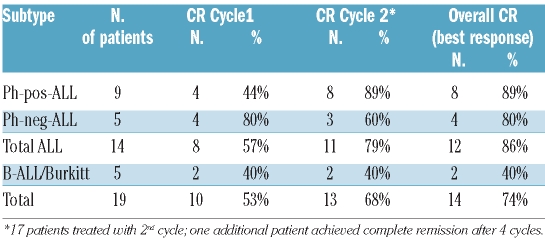
Fourteen patients achieved a CNS cytological response at some time-point. The overall rate of CNS cytological response (best response) was 74%. The patients with CNS cytological response as best response included one patient with a complete response after one cycle and progression after two cycles and four patients with first detection of complete response after two cycles. The complete cytological response rate was 86% in B-precursor/T-ALL compared to 40% in Burkitt’s lymphoma (Table 2). One patient with a cerebral lesion showed a good response on CT scans after the first cycle of liposomal cytarabine with further improvement after the following cycles. Clinical symptoms improved significantly. At the end of treatment (8 cycles) the patient had achieved a stable partial response for more than 9 months before progression occurred.
Table 2.
Response after cycle 1 and 2 according to subtype of disease.
Outcome
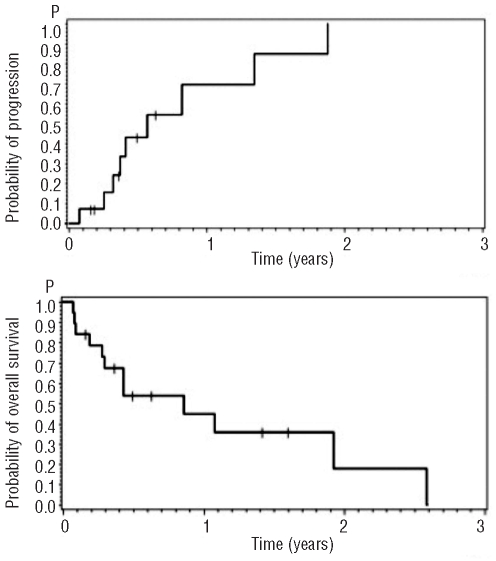
Nine out of the 14 patients with a cytological response relapsed and five remained in complete remission. The site of the relapse was the CNS in three patients, bone marrow in two and CNS together with bone marrow in four patients. The median time to progression was 7 months (range, 1–22 months) (Figure 2A). No patient died in complete remission on treatment. Overall, seven patients (37%) were alive at last follow-up. The median overall survival is 11 months (range, 1–31 months). The probability of survival was 45% after 1 year and 18% after 2 years (Figure 2B).
Figure 2.
(A) Probability of progression in 14 patients with complete remission; 69% at 1 year; median 7 months. (B) Probability of overall survival in 19 evaluable patients; 18% at 2 years; median 11 months.
Prognostic factors for response
The correlation between cytological response rate (best response) and potential prognostic factors is shown in Table 3. The difference in terms of complete response rate comparing B-precursor-/T-ALL to Burkitt’s lymphoma was statistically significant (86% versus 40%; P=0.05). There was a trend towards better response in younger patients compared to patients older than 60 years (82% versus 63%) and in female patients compared to male ones (86% versus 67%). The response rate was higher in patients with isolated CNS relapse than in those with combined relapse (82% versus 62%). Interestingly, patients who had previously received radiotherapy (prophylactically or for the treatment of previous CNS relapse) all achieved complete remission compared to 55% of patients in the group who had not previously received radiotherapy (100% versus 55%; P=0.03). However, all of the latter had Burkitt’s lymphoma.
Table 3.
Best response after cycle 1–2 according to prognostic factors.
