Abstract
We prospectively evaluated the bone changes associated with proteasome inhibition using single agent bortezomib in relapsed or refractory myeloma patients. Ten patients received bortezomib 1.3 mg/m2 per days 1, 4, 8 and 11 for three 21-day cycles, and 6 patients received 1 mg/m2 per day with the same schedule. Bone architecture and metabolism changes were assessed by bone markers, micro-CT, bone histomorphometry, tetracycline labeling and serum parathormone levels. Bone parameter variations were compared by response to treatment. Microarchitectural changes were observed in all evaluable responsive patients. Bone alkaline phosphatase changes were associated with disease response (≥PR vs. others P=0.03 cycle 1, day 11) serum parathormone levels were also significantly increased (P=0.04 on days 11, 21, 33) in responding individuals.
This study demonstrates that the myeloma control produced by proteasome inhibition is associated with bone changes and to a discrete pattern of hormonal variation. (Clinicaltrials.gov identifier: NCT00569868)
Keywords: bone, bortezomib, PTH, myeloma
Introduction
Multiple myeloma is a plasma cell malignancy with high osteolytic capacity and impaired bone formation. The receptor activator of NFκB ligand (RANKL) signaling pathway plays a critical role in normal bone remodeling and myeloma bone disease. In myeloma, RANKL expression is markedly increased while osteoprotegerin, its decoy receptor for RANKL, is decreased.1,2 In addition, the Wnt signaling pathway has also been associated with the characteristic osteoblastic dysfunction in myeloma. Supporting this idea, the expression of the Wnt signaling inhibitor, DKK1, is significantly increased in patients with myeloma and correlates with the extent of bone disease.3,4 The ubiquitin-proteasome pathway which is an essential cellular degradative system in myeloma cells can also regulate bone formation via effects on osteoblast differentiation.5,6 The proteasome inhibitor, bortezomib, has been shown to inhibit myeloma progression in the myeloma SCID-hu model in vivo. In these studies, bortezomib treatment (0.5 mg/kg twice a week) of myeloma-bearing scid-mice was associated with an increase in bone mineral density (20±14%), while in untreated animals, myeloma growth induced a decrease in bone mineral density (13±12%). Exposure of non-myeloma-bearing control mice to bortezomib also resulted in a significant increase in bone mineral density.7
Retrospective analysis of the variation of bone alkaline phosphatase during bortezomib treatment has indicated a close correlation between myeloma response and drug activity.8 In the study described here, we report the first prospective study of bortezomib-associated bone and hormonal changes
Design and Methods
Bortezomib naïve myeloma patients with histologically documented relapsed or progressive disease after one line of prior therapy were enrolled in the study. Measurable disease was defined as serum M-protein level 1.0 gm/dL or over urinary M-protein excretion 200 mg/24 h or more; serum free light chains greater than twice the upper limit of normal, or bone marrow plasmacytosis of 30% or more. Patients were not allowed to have received chemotherapy, bisphosphonates or radiotherapy within four weeks prior to enrollment and if they had grade 2 or more baseline neuropathy, renal or hepatic impairment. Patients were not allowed to receive bisphosphonates or corticosteroids for the entire study period.
Patients were enrolled in groups of 10 in the following treatment cohorts: Cohort A, bortezomib 1.3 mg/m2 (patient 1–10); Cohort B, bortezomib 1.0 mg/m2 (patient 11–17). Bortezomib was administered on days 1, 4, 8, and 11 on a 21-day cycle for a total of three cycles. Patients who achieved at least stable disease were allowed to continue the treatment regimen and were followed until evidence of disease progression.
Serum bone biochemical markers, osteocalcin, bone alkaline phosphatase, serum calcium, magnesium, phosphate and intact parathormone (1–84) were measured on days 1, 4, 8, and 11 before and after each dose and every four hours post dose for 2 more samples; daily samples were obtained the other days of the treatment cycle. Responses were assessed according to the European Group for Blood and Marrow Transplant criteria.9 Response was defined as the sum of complete response plus partial response. The changes from baseline in bone biochemical markers were compared between responders and non-responders during treatment.
