Abstract
Background
In 2009 the declaration by the World Health Organization of a global pandemic of influenza-H1N1 virus led to a vaccination campaign to ensure protection for immunocompromised patients. The goal of this study was to determine the efficacy of the 2009 H1N1 vaccine in patients with hematologic malignancies.
Design and Methods
We evaluated humoral and cellular immune responses to 2009 H1N1 vaccine in 97 adults with hematologic malignancies and compared these responses with those in 25 adult controls. Patients received two injections of vaccine 21 days apart and the controls received one dose. Antibody titers were measured using a hemagglutination-inhibition assay on days 0, 21 and 49 after injection of the first dose. Cellular immune responses to H1N1 were determined on days 0 and 49.
Results
By day 21 post-vaccination, protective antibody titers of 1:32 or more were seen in 100% of controls compared to 39% of patients with B-cell malignancies (P<0.001), 46% of allogeneic stem cell transplant recipients (P<0.001) and 85% of patients with chronic myeloid leukemia (P=0.086). After a second dose, seroprotection rates increased to 68%, (P=0.008), 73%, (P=0.031), and 95% (P=0.5) in patients with B-cell malignancies, after allogeneic stem cell transplantation and with chronic myeloid leukemia, respectively. On the other hand, T-cell responses to H1N1 vaccine were not significantly different between patients and controls.
Conclusions
These data demonstrate the efficacy of H1N1 vaccine in most patients with hematologic malignancies and support the recommendation for the administration of two doses of vaccine in immunocompromised patients. These results may contribute towards the development of evidence-based guidelines for influenza vaccination in such patients in the future.
Keywords: H1N1 vaccine, efficacy, immunocompromised host, leukemia, CML, allogeneic SCT, lymphoma, influenza, hematologic malignancies, hematology, cancer
Introduction
In 2009 the spread of influenza A (H1N1) satisfied the World Health Organization (WHO) criteria of a global pandemic and led to the initiation of a vaccination campaign to ensure protection for the most vulnerable patients, including those with hematologic malignancies. However, the immunogenicity of the 2009 H1N1 vaccine in immunocompromised patients has not been specifically tested. Furthermore, the number of doses of vaccine required for effective immunization against the novel influenza A (H1N1) has not been established. Whereas the European Medicines Agency1 and the UK Department of Health (DoH)2 recommend the injection of two doses of inactivated H1N1 vaccine with a minimum of 3 weeks between doses for immunocompromised individuals, the Centers for Disease Control and Prevention recommend immunization with one dose of inactivated H1N1 vaccine for patients with cancer receiving chemotherapy, followed by a booster vaccine 3 months after completion of treatment if the pandemic continues.3
We conducted a prospective study to determine the safety and immunogenicity of the vaccination program against the 2009 pandemic H1N1 in patients with hematologic malignancies and healthy controls and to characterize the different components of the immune response to H1N1. The results provide a more complete picture of the host response to the vaccination, and facilitate the development of improved vaccination strategies for immunosuppressed individuals.
Design and methods
Study design
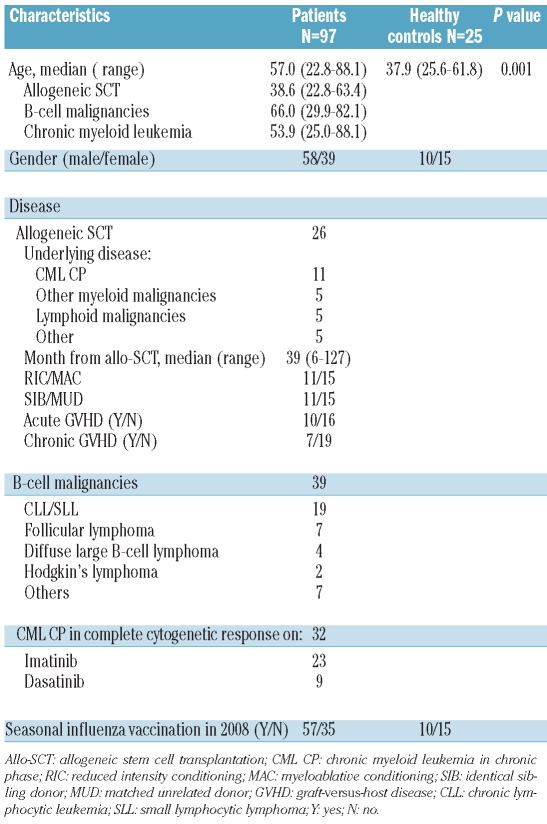
From 28th October until 18th December 2009, 97 adult patients with hematologic malignancies and 25 adult controls were vaccinated in compliance with UK DoH guidelines.2 All patients and donors gave informed consent and the study protocol was approved by the local research ethics committee. Of the 97 patients, 32 had chronic myeloid leukemia (CML) in chronic phase in complete cytogenetic response on the tyrosine kinase inhibitors imatinib or dasatinib, 39 had a B-cell malignancy in complete remission or untreated, including non-Hodgkin’s lymphoma, Hodgkin’s lymphoma or chronic lymphocytic leukemia, and 26 were recipients of an allogeneic hematopoietic stem cell transplant (SCT) in complete remission at least 6 months after transplantation and without evidence of active graft-versus-host disease. Healthy controls were recruited from hospital staff who were offered vaccination because they were front-line healthcare workers. Patients and controls who had been previously exposed to 2009 H1N1 infection, as confirmed by reverse transcriptase polymerase chain reaction (RT-PCR), were excluded from this study.
