Abstract
Background
Optimal lymphocyte parameters and thresholds for the diagnosis of chronic lymphocytic leukemia have been proposed by The National Cancer Institute sponsored Working Group and recently updated by the International Workshop on chronic lymphocytic leukemia. However, it is not clear how these criteria apply to patient management in daily clinical practice and whether the lymphocyte thresholds recommended truly predict clinical outcome in early chronic lymphocytic leukemia.
Design and Methods
For the purpose of this study, an observational database of the GIMEMA (Gruppo Italiano Malattie Ematologiche dell’Adulto) which included 1,158 patients with newly diagnosed Binet stage A chronic lymphocytic leukemia who were observed at different primary hematology centers during the period 1991–2000, was used.
Results
Among 818 consecutive chronic lymphocytic leukemia patients with Rai stage 0 (i.e. no palpable lymphadenopathy or hepatosplenomegaly) who had flow cytometry evaluations at the time of diagnosis and were included in a GIMEMA database, both absolute lymphocyte count and B-cell count were of a similar value in predicting time to first treatment as continuous variables (P<0.0001). Receiver operating characteristic analysis identified an absolute lymphocyte count of 11.5×109/L and an absolute B-cell count of 10.0×109/L as the best thresholds capable of identifying patients who will require treatment from those with stable disease. However, in a Cox’s multivariate analysis only the B-cell count retained its discriminating power (P<0.0001) and the estimated rate of progression to chronic lymphocytic leukemia requiring treatment among subjects with a B-cell count less than 10.0×109/L was approximately 2.3% per year (95% CI 2.1–2.5%) while it was 2-fold higher for patients with a B-cell count of 10.0×109/L or over (i.e. 5.2% per year; 95% CI 4.9–5.5%). Finally, in this community-based patient cohort, the B-cell threshold defined by investigators at the Mayo Clinic (i.e. 11.0×109/L) allowed patients to be divided into two subsets with a higher and lower likelihood of treatment (P<0.0001).
Conclusions
Our results, based on a retrospective patients’ cohort, provide a clear justification to retain the B-cell count as the reference gold standard of chronic lymphocytic leukemia diagnosis and imply that a count of 10×109/L B cells is the best lymphocyte threshold to predict time to first treatment. The use of clinical outcome to distinguish chronic lymphocytic leukemia from other premalignant conditions, such as monoclonal B-cell lymphocytosis, is a pragmatic approach meeting the patients’ need to minimize the psychological discomfort of receiving a diagnosis of leukemia when the risk of adverse clinical consequences is low.
Keywords: B-cell count, CLL diagnosis, MBL, prognosis
Introduction
The incidence and presenting features of chronic lymphocytic leukemia (CLL) have changed significantly in the last thirty years1–3 Both the introduction of automated blood counters in routine clinical practice and the evolution of flow cytometry have led to a lowering of the absolute lymphocyte count (ALC) required for a diagnosis of chronic lymphocytic leukemia (i.e. ≥5.0×109/L).4 In addition, the sensitivity of these diagnostic procedures has allowed the identification of a new clinical entity defined as monoclonal B-cell lymphocytosis (MBL), whose natural history still has to be conclusively defined.5,6
From a clinical standpoint, the development of formal criteria for the diagnosis of monoclonal B-cell lymphocytosis proposed in 2005 guided the 2008 revisions of minimal requirements for chronic lymphocytic leukemia diagnosis.7 Briefly, the new criteria recommend using the B-cell count rather than the absolute lymphocyte count as a basis for the diagnosis of chronic lymphocytic leukemia, and suggest a B-cell threshold of 5.0×109/L to distinguish chronic lymphocytic leukemia from monoclonal B-cell lymphocytosis.7
However, the selection of the B-cell threshold for the diagnosis of chronic lymphocytic leukemia was arbitrary and was not based on objective data of clinical outcome. Given the seriousness of a diagnosis of leukemia and the evolution of the diagnostic criteria of chronic lymphocytic leukemia, information on how the quantitative evaluation of the B-cell clone relates to the clinical outcome of the disease appears mandatory. Recently, the Mayo Clinic Group looked at the correlation between diagnostic lymphocyte parameters and the clinical outcome in a cohort of Rai stage 0 patients who had molecular markers available and whose follow-up information allowed an association with either time to first treatment or overall survival.8,9
In order to contribute to this debate, and possibly to confirm these observations, the GIMEMA (Gruppo Italiano Malattie EMatologiche dell’Adulto) has used an observational database that includes newly diagnosed chronic lymphocytic leukemia patients in Binet stage A and managed over a 10-year period outside the setting of clinical trials according to a “wait and watch” policy. In this large community-based cohort of patients, not influenced by any referral bias, we focused on Rai stage 0 patients and analyzed the relationship between lymphocyte count and clinical outcome. This analysis addresses critical issues, such as the choice of the lymphocyte parameter (i.e. absolute lymphocyte count or B-cell count) to be used for a correct diagnosis of chronic lymphocytic leukemia and the identification of the lymphocyte threshold that better predicts the risk of transformation into an active disease requiring therapy.
