Abstract
Aberrant CD117 expression is associated with a favorable outcome in multiple myeloma. We analyzed 106 patients with symptomatic multiple myeloma (n=50), smoldering multiple myeloma (n=38) and monoclonal gammopathy of undetermined significance (n=18) to elucidate biological features of CD117+ versus CD117− monoclonal gammopathies. CD117+ (mono)clonal plasma cells were detected in 30% symptomatic multiple myeloma, 45% smoldering multiple myeloma and 72% monoclonal gammopathy of undetermined significance patients. CD117 expression was associated with higher percentages of normal bone marrow plasma cells, CD117+ myeloid precursors and CD38+ B lymphocytes in all monoclonal gammopathies. Conversely, the number of bone marrow CD34+ myeloid cells and peripheral blood neutrophils was reduced among CD117+ multiple myeloma but not monoclonal gammopathy of undetermined significance patients.
CD117 expression by (mono)clonal plasma cells is associated with uniquely altered patterns of production of hematopoietic bone marrow cells with decreased peripheral blood neutrophil counts and persistence of normal residual bone marrow plasma cells.
Keywords: CD117 expression, monoclonal gammopathy, multiparameter flow cytometry, myeloid maturation, lymphoid maturation, normal residual plasma cells
Introduction
CD117 (ckit), a class III receptor tyrosine kinase, is normally expressed by mast cells and a subset of precursor cells in the bone marrow.1 Despite the fact that early bone marrow B-cell precursors might be partly CD117+ in rodents, in humans all CD19+ B cells are typically CD117− from early bone marrow precursors to plasma cells.2–4 Noteworthy, aberrant CD117 expression has sporadically been reported in patients with B-cell non-Hodgkin’s lymphoma, but occurs in about 30% of all multiple myeloma (MM) patients.5–7 Interestingly, CD117 expression by (mono)clonal plasma cells confers a favorable prognosis in multiple myeloma, albeit ckit has been associated with oncogenic transformation in other malignancies such as gastrointestinal stromal tumor and mastocytosis, in which it may represent an important target molecule for specific therapies (e.g. imatinib).2,6,8,9 Previous studies in systemic mastocytosis and myeloid neoplasms have shown that ckit might promote activation of pro-oncogenic regulatory molecules such as the signal transducer and activator of transcription 5.10
Based on these observations it could be hypothesized that, more than an oncogenic receptor, CD117 expression by (mono)clonal plasma cells could act as an adhesion molecule, which could favor anchoring of bone marrow plasma cells to inappropriate myeloid precursor-associated bone marrow niches through ckit ligand expressing stromal cells.
We investigated the impact of CD117 expression by (mono)clonal plasma cells on bone marrow hematopoiesis in different subtypes of monoclonal gammopathy and correlated it with other biological and cytogenetic features of the disease. Overall, our results suggest that CD117 expression by (mono)clonal plasma cells is associated with a uniquely altered pattern of production of hematopoietic cells consisting of a decreased neutrophil production and a preserved normal residual bone marrow plasma cell compartment.
Design and Methods
Patients and samples
Patients with newly diagnosed symptomatic multiple myeloma (n=50), smoldering multiple myeloma (n=38) and monoclonal gammopathy of undetermined significance (n=18) were included in this study.11 Patients were 43% females and 57% males with a median age of 65 years (range 39–89 years). All patients gave written informed consent before entering this study, which was approved by the local ethics committee.
Multiparameter flow cytometry studies, fluorescence in situ hybridization and DNA ploidy analyses
Immunophenotyping of (mono)clonal plasma cells was carried out in EDTA-anticoagulated, erythrocyte-lysed peripheral blood or whole bone marrow samples with a conventional direct immunofluorescence stain-and-then-lyse technique previously described.12,13 For data acquisition, a standard FACSCanto II flow cytometer equipped with the FACS Diva software (Becton Dickinson Biosciences (BD), San José, CA, USA) was used; the Infinicyt software (Cytognos SL, Salamanca, Spain) was used for data analysis (Online Supplementary Appendix). Fluorescence in situ hybridization (FISH) and DNA ploidy analyses were carried out as previously reported (Online Supplementary Appendix).6,14
Statistical methods
Group comparisons with respect to frequencies were performed with the χ2 or Fisher’s exact test, while for continuous measurements Mann-Whitney’s U test was used. Spearman’s test was used to evaluate the correlation between quantitative variables. P values below 0.05 (two-sided) indicated statistical significance. For all statistical analyses the commercially available PASW software (Version 18.0, Chicago, IL, USA) was used.