Vaccine
Consistent with UK DoH guidelines all patients received an inactivated split-virion preparation of the influenza A/California/2009 (H1N1)v-like strain containing 3.75 μg of hemagglutinin and AS03 adjuvant (Pandemrix GSK, UK). The vaccine was administered by intramuscular injection into the deltoid muscle of the non-dominant arm by the patient’s primary care physician, according to DoH guidelines. Over the same period, 89 of 97 patients and 15 of 25 controls also received one dose of a seasonal influenza vaccine containing 15 μg of hemagglutinin antigens of the following three strains: A/Brisbane/59/2007 (H1N1)-, A/Brisbane/10/2007 (H3N2)- and B/Florida/30/2008-like strain; in the majority of patients and controls the vaccines were not given concomitantly.
Safety assessments and assessment of influenza-like illness
We solicited reports of local (pain, tenderness, redness, induration and ecchymosis) and systemic (fever, headache, malaise, myalgia, chills and nausea) adverse events by 2-weekly phone calls performed by trained medical students, starting 1 week after the first injection. All local and systemic adverse events reported in response to solicitation within 7 days after administration of the vaccine were considered to be related to the vaccine. Symptoms were graded as follows: none, mild if they did not interfere with normal activities, moderate if they resulted in interference with normal activities, and severe if they prevented engagement in daily activities or necessitated medical attention.
An influenza-like illness was defined as an oral temperature of more than 38°C or a history of fever or chills and at least one influenza-like symptom.
Immunological investigations
Serum and peripheral blood mononuclear cells were collected before vaccination and on days 21 and 49 after the first vaccine dose, and cryopreserved. Antibody responses were detected by means of hemagglutination-inhibition assays, according to standard methods and as previously described, at the Centre for Infections, Health Protection Agency (London, UK).4 Serum samples obtained from subjects were tested in duplicate using 1:2 serial dilutions, starting at an initial dilution of 1:8 and finishing at a final dilution of 1:1024. Hemagglutination-inhibition antibody titers were reported according to the criteria conventionally used to assess the immunogenicity of H1N1 influenza vaccines, i.e. geometric mean titer, geometric mean titer ratio, seroprotection rate (proportion with titers ≥1:32) and seroconversion rate (proportion with pre-vaccination titer <1:8 and a post-vaccination titer ≥1:32, or a pre-vaccination titer ≥1:8 and an increase in the titer by a factor of four or more).5
Specific humoral responses to the seasonal flu vaccine were not measured as these have been extensively described previously.3,6–8
To assess T-cell responses, peripheral blood mononuclear cells collected before vaccination and on day 49 were thawed and stimulated for 24 h with or without H1N1 vaccine (A/California/07/2009(H1N1)v-like strain, Baxter, UK) or seasonal influenza vaccine (A/Brisbane/59/2007(H1N1), A/Brisbane/10/2007(H3N2)- and B/Florida/30/2008-like strain, CSL Biotherapies, Germany) (used as a positive control) at a final concentration of 1.5 μg/mL of hemagglutinin antigens. The effector function of antigen-specific CD8+ and CD4+ T cells was assessed by intracellular-cytokine staining for interferon-γ (INF-γ) and tumor necrosis factor-α (TNF-α), as previously described.9 Allophycocyanin-conjugated antibodies to INF-γ and TNF-α were employed to detect the frequencies of INF-γ- or TNF-α-producing T cells. A response was considered positive if the combined percentage of H1N1-specific TNF-α plus IFN-γ-producing CD4+ or CD8+ T cells was 2-fold or higher compared to the background level (non-stimulated peripheral blood mononuclear cells) and if there was a minimum of 0.05% H1N1-specific TNF-α plus IFN-γ-producing CD4+ or CD8+ T cells (after subtracting the background).
Statistical analysis
Groups were compared using Fisher’s exact test for categorical data and the Mann-Whitney test for continuous variables. To evaluate the effect of a second vaccine dose, paired sample analysis was performed using a McNemar test. Geometric mean titer values, with 95% confidence intervals, were calculated by use of the mean, and lower and upper limits of the 95% confidence intervals of log-transformed titers. The influence of variables on the rates of seroprotection or seroconversion was studied using a logistic regression model. All reported P values are two-sided and without adjustment for multiple testing. Analyses were done for the full-analysis set using the software package SPSS (version 17).
Results
Patients’ characteristics
The clinical characteristics of the patients and healthy controls are summarized in Table 1. Of the 97 patients, 89 received the recommended booster at a median of 27 days (range, 18–57 days) after the first dose. Eight patients failed to receive a booster dose, either due to the patients’ refusal (n=3) or limited access to their primary health care physician (n=5). Twenty-five healthy controls received one dose of the vaccine only, in accordance with UK DoH guidelines.