Design and Methods
Patients
The GIMEMA CLL database includes previously untreated chronic lymphocytic leukemia patients in Binet stage A who were observed at different GIMEMA primary hematological centers during the period 1991–2000. All patients were managed outside therapeutic protocols according to a “wait and watch” policy. Overall, 1,158 patients from 25 centers were merged into a preliminary working file. Information regarding age, gender, Rai stage, absolute peripheral blood lymphocytosis and number of lymph node sites involved were available for all 1,158 patients. We excluded 20 patients (1.7%) because of inadequate follow up.
We used this database to identify 858 Rai stage 0 patients. All patients had an absolute lymphocyte count of at least 5.0×109/L at the time of diagnosis, thus fulfilling both 1988 and 1996 National Cancer Institute-sponsored Working Group (NCI-WC) guideline criteria for chronic lymphocytic leukemia.4,10 An immunologically confirmed diagnosis by flow cytometry (CD5+/SmIg weak and/or CD5/CD19 co-expression) was available in 818 patients (96.1%). The proportion of B cells and T cells was determined in each patient and the absolute B-lymphocyte count was calculated multiplying the percentage of CD19-positive cells by baseline absolute lymphocyte count. In the majority of patients with B-cell lymphocytosis less than 5.0×109/L (i.e. 102 of 124, 82.2%), in addition to CD19 positive cells, we assessed the number of clonal B cells by CD5/CD19 co-expression.
Data management and analyses were performed in accordance with the ethical guidelines of the GIMEMA Review Board and the tenets of the Declaration of Helsinki. The study was also evaluated and approved by the ethical committee of the Azienda Ospedaliera Pugliese-Ciaccio, Catanzaro, Italy.
Indication for therapy
Patients underwent sequential monitoring and the frequency of follow-up visits was individualized according to patient risk; this ranged from between three and 12 months (median 6 months). All physicians who registered patients into this observational GIMEMA database stated that they had used the NCI-WG guidelines as a reference criteria for starting therapy.4,10 In detail, the absolute lymphocyte count was not used as the sole indicator for treatment. Active disease, requiring therapy, was defined on the basis of at least one of the criteria set out in the National Cancer Institute-sponsored Working Group guidelines.4
Statistical analysis
Time to first treatment was defined as the time between the date of diagnosis and the date of initiation of first treatment or the date of the last follow up at which the patient was known to be untreated. Estimates of time to first treatment were calculated using the Kaplan-Meier method. Likelihood ratio tests were used to test effects of individual factors, either in univariate analysis or jointly. Hazard ratios (HR) and confidence intervals (CI) for hazard ratios were calculated from the Cox’s models. Optimal thresholds for absolute lymphocyte counts and B-cell counts relating to time to first treatment were determined using the receiver operating characteristic (ROC) analysis. The area under the ROC curve (AUC) can range from 0.5 (which indicates a test with no information) to 1.0 (which indicates a perfect test). Because hazard ratio calculates the magnitude of risk rather than the model’s capacity to accurately classify patient outcome, Harrell’s C-statistics were used to further evaluate the discriminatory power of lymphocyte variables in terms of time to first treatment.11
Results
Among 818 consecutive chronic lymphocytic leukemia patients with Rai stage 0 who had flow cytometry evaluation at the time of diagnosis and who were included in the GIMEMA database, none had palpable lymphadenopathy or hepatosplenomegaly. Patients underwent sequential monitoring and median follow up for vital status and treatment status were 47 months and 32 months (range 2–120 months), respectively. As of last follow up, 48 (5.8%) patients had died and 143 (17.4%) had experienced a disease progression that required treatment. The number of deaths recorded in the GIMEMA database was considered insufficient to perform an analysis of association between lymphocyte parameters and overall survival.