Results and Discussion
Patients’ characteristics
CD117 expression occurred more frequently (P=0.004) in monoclonal gammopathy of undetermined significance (72%) than in smoldering (45%) or symptomatic multiple myeloma (30%). No statistically significant differences were found among the three different patient groups as regards the median percentage of CD117+ (mono)clonal plasma cells among CD117+ cases. There was no significant difference in either the median age nor the distribution by sex or the International Staging System stages between CD117+ and CD117− multiple myeloma patients (data not shown). The fact that CD117 expression by (mono)clonal plasma cells decreased from monoclonal gammopathy of undetermined significance to smoldering and symptomatic multiple myeloma in our series supports the hypothesis that its expression is related to the aggressiveness and/or prognosis of the underlying monoclonal gammopathy.6
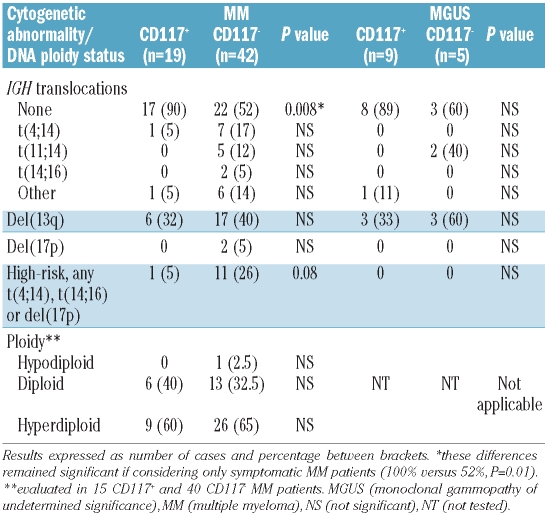
In this regard, immunoglobulin heavy chain (IGH) translocations were more common among CD117− versus CD117+ patients in all three subgroups of monoclonal gammopathy and high-risk karyotypes were prevailing in CD117− versus CD117+ multiple myeloma patients (Table 1) with a similar distribution in symptomatic versus smoldering multiple myeloma (data not shown).
Table 1.
Cytogenetic abnormalities in CD117+ versus CD117− MM and MGUS patients.
DNA ploidy did not differ significantly between CD117− and CD117+ multiple myeloma patients: 8 of 10 (80%) and 13 of 19 (68%) hyperdiploid cases in the absence of IGH translocations in CD117+ versus CD117− cases (not significant, Table 1) with comparable DNA indices (median of 1.21 versus 1.29, range 1.08–1.27 and 1.10–2.00). IgG isotype was prevailing in CD117+ multiple myeloma patients (73% versus 37%, P=0.02), whereas IgA and light chain multiple myeloma were more common in the CD117− cohort: 13% versus 42% (P=0.06) and 7% versus 17% (not significant). There was no significant difference in total serum Ig levels between CD117+ versus CD117− multiple myeloma patients, neither when considering involved nor none-involved Igs (data not shown). Immunoparesis (serum Ig levels decreased by more than 25% of the lower normal values) was present in a similar proportion of CD117+ (82%) and CD117− (86%) multiple myeloma patients (not significant).