Adverse events
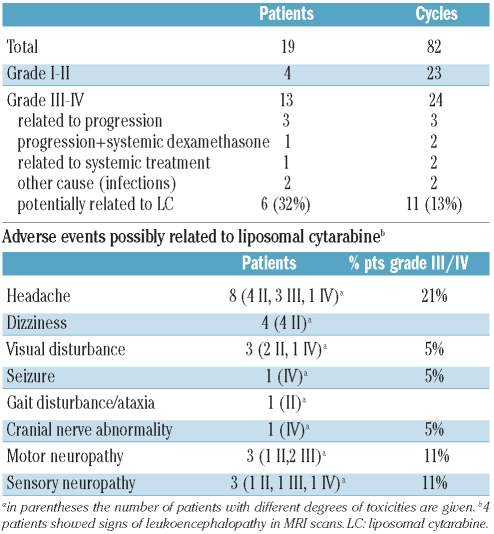
Nineteen patients and a total of 82 cycles were evaluable for adverse events. Seventeen patients (89%) experienced an adverse event of some degree during at least one cycle. Adverse events occurred in 47 of the 82 cycles (57%). Further analysis was focused on grade III/IV adverse events and on those events with potential correlation with liposomal cytarabine (Table 4). Grade III/IV events occurred in 24 of 82 cycles (29%) and in 13 out of 19 patients (68%). In four patients the adverse events were associated with progressive disease and/or side effects of systemic dexamethasone therapy. In three additional patients the adverse events were clearly not correlated to liposomal cytarabine. Thus, the overall incidence of grade III/IV adverse event possibly related to liposomal cytarabine was 32% (6 of 19 patients). Headache was the most frequent adverse event and occurred in four patients (21%) in eight of the 82 cycles (10%). The incidence of adverse events seemed to increase with the number of cycles administered. Beside headache, none of the other neurological adverse events was observed within the first two cycles. In most patients, the neurological adverse events resolved or improved.
Table 4.
Adverse events.
One patient with isolated CNS relapse of B-lymphoblastic lymphoma after intensive high-dose methotrexate/cytarabine-based chemotherapy achieved a complete response after one cycle of liposomal cytarabine. After the fourth application, the patient developed focal seizures and subsequently bilateral 80% reduction of vision. Optical nerve neuritis was assumed as a potential reason for the loss of vision. The patient also developed functional impairment of the oculomotor nerves and lumbar radiculopathy with saddle anesthesia and sphincter dysfunction. The focal seizures were controlled, but the loss of vision and radiculopathy improved only minimally. No further treatment with liposomal cytarabine was administered. In four patients, leukoencephalopathy was detected on MRI scans. In two of these patients, it had already been present on MRI scans prior to starting the study. In two other patients no imaging had been performed prior to starting the liposomal cytarabine. All four of these patients had Philadelphia chromosome-positive ALL with at least one prior relapse (range, 1–4). They had all previously been treated with SCT, tyrosine kinase inhibitors and three of the four had received cranial irradiation in the past.
Discussion
In adult ALL patients, CNS as well as bone marrow relapse are associated with a poor long-term survival rate of less than 5%.3–7 Although CNS disease generally responds to various treatment approaches, the rate of local or systemic relapse is high and patients are at increased risk of developing neurotoxicity. The quality of life of the patients is affected by frequent intrathecal treatments.
The pharmacological approach to treatment of CNS relapse should be reconsidered. Methotrexate and cytarabine are cell-cycle specific agents acting particularly in the S-phase. Prolonged exposure would, therefore, be required to achieve optimal efficacy, particularly in the cerebrospinal fluid in which the rate of proliferation of leukemic blasts is expected to be slow. In this setting, liposomal cytarabine has several theoretical advantages. In contrast to methotrexate and cytarabine with half-lives of 3.4 hours and 4.5 hours, respectively, liposomal cytarabine has a half-life of 141 hours, although interindividual variability has been described.11,13 Promising efficacy data have been reported for lymphomatous meningitis.12 A prospective study in adult ALL did, therefore, seem warranted.