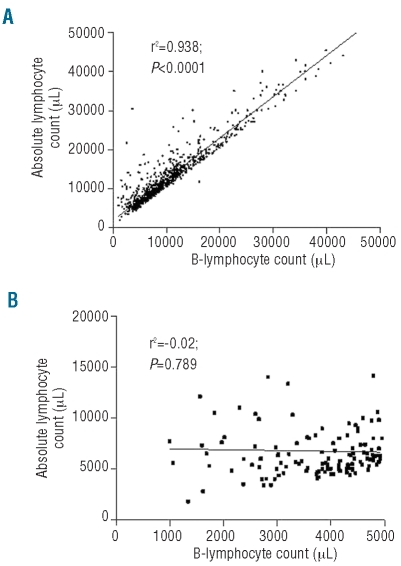
All patients included in the GIMEMA database had at the time of first diagnosis an absolute lymphocyte count of at least 5.0×109/L (median 11.2×109/L; range 5.0–220.0) and met the diagnostic criteria of chronic lymphocytic leukemia as established by both 1988 and 1996 NCI-WC guidelines.4,10 In this patient cohort, B-cell counts ranged from 1.0 to 209.0×109/L (median 9.0×109/L). At the time of the present investigation, 124 patients (15.1%) would be reclassified as monoclonal B-cell lymphocytosis using the 2008 chronic lymphocytic leukemia diagnostic criteria7 and the median B-cell count of this patient subgroup was 3.9×109/L (range 1.0–4.9×109/L); therefore, slightly higher than the median B-cell count reported in two large monoclonal B-cell lymphocytosis series (2.7×109/L and 3.3×109/L, respectively).6,9 While absolute lymphocyte count and B-cell count were strongly correlated for the overall cohort of patients (r=0.938; P<0.0001) (Figure 1A), no correlation between absolute lymphocyte count and B-cell count was observed when the analysis was restricted to subjects with a B-cell count less than 5.0×109/L (r=−0.02; P=0.789) (Figure 1B).
Figure 1.
(A) Relationship between ALC and B-cell count in 818 patients who met the 1988 and 1996 IWCLL-NCI diagnostic CLL criteria (4) (i.e. ALC of at least 5.0×109/L and immunologically confirmed diagnosis of CLL by flow cytometry). (B) Relationship between ALC and B-cell count in 124 patients who would be reclassified as MBL using the 2008 CLL diagnostic criteria.7
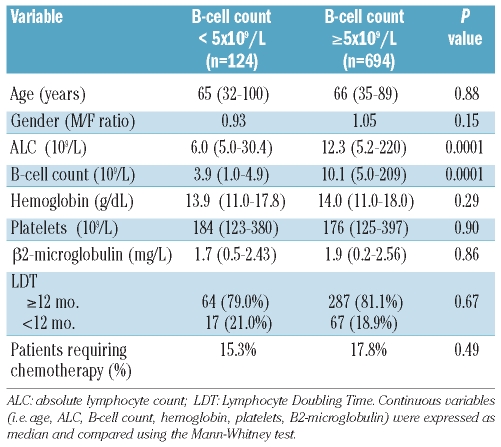
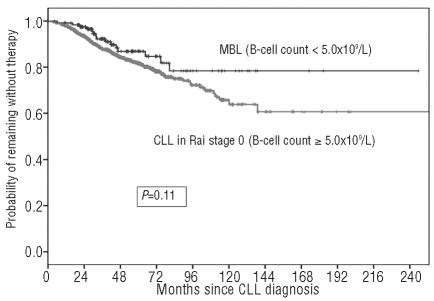
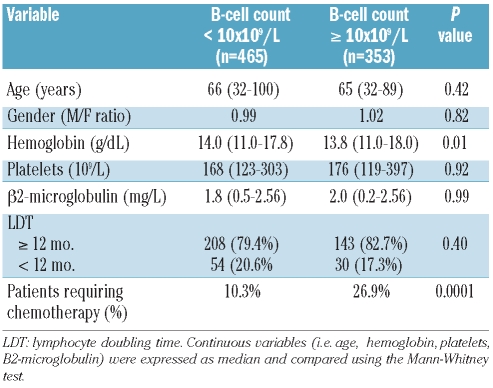
A comparison of the demographic and clinico-hematological characteristics at the time of first diagnosis between patients reclassified as monoclonal B-cell lymphocytosis and those with Rai stage 0 failed to show any difference between the two groups (Table 1). Also the likelihood of treatment for monoclonal B-cell lymphocytosis patients was substantially similar to that of patients with chronic lymphocytic leukemia Rai stage 0 (HR 1.55; 95% CI 0.91–2.65; P=not significant) (Figure 2). Since patient stratification into the two sub-groups (i.e. MBL and CLL, respectively) reflected the absolute number of CD19 positive cells, we wondered whether results would have been biased by an excess of polyclonal B-cells among subjects with monoclonal B-cell lymphocytosis. A sub-analysis carried out to assess the relationship between absolute B-cell count and monoclonal B cells (i.e. CD5/CD19 positive) revealed that 85 of 102 patients with monoclonal B-cell lymphocytosis had rare polyclonal B cells (i.e. < 0.1×109/L) while in the remaining 17 cases the absolute number of polyclonal B cells did not exceed 0.5×109/L. These observations, similar to those reported by Shanafelt et al.,8 seem to confirm that absolute CD19 positive B cells may be considered a reliable surrogate of clonality in this subset of patients.