Histomorphometric analysis was performed at baseline and at the end of three treatment cycles. Patients who agreed to undergo bone histomorphometric analysis received doxycycline 200 mg daily on days −21, −20, −19 and repeated on days −3, −2, −1 before the first cycle and after completion of the third cycle to measure dynamic bone indices.
Under general anesthesia, bone biopsy specimens of approximately 7 mm in diameter, which included the two cortices and the intervening cancellous bone, were obtained for histomorphometric evaluation. Samples were fixed in iced Millonig’s phosphate-buffered 10% formalin at pH 7.4.
Prior to histological preparation of the biopsy, all specimens were examined by high-resolution micro-computed tomography (micro-CT) using a μCT40 system (Scanco Medical, Bassersdorf, Switzerland) with a spatial resolution of 28 μm. The harvested bone biopsy samples were immediately scanned in Millonig’s phosphate-buffered 10% formalin at pH 7.4. Within 24 h, the samples were dehydrated in graded ethanol solutions before being embedded undecalcified in methylmethacrylate.10
Microarchitectural micro-CT data were acquired by scanning at 55 keV and 145 mA, with a field of vision of 36.8 mm and a matrix of 1024 x 1024 x 808 with an isotropic voxel size. For each analysis, an average of 100 contiguous slices were contoured by user-defined thresholds for trabecular bone and iterated across slices using the Scanco software to derive measures of architectural parameters. Three-dimensional micro-CT data obtained included bone volume to total volume fraction (BV/TV), trabecular number (Tb.N), thickness (Tb.Th), and separation (Tb.Sp). A standard bone phantom was analyzed weekly to assess reproducibility of the micro-CT analyses.
After micro-CT scanning and subsequent dehydration in graded ethanol the specimens were embedded without decalcification in methyl methacrylate and prepared for routine histomorphometric analysis. Longitudinal sections, approximately 5 μm in thickness, were taken from one-third and one-half the depth of the specimens. Two sections from each depth were left unstained for examination of the tetracycline labels, and two sections were stained with Masson’s trichrome. Histomorphometric examination was performed in all available and suitable samples.
The histomorphometric examination of the samples was performed with the use of a digitizer tablet (Osteo-Metrics) as described previously11,12 using the Parfitt terminology.12 The cancellous bone measurements were two-dimensional and made at a magnification of 20X. Routine bone marrow for histology and cytogenetic analysis was also obtained at baseline from all patients and at the end of the study period.
All patients enrolled in this analysis signed an informed consent approved by the Institutional Review Board.
Results
A total of 16 patients, 10 in the first cohort and 6 in the second cohort were enrolled in the study; median age was 62 years and 7 patients were male. All enrolled patients had relapsing or refractory disease, 75% after being exposed to autologous bone marrow transplant and with a median time from diagnosis of 48 months. Seven of the 16 evaluable patients achieved at least partial response during treatment.
The median osteocalcin value increased from 1.95 mg/ml at baseline to 7.16 mg/mL at the end of study. Bone alkaline phosphatase ranged from 4.5 to 48.4 mg/mL at baseline and was significantly increased during treatment in responsive patients following bortezomib exposure (P=0.03 on day 12, cycle 1). No significant serum variations from baseline of calcium, magnesium and phosphorus were recorded during bortezomib treatment.