Table 1.
Characteristics of the patients and healthy controls.
Toxicity profile following vaccination with 2009 H1N1 and seasonal influenza vaccines
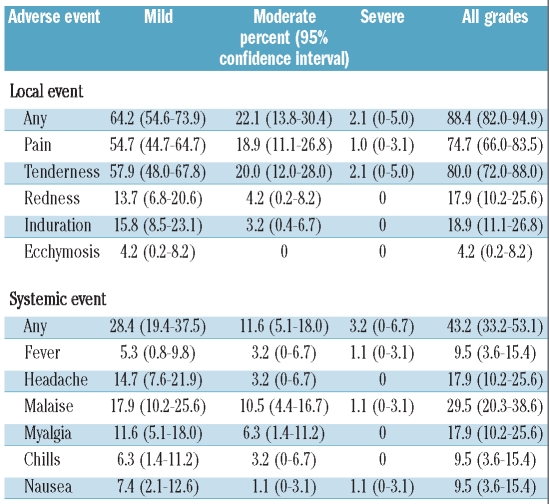
In general the vaccines were well tolerated. Table 2 shows the adverse events during the first 7 days after the first dose. Overall 86/95 evaluable patients (90.5%) reported adverse reactions after the first vaccine dose, including local reactions in 84/95 (88.4%) and systemic adverse events in 41/95 (43.2%), of which 2.1% and 3.2%, respectively, were reported as severe adverse events. We solicited information from 72 patients on side effects after the second vaccine dose: nine (12.5%) reported worsening side effects, of whom six had exacerbated local reactions (pain or tenderness) and three had exacerbated systemic adverse events (fever, nausea or malaise). No patient required hospital admission as a consequence of vaccine-related adverse events.
Table 2.
Injection-site and systemic adverse effects within 7 days after the first dose of vaccine among patients.
In comparison 22/25 healthy controls (88%) reported adverse events, of whom 22/25 had local reactions (88%) and 10/25 (40%) had systemic adverse events. There were no obvious differences in the side effect profiles or frequencies of adverse events between patients and controls (data not shown).
Clinical efficacy of vaccination
Five patients reported an influenza-like illness by the end of the influenza season on 31st March 2010, of whom one required admission to hospital. None of these five patients had an RT-PCR-confirmed H1N1 influenza illness or received antiviral therapy, and all had achieved seroconversion after vaccination. Similarly, H1N1 infection was not diagnosed in any of the vaccinated controls during follow-up.
Seroprotection rates to 2009 H1N1 in controls and patients
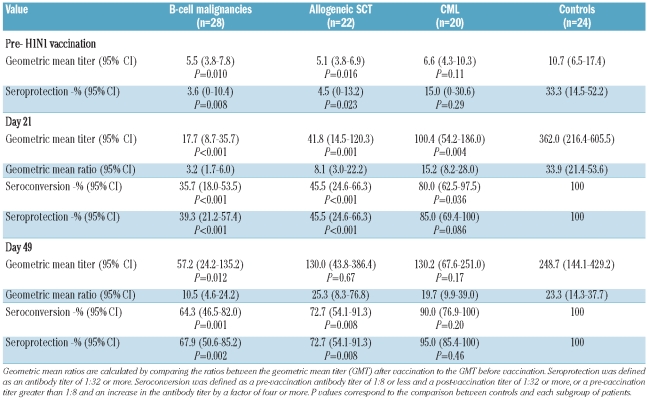
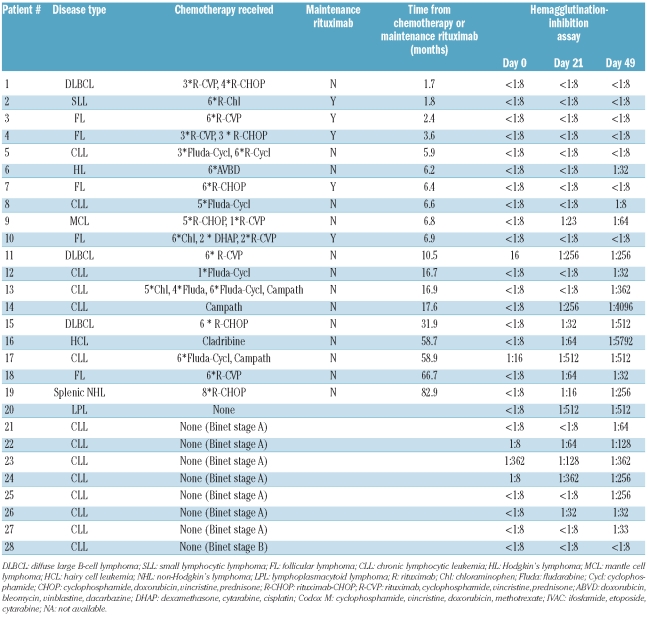
We evaluated the humoral response in 70 patients and 24 healthy controls in whom antibody titers were available at all study time-points (before vaccination and at days 21 and 49); patients who failed to receive a second dose were also excluded from this analysis. Before vaccination protective antibody titers of 1:32 or more were seen in 8/24 (33.3%) controls compared to 1/28 (3.6%) patients with B-cell malignancies (P=0.008), 1/22 (4.5%) allogeneic SCT recipients (P=0.023) and 3/20 (15.0%) CML patients (P=0.29), as shown in Table 3 and Figure 1.