Table 1.
Comparison of demographic and clinico-hematological features between patients reclassified as MBL according to the 2008 CLL diagnostic criteria and patients in Rai stage 0.
Figure 2.
Time to first treatment (TFT) for patients reclassified, at the time of this analysis, as MBL using the 2008 CLL diagnostic criteria7 (i.e. absolute B-cell count <5.0x109/L) and CLL Rai stage 0 (i.e. absolute B-cell count ≥5.0x109/L).
Since the B-cell threshold proposed in the 2008 CLL diagnostic criteria7 to differentiate monoclonal B-cell lymphocytosis from chronic lymphocytic leukemia (i.e. 5×109/L) did not stratify patients with a different clinical outcome with respect to the rate of progression requiring therapy, we assessed the impact of the absolute lymphocyte count and B-cell count on time to first treatment analyzing these parameters as continuous variables (i.e. measuring the risk of each 1.0×109/L increase in the cell count). Interestingly, both the absolute lymphocyte count and the B-cell count were associated to time to first treatment (HR ALC 1.018, 95% CI 1.012–1.024, P<0.0001; HR B-cell count 1.019, 95% CI 1.013–1.025, P<0.0001).
In order to investigate which lymphocyte parameter (i.e. absolute lymphocyte count or B-cell count) provided a more accurate prediction of time to first treatment, a C-statistic analysis, considered a measure of concordance between observed and predicted time-dependent events, was carried out. The results of this statistical evaluation clearly demonstrated that both the absolute lymphocyte count (c=0.50, P<0.001) and the B-cell lymphocytosis (c=0.51, P<0.001) correctly predicted time to first treatment.
We then considered what threshold at diagnosis best identified patients requiring treatment from those with stable disease. ROC analysis revealed that an absolute lymphocyte count of 11.5×109/L (area under the curve (AUC) 0.664, sensitivity 69.72%, specificity 56.67%; P=0.0001) (Figure 3A) and an absolute B-cell lymphocyte count of 10.0×109/L (AUC 0.651, sensitivity 64.71%, specificity 61.21%; P=0.0001) (Figure 3B) best identified those patients who needed treatment. Interestingly, absolute B-cell count of 10.0×109/L allowed an accurate prediction of time to first treatment (c=0.62; P<0.0001). As expected, the same does not apply for absolute B-cell count of 5.0×109/L (c=0.52; P=0.36).
Figure 3.
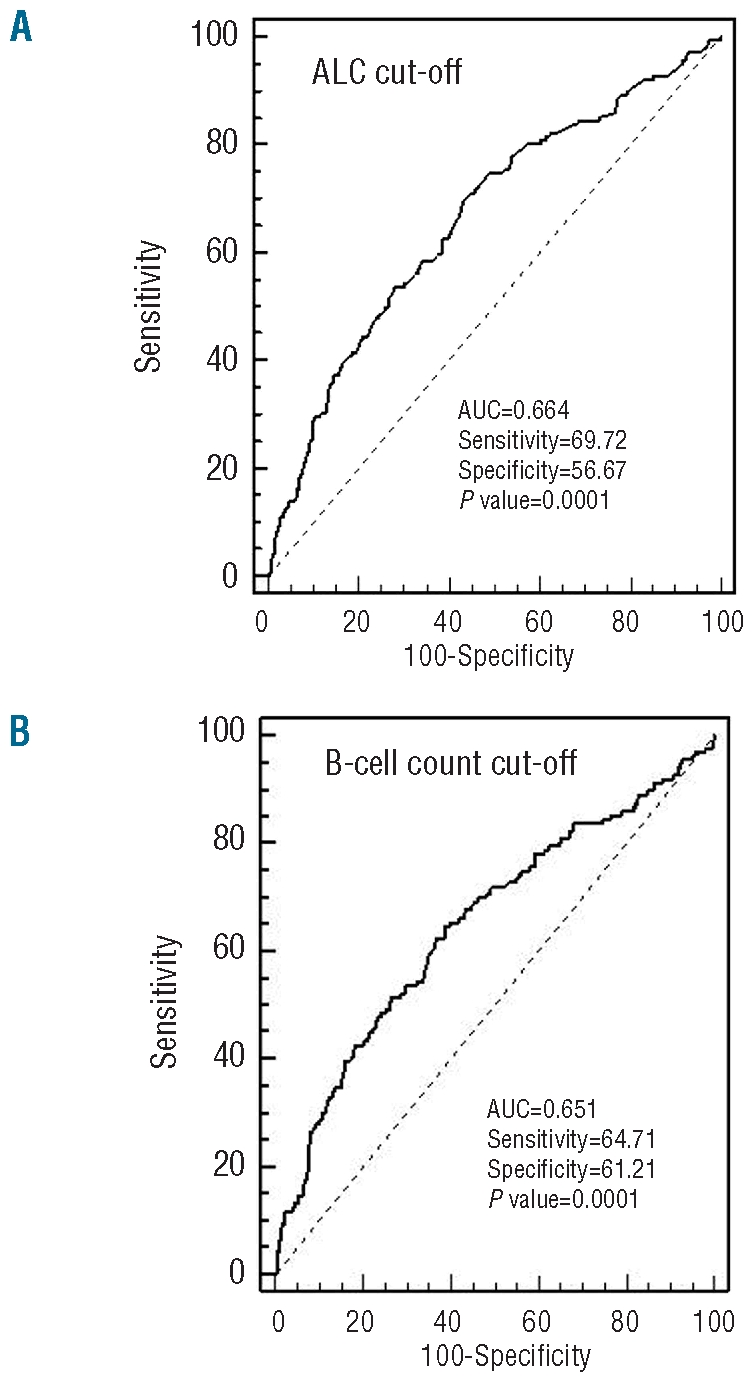