Distribution of plasma cells and other hematopoietic cell subsets in the bone marrow and the peripheral blood
In vitro studies showed that different isoforms of the ckit tyrosine kinase receptor might be induced in (mono)clonal plasma cells by the stem cell factor and that it is efficiently coupled to downstream pathways (e.g. the phosphoinositide-3 kinase/Akt pathway), suggesting ckit is functional in (mono)clonal plasma cells.9,15 Despite this, preliminary studies indicate that inhibition of CD117 by imatinib does not show a marked effect on CD117+ versus CD117− (mono)clonal plasma cells, neither in vivo nor in vitro.16,17 Interestingly, CD117 is rarely expressed in multiple myeloma cell lines indicating that the interaction of the bone marrow microenvironment and (mono)clonal plasma cells might also play a relevant role in the regulation of CD117 expression in these diseases.2,16 Therefore, it could be hypothesized that, more than an oncogenic receptor, CD117 expression by (mono)clonal plasma cells could have other functional roles and act, for example, as an adhesion molecule. This could favor anchoring of (mono)clonal plasma cells to inappropriate myeloid precursor-associated niches in the bone marrow.
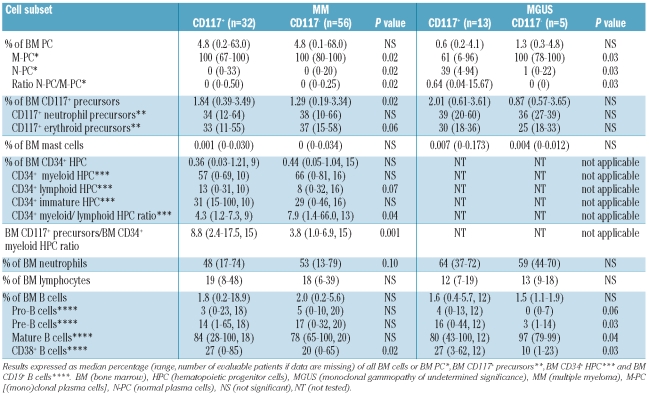
In this regard, our results showed significantly higher median percentages of residual normal plasma cells, CD117+ precursors and CD38+ B cells in the bone marrow of CD117+ versus CD117− multiple myeloma (Table 2). Conversely, CD34+ myeloid hematopoietic progenitor cells tended to be lower in CD117+ versus CD117− multiple myeloma patients, leading to a significantly decreased median ratio between CD34+/CD19− myeloid and CD34+/CD19+ lymphoid progenitors and a significantly increased median ratio between CD117+ precursors and CD34+/CD19− myeloid progenitors in CD117+ versus CD117− multiple myeloma patients (Table 2). These differences were likewise observed in symptomatic and smoldering multiple myeloma when both patient groups were analyzed separately (data not shown). CD117 expression by (mono)clonal plasma cells was further associated with a significantly (P=0.02) decreased percentage of bone marrow neutrophils in symptomatic multiple myeloma patients (median 38%, range 17%–67% versus median 50%, range 13%–72%).
Table 2.
Distribution of bone marrow cell subsets in CD117+ versus CD117− MM and MGUS patients.
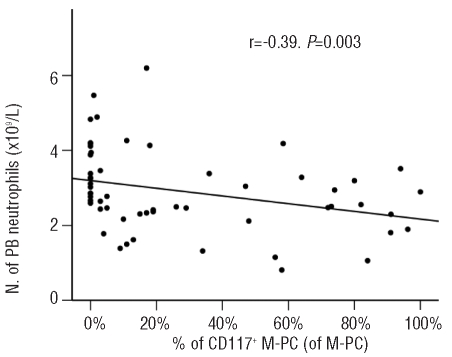
In line with these observations, CD117 expression by (mono)clonal plasma cells was associated with a significantly lower number of peripheral blood neutrophils in multiple myeloma patients (median 2.5 versus 2.9×109/L, P=0.04) leading to a substantially lower median ratio between the number of peripheral blood neutrophils and the percentage of CD117+ precursors in CD117+ multiple myeloma (1.2 versus 2.1, P<0.001; Online Supplementary Tables S1 and S2). Thus, an inverse correlation was found in multiple myeloma between the percentage of bone marrow CD117+ (mono)clonal plasma cells and the number of peripheral blood neutrophils (r=−0.39, P=0.003, Figure 1), whereas the percentage of bone marrow CD117+ precursors positively correlated with the percentage of bone marrow CD117+ (mono)clonal plasma cells (r=0.31, P=0.003). Taken together, these results suggest that neutrophil maturation in the bone marrow is considerably altered in CD117+ versus CD117− multiple myeloma patients with a lower rate of production and release of mature neutrophils into the peripheral blood, despite progressive accumulation of early CD117+ myeloid precursors potentially reflecting an early blockade and altered bone marrow production of neutrophils. This could be attributable to an increased consumption of stem cell factor in the bone marrow by CD117+ (mono)clonal plasma cells; alternatively, it