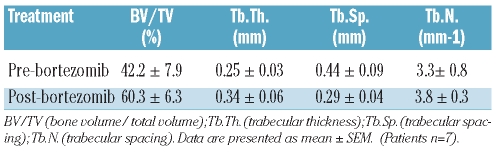
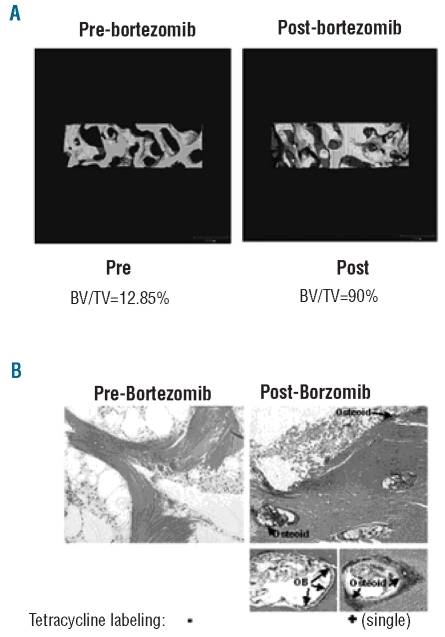
Micro-CT measurements were obtained in 11 patients at baseline and in 9 patients at the end of study; two samples were felt to be inadequate and 7 patients were evaluable for comparative paired evaluation. Architectural parameters such as bone volume/total volume (BV/TV), trabecular number (TbN) and trabecular thickness (TbTh) were determined (Table 1). The measurements of bone volume/total volume ranged from 13% to 90% at baseline and after bortezomib exposure a significant increase in bone volume/total volume was measured in 6 of 7 patients (P<0.02). Interestingly, trabecular thickness also increased from baseline (range 20–45.6%) in 5 of the 7 patients who responded to bortezomib. A 3-dimensional micro-CT rendering from a responding and a non-responding patient is shown in Figure 1A. The bone volume/total volume increased from 12% at baseline to 90% after 12 doses of bortezomib in the responding patient.
Table 1.
Microarchitectural parameters determined by micro-CT.
Figure 1.
Micro-CT rendering of the trabecular microarchitecture of representative bone biopsy specimens at baseline and after 12 infusions of bortezomib in a responsive patient (A). (B) Histomorphometric examination using Goldner’s Trichrome staining at baseline and after 12 infusions of bortezomib in a responsive patient. It shows increased osteoid and an increase in osteoblast (OB) visible on the active bone forming surface (arrows). The areas of increased osteoid correspond to areas of increased tetracycline label incorpotarion that are visible as a single tetracycline label under fluorescent microscopy.
Histologically, a lack of osteoid formation and osteoblast activity was consistently observed at baseline with an increase in both osteoid and osteoblast number after bortezomib treatment observed only in responsive patients. Similarly, bortezomib induced increases in osteoblast activity as observed by increased osteoid, osteoblast number and the appearance of labeled bone surfaces (Figure 1B).
In addition, the measurement of dynamic histomorphometric indices was also performed by the evaluation of tetracycline-labeled bone surfaces.
At baseline, only 2 of the 11 patients’ samples (18%) showed evidence of tetracycline labeling of bone under polarized microscopy. However, after 12 doses of bortezomib, evidence of tetracycline incorporation (single labeled surface) was observed in 7 (63%) biopsy samples (P<0.03 baseline vs. post-treatment score changes). Moreover, the 2 patients with evidence of baseline tetracycline labeling, after bortezomib exposure showed significantly increased double labeled surfaces.
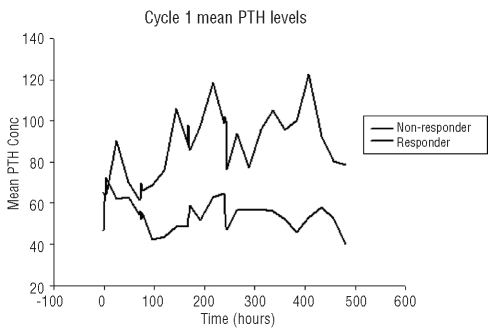
Serial parathormone samples were collected in all enrolled patients. Median parathormone variation across cycle of the entire group and by myeloma response is shown in Figure 2. Comparing patients who achieved at least a partial response versus the others, a significant separation in parathormone levels was evident just after the second bortezomib dose and these levels were statistically higher in responding patients on days 11 (P=0.04), 21 (P=0.04), and 33 (P=0.04) (Figure 2). Comparing the parathormone concentration measured over the dosing interval in responders versus non-responders showed a significant difference in the average concentration over the first cycle (Av ±SD for R = 85.6 ±16.9 NR = 54.4±7.1, P<0.001). There was no difference in the change in parathormone from baseline in control patients.