Table 3.
Antibody response to the first (day 21) and second dose (day 49, patients only) of vaccine as measured by the hemagglutination-inhibition assay.
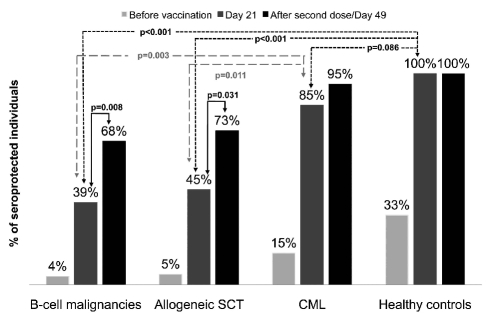
Figure 1.
Frequency of seroprotected individuals after one dose (patients and controls) and two doses (patients only) of vaccine
On day 21 after vaccination, protective antibody titers of 1:32 or more were seen in 24/24 (100%) controls compared to in only 11/28 (39.3%) patients with B-cell malignancies (P<0.001), 10/22 (45.5%) of allogeneic SCT recipients (P<0.001) and 17/20 (85.0%) CML patients (P=0.086) (Table 3 and Figure 1). The geometric mean titer was significantly higher in healthy controls than in all groups of patients, namely 362 versus 18 (P=0.001) in patients with B-cell malignancies, 362 versus 42 (P=0.001) in allogeneic SCT recipients and 362 versus 100 (P=0.004) in CML patients (Table 3). The seroprotection rates achieved in CML patients were significantly higher than those in patients with B-cell malignancies (P=0.003) and recipients of allogeneic SCT (P=0.011). Similar results were obtained when looking at the rate of seroconversion after the first injection (Table 3).
Humoral response to the second dose of vaccine
When we analyzed the antibody response to H1N1 at day 49 post-vaccination the seroprotection rates were significantly lower in patients with B-cell malignancies (P=0.002) and in allogeneic SCT recipients (P=0.008) than in healthy controls (Table 3). The seroprotection rates achieved in CML patients at day 49 were significantly higher than those achieved in patients with B-cell malignancies (19/20 versus 19/28 respectively; P=0.031) but not significantly different to those in recipients of allogeneic SCT (19/20 versus 16/22; P=0.096) or healthy controls (P=0.46).
In order to assess the effect of the second booster dose, we performed a paired sample analysis using a McNemar test. The second vaccine dose induced a significant increase in the seroprotection rates from 39% to 68% (11/28 versus 19/28; P=0.008) in patients with B-cell malignancies and from 45% to 73% (10/22 versus 16/22; P=0.031) in allogeneic SCT recipients. However, after the second booster dose, the seroprotection rate for CML patients did not change significantly (17/20 after the 1st dose and 19/20 after 2nd dose; P=0.5). The seroconversion rates followed the same pattern (data not shown).
Impact of age on the level of seroprotection and seroconversion
The median age of controls was 37.9 years (range, 25.6–61.8 years) compared to 66.0 years (range, 29.9–82.1 years) in patients with B-cell malignancies, 38.6 years (range, 22.8–63.4 years) in allogeneic SCT recipients and 53.9 years (range, 25.0–88.1) in CML patients. We studied the relationship between age and the rate of seroconversion or seroprotection by constructing a logistic regression model for each outcome in which we entered the baseline disease (B-cell malignancies, CML, allograft or control) and the age of the patient or control. Age, either as continuous variable or as a categorical variable (quartiles), did not influence the seroconversion or seroprotection rates as measured on day 21 or day 49 (data not shown).
Effect of chemotherapy and rituximab on the humoral response to vaccination
Among the 28 evaluable patients with B-cell malignancies, nine patients had not received chemotherapy. Of the 19 treated patients 12 had received rituximab-based treatment or were on maintenance rituximab (Table 4). The period between chemotherapy and vaccination was significantly longer in patients who were seroprotected at day 49 compared to those who were not (4.7 versus 17.5 months, P=0.001). Of the 19 patients who had received prior chemotherapy, all eight (100%) patients vaccinated more than 12 months after chemotherapy achieved seroprotection after two doses of the vaccine, compared to three of six (50%) vaccinated between 6–12 months and none of five (0%) vaccinated within 6 months of chemotherapy (P=0.001, χ2 trend test). There were no significant differences in the seroprotection rates of the eight patients who were vaccinated more than 12 months following chemotherapy compared to tose of the nine patients who had not been previously treated (100% versus 89%, P=0.99) (Table 4). Importantly, when restricting the analysis to the 17 patients with B-cell malignancies who were vaccinated more than 12 months after receiving chemotherapy (n=8) or those who had never been treated with chemotherapy (n=9), 16/17 (94%) achieved a level compatible with seroprotection after a second dose, which was not statistically significantly different from the rate in healthy controls (P=0.415). However, it appears that two doses of vaccine are still necessary to induce a significant antibody titer in these patients as only 10 of the 17 seroconverted after the first dose (P=0.031).