could also be associated with an altered homing of CD117+ (mono)clonal plasma cells into bone marrow niches. Dysregulated hematopoietic niches have already been related to various diseases e.g. myeloproliferative syndromes and our observations led to the assumption that these niches could also be altered in CD117+ monoclonal gammopathies.18 The fact that also neutrophil lineage cells within the CD117+ precursor cell compartment, CD34+ myeloid cells, and total bone marrow neutrophils tended to decrease in CD117+ versus CD117− multiple myeloma patients (Table 2) suggests that different maturation-associated compartments of myeloid cells (including the very immature cell compartments) could be involved in the dysregulated myelopoiesis. Thus, our observations indicate that CD117 expression by (mono)clonal plasma cells could alter their homing and lead to an interference with neutrophil precursor niches, with progressively more marked downregulation of neutrophil production. Hereby, CD117 expression by (mono)clonal plasma cells might act as an additional anchor molecule, resulting in a decrease in the spread of (mono)clonal plasma cells to the plasma cell/B-cell precursor niches. In turn, this would lead to a greater maintenance of the homeostatic role of residual normal plasma cells in CD117+ versus CD117− multiple myeloma.
Figure 1.
Correlation between the percentage of CD117+ bone marrow (mono)clonal plasma cells and peripheral blood neutrophil counts in MM. M-PC [(mono)clonal plasma cells], N. (number), PB (peripheral blood).
In line with this hypothesis, we also found altered patterns of lymphoid maturation in CD117+ versus CD117− multiple myeloma. Accordingly, the percentage of early bone marrow CD38+ B cells was significantly increased in CD117+ versus CD117− multiple myeloma and also the percentage of CD34+CD19+ lymphoid cells tended to be higher in these patients (Table 2). However, the percentage of B lymphocytes in the peripheral blood was decreased in CD117+ versus CD117− multiple myeloma (56 versus 94 ×106/L, not significant; Online Supplementary Table S1). Since B cell precursors are exclusively CD117− in humans, our data led to the assumption that the dysregulated hematopoiesis in CD117+ multiple myeloma is not restricted to the CD117+ bone marrow cell compartments, but it also involves CD117− B cells.4
The alterations of both bone marrow myeloid and lymphoid maturation in CD117+ versus CD117− cases discussed above affected both symptomatic and smoldering multiple myeloma, but not monoclonal gammopathy of undetermined significance patients. Also in these latter monoclonal gammopathy of undetermined significance cases, the median percentage of bone marrow CD117+ precursors and CD38+ B cells was higher in CD117+ versus CD117− patients (Table 2). However, in contrast to multiple myeloma, the median number of peripheral blood neutrophils was not decreased in CD117+ (4.4×109/L, range 3.4–13.1×109/L) versus CD117− (3.2×109/L, range 2.1–4.8×109/L) monoclonal gammopathy of undetermined significance patients. Altogether, these results suggest that CD117 expression by (mono)clonal plasma cells might have a comparable impact on bone marrow myeloid and lymphoid maturation patterns in different subgroups of monoclonal gammopathies, but that the release of mature neutrophils into the peripheral blood would only be impaired in multiple myeloma. Conversely, the distribution of normal versus (mono)clonal plasma cells is already altered differentially in CD117+ versus CD117− in both multiple myeloma and monoclonal gammopathy of undetermined significance cases. Accordingly, the percentage of residual bone marrow normal plasma cells was also significantly higher in CD117+ versus CD117− monoclonal gammopathy of undetermined significance patients (Table 2).