Receiving operating characteristic (ROC) analysis of the accuracy for ALC (A) and B-cell count (B). The accuracy is measured by the area under the ROC curve.
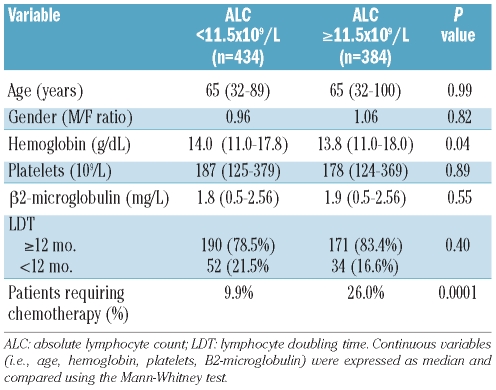
A comparison of the demographic and clinico-hematological characteristics of the patients obtained at the time of first diagnosis and carried out according to these cut-off values demonstrated that age, gender distribution, platelet count, beta2-microglobulin level and lymphocyte doubling time (LDT) were similar among patients with low and higher B-cell counts or absolute lymphocyte counts (Tables 2 and 3). The only difference between groups relied on a significantly lower hemoglobin level that characterized patients with an absolute lymphocyte count greater than 11.5×109/L (P=0.04) or a B-cell count greater than 10×109/L (P=0.01).
Table 2.
Comparison of demographic and clinico-hematological features of Rai stage 0 patients stratified according to the absolute lymphocyte count (ALC) threshold (i.e. 11.5x109/L) selected on the basis of clinical outcome (i.e. time to first treatment).
Table 3.
Comparison of demographic and clinico-hematological features of Rai stage 0 patients stratified according to the absolute B-cell count threshold (i.e. 10x109/L) selected on the basis of clinical outcome (i.e. time to first treatment).
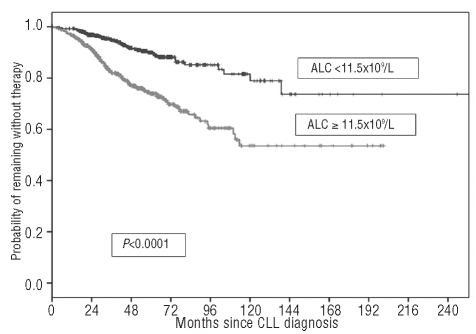
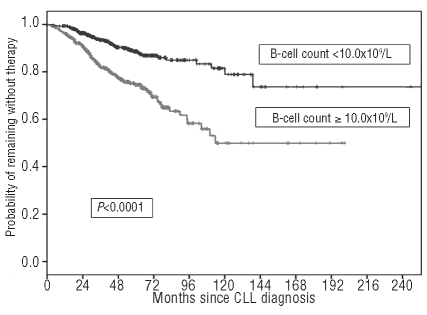
A graphic representation of how these thresholds work in predicting time to first treatment is presented in Figures 4 and 5, respectively. It would appear that both the absolute lymphocyte count and the B-cell count could be considered interchangeable lymphocyte variables to predict patient clinical outcome. In fact, the likelihood of treatment for patients with an absolute lymphocyte count of 11.5×109/L or greater (HR 2.78; 95% CI 1.94–3.99; P<0.0001) or a B-cell lymphocyte count of 10.0×109/L or greater (HR 2.68; 95% CI 1.88–3.81; P<0.0001) was substantially higher than that of patients with lower counts. However, in a Cox’s multivariate analysis only the B-cell count retained its discriminating power (P<0.0001) and during the first five years of follow up the estimated rate of progression to an active phase of the disease requiring treatment was approximately 2.3% per year (95% CI 2.1–2.5%) among subjects with a B-cell count less than 10.0×109/L. Interestingly, such an estimate was 2-fold higher for patients with a B-cell count of 10.0×109/L or greater (i.e. 5.2% per year; 95% CI 4.9–5.5%).