Patients included in this trial were heavily pretreated, had a high incidence of unfavorable features and a rather high median age (42% were older than 60 years). They, therefore, constituted a negatively selected group compared to other published series with CNS relapse of ALL,3–7 with a high proportion of resistant disease and increased risk of toxicities. The efficacy of treatment with liposomal cytarabine in terms of CNS cytological response rate was high (74% after 1–2 cycles). Response was significantly better in ALL (86%) than in Burkitt’s lymphoma (40%). It is well known that after relapse the outcome of Burkitt’s lymphoma is extremely poor. Intrathecal therapy was apparently insufficient to control CNS involvement. Additional treatment elements should, therefore, be considered including systemic therapy, and CNS irradiation in previously non-irradiated patients. Intrathecal rituximab is another option. In seven patients with pediatric B-precursor ALL and refractory CNS relapse, intrathecal rituximab was administered weekly for 4 consecutive weeks and all of these patients achieved a complete remission without relevant toxicity.14
In the majority of patients in this trial, one administration of liposomal cytarabine was sufficient to induce complete remission. Surprisingly, the drug was also effective in one patient with Burkitt’s lymphoma involving the brain, supporting the observation that liposomal cytarabine is able to diffuse into the cerebral parenchyma. Patients with Philadelphia chromosome-positive ALL needed more cycles of treatment to achieve complete remission but still responded well.
Our results underline the strong effectiveness of a single drug, liposomal cytarabine, which, according to the reported response rates, appears to be at least equal to that of intrathecal triple therapy with or without systemic therapy at 32% to 94%.3–7 Previously, the efficacy of liposomal cytarabine had been reported in individual cases or case series with mixed hematologic malignancies. Complete response rates ranged between 60% and 100% for adult and pediatric ALL,15–17 various lymphomas,18 human immunodeficiency virus-associated lymphoma,19 acute myeloid leukemia20 and chronic myeloid leukemia in myeloid blast crisis.21 The populations of patients and the treatments were not very well defined in these retrospective case series. In particular, treatment with other CNS-active drugs was not prohibited and patients with CNS relapse or initial CNS involvement were included.
Overall, liposomal cytarabine was well tolerated. As expected, it was often difficult to differentiate whether adverse events were due to CNS involvement, cumulative toxicities of prior therapies, or to acute toxicity of liposomal cytarabine. The large majority of patients (84%) already presented with neurological signs and symptoms at entry into the study, which indicates rather advanced CNS involvement. Grade III/IV toxicities possibly related to liposomal cytarabine occurred in 32% of the patients and in 13% of the cycles. Toxicity accumulated in individual patients and appeared to increase with the number of cycles. This underlines the observation that cumulative neurotoxicity is a prominent problem in the treatment of CNS relapse in ALL. To avoid this, treatment with liposomal cytarabine could be shifted earlier in the course of treatment to a monthly maintenance schedule, e.g. after two initial administrations. It also remains undetermined whether longer intervals between doses and a limited number of administrations in maintenance would be equally effective.
In phase II studies, it has been shown that systemic prophylaxis with dexamethasone can considerably reduce the risk of arachnoiditis and headache.13 In our trial, two patients suffered from side effects of systemic steroid therapy. This led to the decision to replace oral dexamethasone by intrathecal dexamethasone. The need for steroid co-medication has to be taken into account, if future integration of liposomal cytarabine into CNS prophylaxis is planned. Chemotherapy for ALL generally includes several cycles of steroids. Here, it may not always be feasible to administer additional systemic steroids as part of CNS prophylaxis. It will be important to develop liposomal cytarabine regimens using less systemic steroids.