Recent clinical observations led to the assumption that co-expression of CD28 by (mono)clonal plasma cells might attenuate the positive prognostic impact of CD117 expression in multiple myeloma.6 In the present study, CD28 expression by (mono)clonal plasma cells occurred in 11 of 32 (34%) CD117+ versus 21 of 54 (39%) CD117− multiple myeloma patients (not significant). In monoclonal gammopathy of undetermined significance, CD28 expression was observed in 6 of 12 (50%) CD117+ versus 3 of 5 (60%) CD117− cases (not significant). CD28 co-expression by (mono)clonal plasma cells did not have any significant impact on any bone marrow or peripheral blood cell subset in CD117+ multiple myeloma or monoclonal gammopathy of undetermined significance patients (data not shown).
In summary, our results show that CD117 expression is associated with characteristic biological features in different types of monoclonal gammopathies consisting of a uniquely altered myeloid and lymphoid maturation pattern, preponderance of IgG isotype and a lower frequency of adverse cytogenetic characteristics. These results suggest that CD117 expression by (mono)clonal plasma cells alters their homing in the bone marrow and could redirect them into neutrophil precursor niches, where CD117 might act as an anchor molecule; such redistribution of (mono)clonal plasma cells in the bone marrow could contribute to the greater maintenance of the homeostatic role of residual normal plasma cells and a more limited spread of (mono)clonal plasma cells in CD117+ monoclonal gammopathies.
Acknowledgments
The authors would like to thank C Teodosio, MV Mateos, L López-Corral, A Oriol, MT Hernández, F de Arriba, A García de Coca, MJ Terol, J Rubia, Y González, A Martín, A Sureda, JJ Lahuerta and J Bladé for participating in this study.
Footnotes
Funding: this work was supported by the Cooperative Research Thematic Network (RTICs; RTICC RD06/0020/0035, RD06/0020/0006 and G03/136), MM Jevitt, SL firm, Instituto de Salud Carlos III/Subdirección General de Investigación Sanitaria (FIS: PI060339; 02/0905; 01/0089/01-02; PS09/01897) and Gerencia Regional de Salud de Castilla y León; Ayuda de Excelencia de Castilla y León, Consejeria de Educación (EDU/894/2009, GR37), and Consejería de Sanidad (557/A/10), Junta de Castilla y León, Valladolid, Spain. This work was further supported by a grant from the Dr. Werner Jackstädt-Foundation (Wuppertal, Germany).
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Escribano L, Ocqueteau M, Almeida J, Orfao A, San Miguel JF. Expression of the c-kit (CD117) molecule in normal and malignant hematopoiesis. Leuk Lymphoma. 1998;30(5–6):459–66. doi: 10.3109/10428199809057558. [DOI] [PubMed] [Google Scholar]
- 2.Bataille R, Pellat-Deceunynck C, Robillard N, Avet-Loiseau H, Harousseau JL, Moreau P. CD117 (c-kit) is aberrantly expressed in a subset of MGUS and multiple myeloma with unexpectedly good prognosis. Leuk Res. 2008;32(3):379–82. doi: 10.1016/j.leukres.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 3.Nagasawa T. Microenvironmental niches in the bone marrow required for B-cell development. Nat Rev Immunol. 2006;6(2):107–16. doi: 10.1038/nri1780. [DOI] [PubMed] [Google Scholar]
- 4.Matarraz S, López A, Barrena S, Fernandez C, Jensen E, Flores-Montero J, et al. Bone marrow cells from myelodysplastic syndromes show altered immunophenotypic profiles that may contribute to the diagnosis and prognostic stratification of the disease: a pilot study on a series of 56 patients. Cytometry B Clin Cytom. 2010;78(3):154–68. doi: 10.1002/cyto.b.20513. [DOI] [PubMed] [Google Scholar]