Table 4.
Comparison of antibody response to 2009 H1N1 vaccination in patients with B-cell malignancies according to time from chemotherapy. Only patients in whom antibody titers were available at all time points and who received two vaccine doses are included in this table.
Impact of time from transplant on humoral response to vaccination
In the allogeneic SCT recipient group, we studied the impact of a number of factors including conditioning regimen (myeloablative versus reduced-intensity), donor type (sibling or matched unrelated donor), time from transplant, previous history of acute or chronic graft-versus-host disease, and underlying disease on seroconversion and seroprotection rates following H1N1 vaccination (Table 1). The time from transplantation was the only significant predictive variable: patients who achieved seroprotection had a significantly longer transplant-to-vaccination interval compared to patients who failed to achieve seroprotection (6.5 versus 48 months; P=0.015). Of note only two patients were on low dose immunosuppressive therapy with cyclosporine A, neither of whom developed a seroprotective humoral response to vaccination.
H1N1-specific T-cell response to vaccination
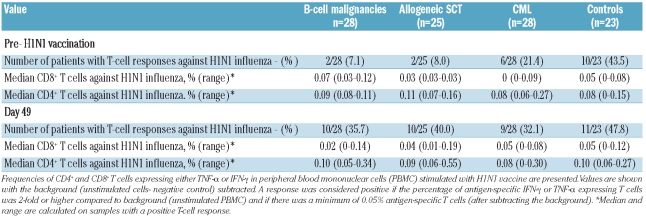
The induction of virus-specific T-cell responses by H1N1 vaccination was assessed directly ex vivo by flow cytometric enumeration of antigen-specific CD8+ and CD4+ T lymphocytes using an intracellular cytokine assay for IFN-γ and TNF-α (Th1 effector cytokines). Peripheral blood mononuclear cells were available for analysis at baseline and at day 49 in 23 controls and 81 patients. Prior to H1N1 vaccination, pre-existing T-cell responses against 2009 H1N1 influenza could be detected in 10/23 (43%) controls compared to in 2/25 (8%) allogeneic SCT recipients (P=0.007), 2/28 (7%) patients with B-cell malignancies (P=0.003) and 6/28 (21%) of CML patients (P=0.131).
Following vaccination, H1N1-specific T cells were induced in a significant proportion of allogeneic SCT recipients (2/25 pre-vaccine versus 10/25 post-vaccine; P=0.008, McNemar’s test) and patients with B-cell malignancies (2/28 pre-vaccine versus 10/28 post-vaccine; P=0.008). There appeared to be no effect of prior chemotherapy or time from transplant on the induction of H1N1-specific T cells after two doses of vaccine (data not shown). In contrast, there was no significant increase in the proportion of individuals with an H1N1-specific T-cell response following H1N1 vaccination in CML patients (6/28 pre-vaccine versus 9/28 post-vaccine; P=0.51) and healthy controls (10/23 pre-vaccine versus 11/23 post-vaccine; P=0.51) (Table 5). Online Supplementary Figure S1 depicts the fluorescent activated cell sorting plots from three representative patients and a control with robust T-cell responses to H1N1 vaccines.
Table 5.
T-cell responses against 2009 influenza A H1N1.
Furthermore, we did not find an association between vaccine-induced T- and B-cell responses following H1N1 vaccination in the 81 patients for whom both day 49 peripheral blood mononuclear cells and sera were available; 19/81 patients mounted both cellular and humoral responses to H1N1 vaccination, 10/81 patients had only T-cell responses, and 41/81 patients had only antibody responses. (P=0.32).
Discussion
A number of studies have examined the efficacy of vaccination with seasonal influenza in protecting against influenza-like illnesses.3,6 Although the incidence of proven influenza infection in the vaccinated population is the preferred clinical endpoint, the low incidence of influenza-like illnesses in these studies makes the seroprotection rate an acceptable surrogate endpoint in normal controls10–13 and in the immunocompromised population.3,6–7,14 In contrast, T-cell protection against influenza remains poorly understood. In a study performed in elderly patients, the antibody response to influenza was reported not to be reliable at predicting risk for laboratory-diagnosed influenza while the T-cell response – as assessed by cytokine and granzyme B production – was predictive for laboratory-diagnosed influenza.15 The same group recently suggested a link between cell-mediated immunity and influenza A/H3N2 illness severity in vaccinated older adults.16
The benefit of a seasonal influenza booster vaccine in patients with hematologic malignancies remains controversial despite a number of well-designed studies.14,17,18 Even less is known about the safety, immunogenicity and optimal dosing regimen of 2009 H1N1 vaccine in this group of patients, although several investigators have reported efficacy of single dosing in healthy adults and children.10–13
Our data demonstrate that vaccination against 2009 pandemic H1N1 is associated with an acceptable safety profile in patients with treated and untreated hematologic malignancies. As previously reported,10–13 100% of healthy controls in our study seroconverted after one dose of vaccine. In contrast, the level of humoral immunity induced by the vaccine in patients appears to be influenced by both the underlying malignancy and the time from last chemotherapy or transplantation. The seroprotection rates were significantly higher in patients with CML, patients with B-cell malignancies who had never received chemotherapy or who were vaccinated more than 12 months after chemo-immunotherapy and in intermediate to long-term survivors of transplantation, compared to all other groups.