Figure 2.
The averaged individual parathormone response during the first cycle of bortezomib (n=16 patients). The response shows the point averaged comparison of the responders (n=7) versus non-responders (n=9) and highlights both the difference in parathormone magnitude and response frequency in the responder group.
Discussion
In the SUMMIT and APEX trials we had previously described a statistically significant elevation of alkaline phosphatase in bortezomib responding patients.8 The median increment (25% increase in alkaline phosphatase, n=105) at week 6 was the strongest indicator associated with quality of response (P<0.0001) and also with the time to progression (206 vs. 169 days) relative to patients with less than a 25% increase in alkaline phosphatase (n=228; P=0.01).13 Our observation was later confirmed by other investigators. Increased serum levels of bone alkaline phosphatase and osteocalcin were observed in a group of 34 relapsed multiple myeloma patients. Patients who achieved a complete response or very good partial response after 4 cycles of bortezomib demonstrated greater elevations of bone alkaline phosphatase levels than those not achieving a complete response or very good partial response. The increase in bone formation markers was accompanied by a reduction in serum Dkk1 levels, which was similar among responders and non-responders after bortezomib therapy.14 Bone marrow biopsies of responding individuals showed a significant increase in the total number and in Runx/Cbfal-positive osteoblastic cells/mm2 of bone tissue.15
This prospective study confirms the in vivo anabolic effect of bortezomib on bone structure by micro-CT, by tetracycline labeling and morphometric analysis. This study has demonstrated for the first time that only a small percentage (20%) of relapsing myeloma patients retain any measurable osteoblastic activity, as determined by the extensive evaluation of serial bone biopsies. In addition, treatment with bortezomib induces significant increases in osteoblast activity and leads to increases in bone architectural parameters, such as bone volume to total volume fraction and trabecular thickness in 80%and 70%, respectively, of the responsive patients.
Since bortezomib exposure had little or no effect on DKK1 or FOSB RNA expression in purified myeloma cells (M Zangari and F Zhan, unpublished data, 2008), the results obtained from whole bone biopsy suggest that bortezomib acts via the microenvironment, as suggested by pre-clinical studies demonstrating that bortezomib inhibits RANK-L induced osteoclastogenesis.16 Interestingly, the significant bone anabolism following bortezomib treatment was preceded only in responsive patients by a significant pulsatile increase in serum parathormone levels without concomitant significant changes in calcium, magnesium or phosphorus levels.
The rapid increases in serum parathormone levels following daily administration of the hormone17–19 are directly associated with the increased bone anabolism observed in post-menopausal osteoporosis patients, even after treatment of only one month duration.20 The similar rapid increases in parathormone observed following bortezomib treatment suggests that the change in parathormone could be the trigger for osteoblastic activation and bone anabolism associated with the myeloma response.
The novel observation that rapid increases in parathormone secretion precede both the positive osteoblastic response and the suppression of myeloma warrants further investigation in larger patient cohorts and such studies are ongoing. The bone microarchitectural and osteoblast changes observed in this short treatment (three cycles) trial, primarily in responding individuals, could have obscured a more generalized bone anabolic effect as a subset analysis in 135 patients enrolled in the APEX trial showed maximal M protein reduction in 80% of the patients by cycle 8.21 Our findings suggest a central role of proteasome function not only in the treatment of multiple myeloma but perhaps also in other bone metabolic disorders.