- 5.Bravo P, Agustín BD, Bellas C, González D, Cámara C, Fuertes IF, et al. Expression of high amounts of the CD117 molecule in a case of B-cell non-Hodgkin's lymphoma carrying the t(14:18) translocation. Am J Hematol. 2000;63(4):226–9. doi: 10.1002/(sici)1096-8652(200004)63:4<226::aid-ajh11>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 6.Mateo G, Montalbán MA, Vidriales MB, Lahuerta JJ, Mateos MV, Gutiérrez N, et al. PETHEMA Study Group; GEM Study Group. Prognostic value of immunophenotyping in multiple myeloma: a study by the PETHEMA/GEM cooperative study groups on patients uniformly treated with high-dose therapy. J Clin Oncol. 2008;26(16):2737–44. doi: 10.1200/JCO.2007.15.4120. [DOI] [PubMed] [Google Scholar]
- 7.Vakiani E, Cattoretti G, Colovai AI, Murty VV, Alobeid B, Bhagat G. CD117 expression in diffuse large B-cell lymphomas: fact or fiction? Pathol Int. 2005;55(11):716–23. doi: 10.1111/j.1440-1827.2005.01893.x. [DOI] [PubMed] [Google Scholar]
- 8.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577–80. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 9.Orfao A, Garcia-Montero AC, Sanchez L, Escribano L REMA. Recent advances in the understanding of mastocytosis: the role of KIT mutations. Br J Haematol. 2007;138(1):12–30. doi: 10.1111/j.1365-2141.2007.06619.x. [DOI] [PubMed] [Google Scholar]
- 10.Baumgartner C, Cerny-Reiterer S, Sonneck K, Mayerhofer M, Gleixner KV, Fritz R, et al. Expression of activated STAT5 in neoplastic mast cells in systemic mastocytosis: subcellular distribution and role of the transforming oncoprotein KIT D816V. Am J Pathol. 2009;175(6):2416–29. doi: 10.2353/ajpath.2009.080953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23(1):3–9. doi: 10.1038/leu.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caraux A, Klein B, Paiva B, Bret C, Schmitz A, Fuhler GM, et al. for the Myeloma Stem Cell Network (MSCNET) Circulating human B and plasma cells. Age-associated changes in counts and detailed characterization of circulating normal CD138- and CD138+ plasma cells. Haematologica. 2010;95(6):1016–20. doi: 10.3324/haematol.2009.018689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paiva B, Vidriales MB, Mateo G, Pérez JJ, Montalbán MA, Sureda A, et al. GEM (Grupo Español de MM)/PETHEMA (Programa para el Estudio de la Terapéutica en Hemopatías Malignas) Cooperative Study Groups. The persistence of immunophenotypically normal residual bone marrow plasma cells at diagnosis identifies a good prognostic subgroup of symptomatic multiple myeloma patients. Blood. 2009;114(20):4369–72. doi: 10.1182/blood-2009-05-221689. [DOI] [PubMed] [Google Scholar]
- 14.Mateo G, Castellanos M, Rasillo A, Gutiérrez NC, Montalbán MA, Martín ML, et al. Genetic abnormalities and patterns of antigenic expression in multiple myeloma. Clin Cancer Res. 2005;11(10):3661–7. doi: 10.1158/1078-0432.CCR-04-1489. [DOI] [PubMed] [Google Scholar]
- 15.Montero JC, López-Pérez R, San Miguel JF, Pandiella A. Expression of c-Kit isoforms in multiple myeloma: differences in signaling and drug sensitivity. Haematologica. 2008;93(6):851–9. doi: 10.3324/haematol.12171. [DOI] [PubMed] [Google Scholar]
- 16.Pandiella A, Carvajal-Vergara X, Tabera S, Mateo G, Gutiérrez N, San Miguel JF. Imatinib mesylate (STI571) inhibits multiple myeloma cell proliferation and potentiates the effect of common antimyeloma agents. Br J Haematol. 2003;123(5):858–68. doi: 10.1046/j.1365-2141.2003.04706.x. [DOI] [PubMed] [Google Scholar]
- 17.Dispenzieri A, Gertz MA, Lacy MQ, Geyer SM, Greipp PR, Rajkumar SV, et al. A phase II trial of imatinib in patients with refractory/relapsed myeloma. Leuk Lymphoma. 2006;47(1):39–42. doi: 10.1080/10428190500271269. [DOI] [PubMed] [Google Scholar]
- 18.Renström J, Kröger M, Peschel C, Oostendorp RA. How the niche regulates hematopoietic stem cells. Chem Biol Interact. 2010;184(1–2):7–15. doi: 10.1016/j.cbi.2009.11.012. [DOI] [PubMed] [Google Scholar]