Visual disturbances, papilledema and a syndrome resembling optical nerve neuritis were observed in one patient in our trial but also by others.22,23 It is often impossible to distinguish whether this syndrome is due to subclinical leukemic infiltration of the optical nerve or due to toxicity. It may respond to steroids with complete recovery but also to further chemotherapy. In addition, several cases of cauda equina or conus medullaris syndrome were reported.23–25 This syndrome may also respond to steroids and be completely reversible. In one study, in which the affected patients had a history of spinal cord surgery or subarachnoid hemorrhage, the authors speculated that a disturbance of cerebrospinal fluid flow may be one possible explanation for increased lumbar toxicity.25
Four patients with Philadelphia chromosome-positive ALL in our study showed signs of leukoencephalopathy. This is a characteristic result of cumulative neurotoxicity due to a combination of prior CNS irradiation, systemic high-dose therapy, intrathecal therapy and CNS involvement. In the GMALL trials the risk of leukoencephalopathy was 1% in patients with continuous complete remission or bone marrow relapse, but significantly higher (11%) in patients with CNS relapse.3 Despite this knowledge, there is no other clinical choice than to treat these patients regardless of their increased risk of leukencephalopathy. In several studies, liposomal cytarabine was administered in combination with systemic therapy including high-dose cytarabine20,26 or high-dose methotrexate.18 With frequent administrations in combination with the hyper-CVAD regimen a number of severe neurotoxicities was observed24 whereas liposomal cytarabine was well tolerated given less frequently27 and not in combination with systemic high-dose cycles.28
As expected, the majority of patients who achieved complete remission subsequently relapsed. In pediatric ALL, the outcome of CNS relapse appears to be similarly favorable whether treated with intensive chemotherapy and irradiation or SCT, with survival rates of 58% to 71%.29 In contrast, it appears that adult ALL patients who relapse can only be cured by SCT. Unfortunately, none of our patients could undergo. The reason was the high median age, which excluded a large proportion of patients from SCT. Furthermore, nearly half of the patients had already received a prior allogeneic SCT. Although in younger patients a second allogeneic SCT is an option, it is generally not considered feasible in older patients and not in all centers. Nevertheless, a median remission duration of 5 months and a median survival of 11 months was achieved, which should be sufficient to organize and perform SCT in patients eligible for this procedure.
Overall, liposomal cytarabine showed excellent antileukemic activity in CNS relapse of ALL and was well tolerated by the majority of patients. Neurotoxicities were relevant but not unexpected in this heavily pretreated population of patients. In order to improve the long-term outcome after CNS relapse, shorter treatment with liposomal cytarabine as a bridge to allogeneic SCT in a larger proportion of patients would be important. Furthermore, treatment might be optimized by reducing the total number of cycles and administering dexamethasone by the intrathecal route rather than systemically.
Liposomal cytarabine is certainly also of interest for prophylaxis of CNS relapse. The advantages are similar to those in the relapse situation, e.g. lower frequency of administration. Prospective trials are necessary, and are indeed ongoing, to define the optimal schedule, frequency and combination with systemic therapy. In particular the interval between liposomal cytarabine and prior or later systemic high-dose chemotherapy needs to carefully defined. It must be demonstrated whether intrathecal prophylaxis with liposomal cytarabine is as efficient as the triple combination, or whether it could even replace CNS irradiation. The latter is a particularly important goal in the management of adult ALL, since it often leads to treatment interruptions and delays of chemotherapy. The resulting reduction in time-dose-intensity has a detrimental impact on prognosis. In brief, the overall effects of innovative CNS prophylaxis, not only on CNS relapse rates but also on systemic relapse rate, need to be evaluated in future trials. With all modifications, it will be essential to maintain the high efficacy of current treatment regimens for adult ALL, which generally yield CNS relapse rates below 5%.
Acknowledgments
We thank Ms. Sabine Hug for the organizational support of the study. We also thank B. Coiffier, P. Montesinos and G. Pollmeier for recruiting, treating and documenting patients for the study.
Footnotes
Funding: this study was supported by the European Union, Sixth Framework Programme, Contract n. LSHC-CT-2004-503216 (European LeukemiaNet) and partly supported by Mundipharma GmbH, Limburg (study drug, research grant).