Figure 4.
Time to first treatment (TFT) of patients stratified by ALC greater than or less than 11.5×109/L.
Figure 5.
Time to first treatment (TFT) of patients stratified by B-cell count greater than or less than 10.0×109/L.
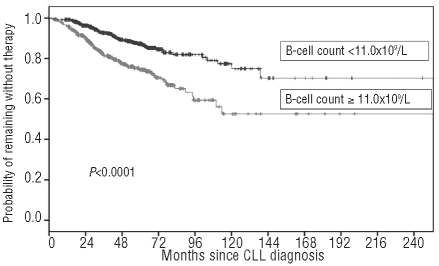
Finally, we wondered whether the B-cell threshold identified by investigators at the Mayo Clinic (i.e. 11.0×109/L)8 was applicable to the patient series included in the GIMEMA database. In this community-based patient cohort, used as a test-set series, we clearly confirmed that the threshold identified by Shanafelt et al.8 allowed patients to be divided into two subsets with a higher and a lower likelihood of treatment, respectively (HR 2.26; 95% CI 1.61–3.18; P<0.0001) (Figure 6).
Figure 6.
Time to first treatment (TFT) of patients stratified by B-cell count according to Shanafelt et al.8 threshold (i.e. greater than or less than 11.0×109/L).
Discussion
The diagnosis of asymptomatic chronic lymphocytic leukemia has been based on the presence of an expanded monoclonal B-cell clone and an increased absolute lymphocyte count. Although the immunophenotypic evaluation of subjects with an absolute lymphocytosis is generally easy in the era of flow cytometry, there is no definitive prospective or retrospective lymphocyte threshold information which can help to establish a diagnosis of chronic lymphocytic leukemia available.12 Our findings, based on a large community-based cohort of patients who underwent evaluation for an asymptomatic lymphocytosis in different Italian hematological institutions referring to the GIMEMA, basically confirm the observations of Shanafelt et al.,8 thus suggesting that compared to absolute lymphocyte count, the B-cell count better identifies those patients who will require treatment from those with stable disease. This is in keeping with the recent International Workshop on chronic lymphocytic leukemia update of the National Cancer Institute 1996 guidelines that modified the definition of chronic lymphocytic leukemia, which now requires a B-cell count of 5.0×109/L cells, rather than the previous absolute lymphocyte count of 5.0×109/L.7
However, this proposed change has some shortcomings, such as the absence of a standardized way to measure B-cell counts. Flow cytometric immunophenotyping for leukemia/lymphoma analysis is not a quantitative test and no standardized approach for determining B-cell counts in chronic lymphocytic leukemia has been proposed.13 On the other hand, the method used for B-cell count measurement in patients included in the GIMEMA multicenter database is less accurate in comparison to the method utilized in the Mayo Clinic studies.8,9 These technical differences reflect the absence of a centralized immunophenotypic standardization in the GIMEMA series and translate into a slight variation in B-cell thresholds between the two series. Furthermore, a shift from an absolute lymphocyte count to a B-cell count could imply repeated flow cytometric analyses to monitor the outcome of chronic lymphocytic leukemia. The relationship between B-cell count and absolute lymphocyte count reported here (Figure 1A), essentially in patients with a diagnosis of chronic lymphocytic leukemia, suggests that a reasonable practical approach would be to monitor the patients with a B-cell count above the diagnostic threshold (i.e. ≥5.0×109/L B-lymphocytes) and to reserve periodic B-cell assessments by flow cytometry to patients with monoclonal B-cell lymphocytosis until the chronic lymphocytic leukemia diagnostic threshold is attained.