The recovery of peripheral blood B-lymphocytes following rituximab-induced B-cell depletion begins 6 months after treatment19,20 and does not return to pre-treatment levels for up to 1 year.21 Although the effect of rituximab on the immunogenicity of seasonal influenza vaccination remains unclear,14,22 it has been suggested that rituximab negatively affects the ability to respond to novel influenza antigens.22 Indeed, we recently showed that patients treated with rituximab with confirmed H1N1 infection fail to mount an antibody response to H1N1.9 In our current study none of the patients treated with rituximab within 6 months of vaccination achieved detectable antibody titers to H1N1. However our results show that the immune responses in untreated patients with B-cell malignancies, those who are more than 6 months from treatment and allogeneic SCT recipients can be substantially improved by a second dose of vaccine, confirming the need for a booster in these groups of patients. In view of the limited efficacy, the advisability of vaccination in recently treated patients remains unclear and must be balanced against the high degree of mortality associated with H1N1 infection in the immunocompromised. Reassuringly, the incidence and severity of side effects were no greater in this group than in any other cohort.
We also had the opportunity to evaluate the immunogenicity of H1N1 vaccine in patients with CML in chronic phase stably treated with the tyrosine kinase inhibitors, imatinib and dasatinib. Some of the tyrosine kinase targets of these drugs play a role in immune responses such that there are theoretical reasons to postulate altered immune reactivity. A number of reports have documented seemingly contradictory immunomodulatory effects of tyrosine kinase inhibitors, ranging from impaired T-cell responses23–25 to enhanced responses to vaccination.26 Although this study was not designed to look at differences in vaccine-induced immune responses between imatinib- and dasatinib-treated patients, our results suggest that patients with CML treated with tyrosine kinase inhibitors can mount effective immune responses to H1N1 vaccination.
Seasonal influenza vaccine fails to produce cross-reactive antibodies to pandemic H1N127 because H1N1 virus and conventional influenza strains differ in their hemagglutinin and neuraminidase sequences, the two surface proteins that are the primary targets of neutralizing antibodies.28 In contrast, recent in vitro data show up to 69% cross-reactivity in CD8+ T-cell epitopes derived from pandemic H1N1 and other seasonal influenza strains.28 Prior to vaccination, pre-existing T-cell responses to H1N1 could be detected in a significant proportion of healthy controls and CML patients, possibly related to previous exposure to 2009 H1N1 virus but more likely due to the presence of cross-reactive seasonal and pandemic H1N1 specific T cells.28 This possibility is supported further by a recent study demonstrating the existence of cross-reactive seasonal and 2009 H1N1-specific T cells of similar avidity with a memory phenotype in healthy controls.29 Following vaccination, H1N1-specific T cells were induced in a significantly greater proportion of allogeneic SCT recipients and patients with B-cell malignancies than in CML patients or healthy controls. The limited ability of vaccination to significantly increase pre-existing influenza-specific T cells has been previously reported although the mechanism for this phenomenon has not yet been fully elucidated.30,31 A potential mechanism could be the exhaustion of influenza-specific T cells upon repeated stimulation with the same influenza antigens.32
Combining cellular and humoral measures of vaccine efficacy may increase the ability to predict the risk of influenza illness. Indeed cellular immune responses to influenza have been shown to correlate with protection against influenza in the absence of strong serum antibody responses among the elderly.15 Moreover, studies in patients vaccinated against hepatitis B virus have demonstrated persistence of HBsAg-specific memory T cells in the circulation for a long time after vaccination, even when serum anti-HBs antibodies were no longer detectable.33 This phenomenon may also apply to rituximab-treated patients in whom no antibody responses were detected, yet cellular responses were present. It is possible that the effector cytotoxic T cells seen in this group can provide protection against H1N1 infection, supporting vaccination for this subgroup of patients. We found no significant correlation between the H1N1 vaccine-induced humoral and cellular immune responses. Furthermore, none of the vaccinated patients in our study contracted H1N1 infection; we are, therefore, unable to evaluate the relationship between the development of influenza illness, serum antibody titers and ex vivo cellular immune responses to 2009 H1N1.
In summary, our results unequivocally support the European Medicines Agency and the UK DoH guidelines for the administration of two vaccine doses in patients with B-cell malignancies and SCT recipients to induce a protective immune response against 2009 H1N1 influenza. These data may also apply to vaccination against other viral agents in the immunocompromised host and warrant further studies. Based on the WHO analyses, it is expected that the pandemic 2009 H1N1 virus will remain globally predominant in 2010–2011.34 Our results may contribute towards the development of evidence-based guidelines for influenza vaccination in patients with hematologic malignancies or other immunocompromised hosts.