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Gökbuget N, Hoelzer D. Treatment of adult acute lymphoblastic leukemia. Sem Hematol. 2009;46(1):64–75. doi: 10.1053/j.seminhematol.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Pui CH, Howard SC. Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol. 2008;9(3):257–68. doi: 10.1016/S1470-2045(08)70070-6. [DOI] [PubMed] [Google Scholar]
- 3.Gökbuget N, Aguion-Freire E, Diedrich H, Digel W, Faak T, Kasper C, et al. Characteristics and outcome of CNS relapse in patients with acute lymphoblastic leukemia (ALL) Blood. 1999;94(10):1287a. [Google Scholar]
- 4.Surapaneni UR, Cortes JE, Thomas D, O'Brien S, Giles FJ, Koller C, et al. Central nervous system relapse in adults with acute lymphoblastic leukemia. Cancer. 2002;94 (3):773–9. doi: 10.1002/cncr.10265. [DOI] [PubMed] [Google Scholar]
- 5.Tavernier E, Boiron JM, Huguet F, Bradstock K, Vey N, Kovacsovics T, et al. Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA-94 trial. Leukemia. 2007;21(9):1907–14. doi: 10.1038/sj.leu.2404824. [DOI] [PubMed] [Google Scholar]
- 6.Fielding AK, Richards SM, Chopra R, Lazarus HM, Litzow MR, Buck G, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109(3):944–50. doi: 10.1182/blood-2006-05-018192. [DOI] [PubMed] [Google Scholar]
- 7.Sancho JM, Ribera JM, Oriol A, Hernandez-Rivas JM, Rivas C, Bethencourt C, et al. Central nervous system recurrence in adult patients with acute lymphoblastic leukemia: frequency and prognosis in 467 patients without cranialirradiation for prophylaxis. Cancer. 2006;106(12):2540–6. doi: 10.1002/cncr.21948. [DOI] [PubMed] [Google Scholar]
- 8.Chamberlain MC. Leukemia and the nervous system. Curr Oncol Rep. 2005;7(1):66–73. doi: 10.1007/s11912-005-0028-7. [DOI] [PubMed] [Google Scholar]
- 9.Gökbuget N, Hoelzer D. Meningeosis leukaemica in adult acute lymphoblastic leukaemia. J Neuro Oncol. 1998;38(2–3):167–80. doi: 10.1023/a:1005963732481. [DOI] [PubMed] [Google Scholar]
- 10.Phuphanich S, Maria B, Braeckman R, Chamberlain M. A pharmacokinetic study of intra-CSF administered encapsulated cytarabine (DepoCyt) for the treatment of neoplastic meningitis in patients with leukemia, lymphoma, or solid tumors as part of a phase III study. J Neurooncol. 2007;81(2):201–8. doi: 10.1007/s11060-006-9218-x. [DOI] [PubMed] [Google Scholar]
- 11.Benesch M, Urban C. Liposomal cytarabine for leukemic and lymphomatous meningitis: recent developments. Expert Opin Pharmacother. 2008;9(2):301–9. doi: 10.1517/14656566.9.2.301. [DOI] [PubMed] [Google Scholar]
- 12.Glantz MJ, LaFollette S, Jaeckle KA, Shapiro W, Swinnen L, Rozental JR, et al. Randomized trial of a slow-release versus a standard formulation of cytarabine for the intrathecal treatment of lymphomatous meningitis. J Clin Oncol. 1999;17(10):3110–6. doi: 10.1200/JCO.1999.17.10.3110. [DOI] [PubMed] [Google Scholar]
- 13.Bleyer WA. Intrathecal depot cytarabine therapy: a welcome addition to a limited armamentarium. Clin Cancer Res. 1999;5 (11):3349–51. [PubMed] [Google Scholar]
- 14.Jaime-Perez JC, Rodriguez-Romo LN, Gonzalez-Llano O, Chapa-Rodriguez A, Gomez-Almaguer D. Effectiveness of intrathecal rituximab in patients with acute lymphoblastic leukaemia relapsed to the CNS and resistant to conventional therapy. Br J Haematol. 2009;144(5):794–5. doi: 10.1111/j.1365-2141.2008.07497.x. [DOI] [PubMed] [Google Scholar]
- 15.Sancho JM, Ribera JM, Romero MJ, Martín-Reina V, Giraldo P, Ruiz E. Compassionate use of intrathecal depot liposomal cytarabine as treatment of central nervous system involvement in acute leukemia: report of 6 cases. Haematologica. 2006;91(3):ECR02. [PubMed] [Google Scholar]