The diagnostic criteria for monoclonal B-cell lymphocytosis were intended to identify individuals with an abnormal B-cell population in the peripheral blood, but who did not meet the current criteria for a B-cell lymphoproliferative disorder.6,8,9,14–16 In a recent study, Rawstron et al.6 reported the clinical outcome of a cohort of 185 CLL-type monoclonal B-cell lymphocytosis cases observed over a 6.7-year period. The B-cell count (< or ≥1.9×109/L) at the time of the diagnosis of monoclonal B-cell lymphocytosis was the only factor independently associated with progressive lymphocytosis; however, no association with overall survival or time to first treatment was reported. Shanafelt et al.9 described a lower likelihood of treatment requirement for patients with a monoclonal B-cell lymphocytosis in comparison to patients with Rai stage 0 chronic lymphocytic leukemia. These findings could be due, at least in part, to the lower median absolute B-cell count at the time of diagnosis (2.7×109/L vs. 3.9×109/L) that characterized the monoclonal B-cell lymphocytosis patient cohort reported by Shanafelt et al.9 in comparison to ours. Additionally, because the number of patients reclassified as monoclonal B-cell lymphocytosis in the GIMEMA database is lower in comparison to the MBL patients of the Mayo Clinic series (15.1% vs. 40.1%), the statistical power to detect the optimal diagnostic threshold for monoclonal B-cell lymphocytosis/chronic lymphocytic leukemia is near the range of the current value of 5.0×109/L.
While the absolute lymphocyte count of 5.0×109/L was selected arbitrarily, there is the suggestion that the selection of an absolute B-lymphocytosis of 5.0×109/L was likewise selected arbitrarily for consistency and was not based on objective clinical data of outcome.12 As clearly illustrated by monoclonal gammopathy of undeterminated significance (MGUS), the distinction between a pre-neoplastic and a neoplastic condition should rely on the individual risk of an adverse clinical outcome rather than on the presence of a clonal cell population. In this respect, we confirm that among patients with a clonal expansion of cells bearing a CLL phenotype in the peripheral blood, the size of the B-cell count relates to a patient’s risk of receiving therapy for active disease. Our analysis clearly demonstrates that the presence of a B-cell clone accounting for 10 ×109/L cells represents the best cut-off value to predict the clinical behavior of the disease in terms of time to first treatment. The same B-cell threshold, on the contrary, did not correlate with overall survival but the number of deaths recorded in the GIMEMA database was considered insufficient to conduct an analysis of association between lymphocyte parameters and overall survival. We would like to point out that among the endpoints used to measure outcome, time to first treatment appears more suitable than overall survival for early chronic lymphocytic leukemia patients. Time to first treatment does not reflect competing risks between successive relapses, histological transformations, deaths in remission and the impact of new therapies.17 However, this does not mean that the association between a B-cell lymphocyte threshold of 11.0×109/L and overall survival reported by the Mayo Clinic group does not hold.
In our retrospective community-based patient cohort, the clinico-hematological characteristics, assessed at the time of diagnosis, did not reflect changes in either the absolute lymphocyte count or B-cell count. An unanticipated finding was represented by the statistically significant difference in the median hemoglobin level between groups. Indeed, there were more patients with mild anemia (i.e. hemoglobin concentrations below the median value for the whole series of 14 g/dL, but above 11 g/dL) in the higher ALC/B-cell count group (51.9% vs. 40.6%; P=0.001).
A limitation of our study is the lack of biologically-based prognostic parameters, which were not always evaluated at the time of diagnosis. This prevented us from performing a comprehensive multivariate analysis that included all these variables. Although Shanafelt et al.8 demonstrated that the B-cell count remained an independent predictor of time to first treatment even after checking for ZAP-70, CD38, IgVH or fluorescence in situ hybridization (FISH), the prognostic independence of the size of the B-cell count should be validated in an independent series of patients monitored prospectively. On the contrary, one specific strength of this report when compared to the studies of Shanafelt et al.8,9 is represented by the characteristics of our patient cohort which was taken from “primary” hematology clinics; consequently, referral/ascertainment bias is not an issue as it was in the Mayo Clinic studies.