Acknowledgments
We acknowledge the support of the National Institute for Health Research (NIHR), Biomedical Research Center (BRC).
Footnotes
Funding: Kay Kendall Leukemia Fund (grant no. KKL 314), Fondation de France, Leuka registered charity 286231.
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.The European Agency for the Evaluation of Medicinal Products (EMEA) European Medicines Agency recommends authorisation of two vaccines for influenza pandemic (H1N1) 2009. http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2009/12/WC500018421.pdf.
- 2.Department of Health. H1N1 swine flu vaccination programme 2009–2010. 2009. http://www.dh.gov.uk/en/Publicationsandstatistics/Lettersandcirculars/Dearcolleagueletters/DH_107169.
- 3.Pollyea DA, Brown JM, Horning SJ. Utility of influenza vaccination for oncology patients. J Clin Oncol. 2010;28(14):2481–90. doi: 10.1200/JCO.2009.26.6908. [DOI] [PubMed] [Google Scholar]
- 4.Miller E, Hoschler K, Hardelid P, Stanford E, Andrews N, Zambon M. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross-sectional serological study. Lancet. 2010;375(9720):1100–8. doi: 10.1016/S0140-6736(09)62126-7. [DOI] [PubMed] [Google Scholar]
- 5.The European Agency for the Evaluation of Medicinal Products (EMEA). Committee for Proprietary Medicinal Products (CPMP) Note for guidance on harmonisation of requirements for influenza vaccines. CPMP/BWP/214/96. 1-3-1997. http://www.emea.europa.eu/pdfs/human/bwp/021496en.pdf.
- 6.Kunisaki KM, Janoff EN. Influenza in immunosuppressed populations: a review of infection frequency, morbidity, mortality, and vaccine responses. Lancet Infect Dis. 2009;9(8):493–504. doi: 10.1016/S1473-3099(09)70175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ljungman P, Avetisyan G. Influenza vaccination in hematopoietic SCT recipients. Bone Marrow Transplant. 2008;42(10):637–41. doi: 10.1038/bmt.2008.264. [DOI] [PubMed] [Google Scholar]
- 8.Ring A, Marx G, Steer C, Harper P. Influenza vaccination and chemotherapy: a shot in the dark? Support Care Cancer. 2002;10(6):462–5. doi: 10.1007/s00520-001-0337-9. [DOI] [PubMed] [Google Scholar]
- 9.Garland P, de Lavallade H, Sekine T, Hoschler K, Sriskandan S, Parind P, et al. Humoral and cellular immunity to primary H1N1 infection in patients with hematological malignancies and following stem cell transplantation. Biol Blood Marrow Transplant. 2010 Aug 10; doi: 10.1016/j.bbmt.2010.08.002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Clark TW, Pareek M, Hoschler K, Dillon H, Nicholson KG, Groth N, et al. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med. 2009;361(25):2424–35. doi: 10.1056/NEJMoa0907650. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg ME, Lai MH, Hartel GF, Wichems CH, Gittleson C, Bennet J, et al. Response to a monovalent 2009 influenza A (H1N1) vaccine. N Engl J Med. 2009;361(25):2405–13. doi: 10.1056/NEJMoa0907413. [DOI] [PubMed] [Google Scholar]
- 12.Liang XF, Wang HQ, Wang JZ, Fang HH, Wu J, Zhu FC, et al. Safety and immunogenicity of 2009 pandemic influenza A H1N1 vaccines in China: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2010;375(9708):56–66. doi: 10.1016/S0140-6736(09)62003-1. [DOI] [PubMed] [Google Scholar]
- 13.Plennevaux E, Sheldon E, Blatter M, Reeves-Hoche MK, Denis M. Immune response after a single vaccination against 2009 influenza A H1N1 in USA: a preliminary report of two randomised controlled phase 2 trials. Lancet. 2010;375(9708):41–8. doi: 10.1016/S0140-6736(09)62026-2. [DOI] [PubMed] [Google Scholar]
- 14.Ljungman P, Nahi H, Linde A. Vaccination of patients with haematological malignancies with one or two doses of influenza vaccine: a randomised study. Br J Haematol. 2005;130(1):96–8. doi: 10.1111/j.1365-2141.2005.05582.x. [DOI] [PubMed] [Google Scholar]
- 15.McElhaney JE, Xie D, Hager WD, Barry MB, Wang Y, Kleppinger A, et al. T cell responses are better correlates of vaccine protection in the elderly. J Immunol. 2006;176(10):6333–9. doi: 10.4049/jimmunol.176.10.6333. [DOI] [PubMed] [Google Scholar]
- 16.Shahid Z, Kleppinger A, Gentleman B, Falsey AR, McElhaney JE. Clinical and immunologic predictors of influenza illness among vaccinated older adults. Vaccine. 2010;28(38):6145–51. doi: 10.1016/j.vaccine.2010.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smithson WA, Siem RA, Ritts RE, Jr, Gilchrist GS, Burgert EO, Jr, Ilstrup DM, et al. Response to influenza virus vaccine in children receiving chemotherapy for malignancy. J Pediatr. 1978;93(4):632–4. doi: 10.1016/s0022-3476(78)80905-6. [DOI] [PubMed] [Google Scholar]