- 16.Holowiecka-Goral A, Holowiecki J, Giebel S, Stella-Holowiecka B, Krawczyk-Kulis M, Kos K, et al. Liposomal cytarabine in advanced-stage acute lymphoblastic leukemia and aggressive lymphoma with central nervous system involvement: experience of the Polish Acute Leukemia Group. Leuk Lymphoma. 2009;50(3):478–80. doi: 10.1080/10428190802702375. [DOI] [PubMed] [Google Scholar]
- 17.Benesch M, Sovinz P, Krammer B, Lackner H, Mann G, Schwinger W, et al. Feasibility and toxicity of intrathecal liposomal cytarabine in 5 children and young adults with refractory neoplastic meningitis. J Pediatr Hematol Oncol. 2007;29(4):222–6. doi: 10.1097/MPH.0b013e318041f112. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Marco JA, Panizo C, Garcia ES, Deben G, Alvarez-Larran A, Barca EG, et al. Efficacy and safety of liposomal cytarabine in lymphoma patients with central nervous system involvement from lymphoma. Cancer. 2009;115(9):1892–8. doi: 10.1002/cncr.24204. [DOI] [PubMed] [Google Scholar]
- 19.Mazhar D, Stebbing J, Lewis R, et al. The management of meningeal lymphoma in patients with HIV in the era of HAART: intrathecal depot cytarabine is effective and safe. Blood. 2006;107(8):3412–4. doi: 10.1182/blood-2005-08-3119. [DOI] [PubMed] [Google Scholar]
- 20.Sancho JM, Deben G, Parker A, Piñana JL, Bolam S, Sánchez-García E, et al. Results of compassionate therapy with intrathecal depot liposomal cytarabine in acute myeloid leukemia meningeosis. Int J Hematol. 2007;86(1):33–6. doi: 10.1532/IJH97.E0704. [DOI] [PubMed] [Google Scholar]
- 21.Aichberger KJ, Herndlhofer S, Agis H, Sperr WR, Esterbauer H, Rabitsch W, et al. Liposomal cytarabine for treatment of myeloid central nervous system relapse in chronic myeloid leukaemia occurring during imatinib therapy. Eur J Clin Invest. 2007;37(10):808–13. doi: 10.1111/j.1365-2362.2007.01859.x. [DOI] [PubMed] [Google Scholar]
- 22.Bomgaars L, Geyer JR, Franklin J, Dahl G, Park J, Winick NJ, et al. Phase I trial of intrathecal liposomal cytarabine in children with neoplastic meningitis. J Clin Oncol. 2004;22(19):3916–21. doi: 10.1200/JCO.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 23.Sommer C, Lackner H, Benesch M, Sovinz P, Schwinger W, Moser A, et al. Neuroophthalmological side effects following intrathecal administration of liposomal cytarabine for central nervous system prophylaxis in three adolescents with acute myeloid leukaemia. Ann Hematol. 2008;87 (11):887–90. doi: 10.1007/s00277-008-0521-9. [DOI] [PubMed] [Google Scholar]
- 24.Jabbour E, O'Brien S, Kantarjian H, Garcia-Manero G, Ferrajoli A, Ravandi F, et al. Neurologic complications associated with intrathecal liposomal cytarabine given prophylactically in combination with high-dose methotrexate and cytarabine to patients with acute lymphocytic leukemia. Blood. 2007;109(8):3214–8. doi: 10.1182/blood-2006-08-043646. [DOI] [PubMed] [Google Scholar]
- 25.Hilgendorf I, Wolff D, Junghanss C, Kahl C, Leithaeuser M, Steiner B, et al. Neurological complications after intrathecal liposomal cytarabine application in patients after allogeneic haematopoietic stem cell transplantation. Ann Hematol. 2008;87(12):1009–12. doi: 10.1007/s00277-008-0546-0. [DOI] [PubMed] [Google Scholar]
- 26.Parasole R, Menna G, Marra N, Petruzziello F, Locatelli F, Mangione A, et al. Efficacy and safety of intrathecal liposomal cytarabine for the treatment of meningeal relapse in acute lymphoblastic leukemia: experience of two pediatric institutions. Leuk Lymphoma. 2008;49(8):1553–9. doi: 10.1080/10428190802216749. [DOI] [PubMed] [Google Scholar]
- 27.Spina M, Chimienti E, Martellotta F, Vaccher E, Berretta M, Zanet E, et al. Phase 2 study of intrathecal, long-acting liposomal cytarabine in the prophylaxis of lymphomatous meningitis in human immunodeficiency virus-related non-Hodgkin's lymphoma. Cancer. 2010;116(6):1495–501. doi: 10.1002/cncr.24922. [DOI] [PubMed] [Google Scholar]