In conclusion, our results, although based on a retrospective patient series, clearly justify retaining the B-cell count as the reference gold standard of chronic lymphocytic leukemia diagnosis, but imply that a B-cell count of 10.0×109/L represents the threshold that best predicts time to first treatment. From a clinical standpoint, an international and integrated clinical observational study including patients with chronic lymphocytic leukemia in early stage (from the time of first presentation) and followed prospectively to better define disease outcome should conclusively address the issue. This is crucial to minimize the unneeded psychological discomfort caused by labeling individuals with laboratory abnormalities and/or low-risk clinical features as having leukemia.18
Footnotes
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Rozman C, Bosch F, Montserrat E. Chronic lymphocytic leukemia: a changing natural history? Leukemia. 1997;11(6):775–8. doi: 10.1038/sj.leu.2400679. [DOI] [PubMed] [Google Scholar]
- 2.Molica S, Levato D. What is changing in the natural history of chronic lymphocytic leukemia. Haematologica. 2001;86(1):8–12. [PubMed] [Google Scholar]
- 3.Abrisqueta P, Pereira A, Rozman C, Aymerich M, Gine E, Moreno C, et al. Improving survival in patients with chronic lymphocytic leukemia (1980–2008): the Hospital Clinic of Barcelona experience. Blood. 2009;114(10):2044–50. doi: 10.1182/blood-2009-04-214346. [DOI] [PubMed] [Google Scholar]
- 4.Cheson BD, Bennett JM, Grever M, Kay N, Keating MJ, O’Brien S, Rai KR. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87(12):4990–7. [PubMed] [Google Scholar]
- 5.Marti GE, Rawstron AC, Ghia P, Hillmen P, Houlston RS, Kay N, et al. Diagnostic criteria for monoclonal B-cell lymphocytosis. Br J Haematol. 2005;130(3):325–32. doi: 10.1111/j.1365-2141.2005.05550.x. [DOI] [PubMed] [Google Scholar]
- 6.Rawstron AC, Bennett FL, O’Connor SJ, Kwok M, Fenton JA, Plummer M, et al. Monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia. N Engl J Med. 2008;359(6):575–83. doi: 10.1056/NEJMoa075290. [DOI] [PubMed] [Google Scholar]
- 7.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111 (12):5446–56. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shanafelt TD, Kay NE, Jenkins G, Call TG, Zent CS, Jelinek DF, et al. B-cell count and survival: differentiating chronic lymphocytic leukemia from monoclonal B-cell lymphocytosis based on clinical outcome. Blood. 2009;113(18):4188–96. doi: 10.1182/blood-2008-09-176149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shanafelt TD, Kay NE, Rabe KG, Call TG, Zent CS, Maddocks K, et al. Brief report: natural history of individuals with clinically recognized monoclonal B-cell lymphocytosis compared with patients with Rai 0 chronic lymphocytic leukemia. J Clin Oncol. 2009;27(24):3959–63. doi: 10.1200/JCO.2008.21.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheson BD, Bennett JM, Rai KR, Grever MR, Kay NE, Schiffer CA, et al. Guidelines for clinical protocols for chronic lymphocytic leukemia: recommendations of the National Cancer Institute-sponsored working group. Am J Hematol. 1988;29(3):152–63. doi: 10.1002/ajh.2830290307. [DOI] [PubMed] [Google Scholar]
- 11.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Marti GE. The changing definition of CLL. Blood. 2009;113(18):4130–1. doi: 10.1182/blood-2008-12-193656. [DOI] [PubMed] [Google Scholar]
- 13.Hanson CA, Kurtin PJ, Dogan A. The pro-posed diagnostic criteria change for chronic lymphocytic leukemia: unintended consequences? Blood. 2009;113(25):6495–6. doi: 10.1182/blood-2008-05-158444. [DOI] [PubMed] [Google Scholar]
- 14.Fung SS, Hillier KL, Leger CS, Sandhu I, Vickars LM, Galbraith PF, et al. Clinical progression and outcome of patients with monoclonal B-cell lymphocytosis. Leuk Lymphoma. 2007;48(6):1087–91. doi: 10.1080/10428190701321277. [DOI] [PubMed] [Google Scholar]
- 15.Rossi D, Sozzi E, Puma A, De Paoli L, Rasi S, Spina V, et al. The prognosis of clinical monoclonal B cell lymphocytosis differs from prognosis of Rai 0 chronic lymphocytic leukaemia and is recapitulated by biological risk factors. Br J Haematol. 2009;146(1):64–75. doi: 10.1111/j.1365-2141.2009.07711.x. [DOI] [PubMed] [Google Scholar]
- 16.Shanafelt TD, Ghia P, Lanasa MC, Landgren O, Rawstron AC. Monoclonal B-cell lymphocytosis (MBL): biology, natural history and clinical management. Leukemia. 2010;24(3):512–20. doi: 10.1038/leu.2009.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molica S, Mauro FR, Callea V, Giannarelli D, Lauria F, Rotoli B, et al. The utility of a prognostic index for predicting time to first treatment (TFT) in early chronic lymphocytic leukemia (CLL): the GIMEMA (Gruppo Italiano Malattie Ematologiche dell’ Adulto) experience. Haematologica. 2010;95(3):464–9. doi: 10.3324/haematol.2009.011767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffbrand VA, Hamblin TJ. Is “leukemia” an appropriate label for all patients who meet the diagnostic criteria of chronic lymphocytic leukemia? Leuk Res. 2007;31(3):273–5. doi: 10.1016/j.leukres.2006.07.006. [DOI] [PubMed] [Google Scholar]