- 18.Lo W, Whimbey E, Elting L, Couch R, Cabanillas F, Bodey G. Antibody response to a two-dose influenza vaccine regimen in adult lymphoma patients on chemotherapy. Eur J Clin Microbiol Infect Dis. 1993;12(10):778–82. doi: 10.1007/BF02098469. [DOI] [PubMed] [Google Scholar]
- 19.Maloney DG, Grillo-Lopez AJ, White CA, Bodkin D, Schilder RJ, Neidhart JA, et al. IDEC-C2B8 (rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood. 1997;90(6):2188–95. [PubMed] [Google Scholar]
- 20.van der Kolk LE, Baars JW, Prins MH, van Oers MH. Rituximab treatment results in impaired secondary humoral immune responsiveness. Blood. 2002;100(6):2257–9. [PubMed] [Google Scholar]
- 21.McLaughlin P, Grillo-Lopez AJ, Link BK, Levy R, Czuczman MS, Williams ME, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16(8):2825–33. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 22.Takata T, Suzumiya J, Ishikawa T, Takamatsu Y, Ikematsu H, Tamura K. Attenuated antibody reaction for the primary antigen but not for the recall antigen of influenza vaccination in patients with non-Hodgkin B-cell lymphoma after the administration of rituximab-CHOP. J Clin Exp Hematop. 2009;49(1):9–13. doi: 10.3960/jslrt.49.9. [DOI] [PubMed] [Google Scholar]
- 23.Fraser CK, Lousberg EL, Kumar R, Hughes TP, Diener KR, Hayball JD. Dasatinib inhibits the secretion of TNF-alpha following TLR stimulation in vitro and in vivo. Exp Hematol. 2009;37(12):1435–44. doi: 10.1016/j.exphem.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Mumprecht S, Matter M, Pavelic V, Ochsenbein AF. Imatinib mesylate selectively impairs expansion of memory cytotoxic T cells without affecting the control of primary viral infections. Blood. 2006;108(10):3406–13. doi: 10.1182/blood-2006-04-018705. [DOI] [PubMed] [Google Scholar]
- 25.Seggewiss R, Lore K, Greiner E, Magnusson MK, Price DA, Douek DC, et al. Imatinib inhibits T-cell receptor-mediated T-cell proliferation and activation in a dose-dependent manner. Blood. 2005;105(6):2473–9. doi: 10.1182/blood-2004-07-2527. [DOI] [PubMed] [Google Scholar]
- 26.Larmonier N, Janikashvili N, LaCasse CJ, Larmonier CB, Cantrell J, Situ E, et al. Imatinib mesylate inhibits CD4+ CD25+ regulatory T cell activity and enhances active immunotherapy against BCR-ABL-tumors. J Immunol. 2008;181(10):6955–63. doi: 10.4049/jimmunol.181.10.6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361(20):1945–52. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 28.Greenbaum JA, Kotturi MF, Kim Y, Oseroff C, Vaughan K, Salimi N, et al. Pre-existing immunity against swine-origin H1N1 influenza viruses in the general human population. Proc Natl Acad Sci USA. 2009;106(48):20365–70. doi: 10.1073/pnas.0911580106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ge X, Tan V, Bollyky PL, Standifer NE, James EA, Kwok WW. Assessment of seasonal influenza A virus-specific CD4 T-cell responses to 2009 pandemic H1N1 swine-origin influenza A virus. J Virol. 2010;84(7):3312–9. doi: 10.1128/JVI.02226-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He XS, Holmes TH, Sasaki S, Jaimes MC, Kemble GW, Dekker CL, et al. Baseline levels of influenza-specific CD4 memory T-cells affect T-cell responses to influenza vaccines. PLoS One. 2008;3(7):e2574. doi: 10.1371/journal.pone.0002574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keynan Y, Card CM, Ball BT, Li Y, Plummer FA, Fowke KR. Cellular immune responses to recurring influenza strains have limited boosting ability and limited cross-reactivity to other strains. Clin Microbiol Infect. 2010;16(8):1179–86. doi: 10.1111/j.1469-0691.2010.03142.x. [DOI] [PubMed] [Google Scholar]
- 32.McElhaney JE, Hooton JW, Hooton N, Bleackley RC. Comparison of single versus booster dose of influenza vaccination on humoral and cellular immune responses in older adults. Vaccine. 2005;23(25):3294–300. doi: 10.1016/j.vaccine.2005.01.080. [DOI] [PubMed] [Google Scholar]
- 33.Wang RX, Boland GJ, van HJ, de Gast GC. Long-term persistence of T cell memory to HBsAg after hepatitis B vaccination. World J Gastroenterol. 2004;10(2):260–3. doi: 10.3748/wjg.v10.i2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization. Recommended viruses for influenza vaccines for use in the 2010–2011 northern hemisphere influenza season. http://www.who.int/csr/disease/influenza/201002_Recommendation.pdf.2010. [PubMed]