Footnotes
Funding: this work was supported by grants from Millennium/Takeda Pharmaceuticals, Cambridge, Massachusetts, the General Clinical Research Center (grant MO1RR14288) and the Carl L. Nelson Chair of Orthopaedic Creativity (LJS).
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Pearse RN, Sordillo EM, Yaccoby S, Wong BR, Liau DF, Colman N, et al. Multiple myeloma disrupts the TRANCE/osteoprotegerin cytokine axis to trigger bone destruction and promote tumor progression. Proc Natl Acad Sci USA. 2001;98(20):11581–6. doi: 10.1073/pnas.201394498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heider U, Kaiser M, Muller C, Jakob C, Zavrski I, Schulz CO, et al. Bortezomib increases osteoblast activity in myeloma patients irrespective of response to treatment. Eur J Haematol. 2006;77(3):233–8. doi: 10.1111/j.1600-0609.2006.00692.x. [DOI] [PubMed] [Google Scholar]
- 3.Giuliani N, Rizzoli V, Roodman GD. Multiple myeloma bone disease: Pathophysiology of osteoblast inhibition. Blood. 2006;108(13):3992–6. doi: 10.1182/blood-2006-05-026112. [DOI] [PubMed] [Google Scholar]
- 4.Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349(26):2483–94. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 5.Garrett IR, Chen D, Gutierrez G, Zhao M, Escobedo A, Rossini G, et al. Selective inhibitors of the osteblast proteasome stimulate bone formation in vivo and in vitro. J Clin Invest. 2003;111(11):1771–82. doi: 10.1172/JCI16198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukherjee S, Raje N, Schoonmaker JA, Liu JC, Hideshima T, Wein MN, et al. Pharmacologic targeting of a stem/progenitor population in vivo is associated with enhanced bone regeneration in mice. J Clin Invest. 2008;118(2):491–504. doi: 10.1172/JCI33102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pennisi A, Li X, Ling W, Khan S, Zangari M, Yaccoby S. The proteasome inhibitor, bortezomib suppresses primary myeloma and stimulates bone formation in myelomatous and nonmyelomatous bones in vivo. Am J Hematol. 2009;84(1):6–14. doi: 10.1002/ajh.21310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zangari M, Esseltine D, Lee CK, Barlogie B, Elice F, Burns MJ, et al. Response to bortezomib is associated to osteoblastic activation in patients with multiple myeloma. Br J Haematol. 2005;131(1):71–3. doi: 10.1111/j.1365-2141.2005.05733.x. [DOI] [PubMed] [Google Scholar]
- 9.Blade J, Samson D, Reece D, Apperley J, Björkstrand B, Gahrton G, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemapoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Brit J Haematol. 1998;102(5):1115–23. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 10.Suva LJ, Seedor G, Endo N, Quartuccio HA, Thompson DD, Bab I, et al. Pattern of gene expression following rat tibial marrow ablation. Bone Miner Res. 1993;8(3):379–88. doi: 10.1002/jbmr.5650080315. [DOI] [PubMed] [Google Scholar]
- 11.Perrien DS, Akel NS, Edwards PK, Carver AA, Bendre MS, Swain FL, et al. Inhibin A is an Endocrine Stimulator of Bone Mass and Strength. Endocrinology. 2007;148(4):1654–65. doi: 10.1210/en.2006-0848. [DOI] [PubMed] [Google Scholar]
- 12.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2(6):595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 13.Zhan F, Huang Y, Colla S, Stewart JP, Hanamura I, Gupta S, et al. The molecular classification of multiple myeloma. Blood. 2006;108(6):2020–8. doi: 10.1182/blood-2005-11-013458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhan F, Hardin J, Kordsmeier B, Bumm K, Zheng M, Tian E, et al. Global gene expression profiling of multiple myeloma, monoclonal gammopathy of undetermined significance, and normal bone marrow plasma cells. Blood. 2002;99(5):1745–57. doi: 10.1182/blood.v99.5.1745. [DOI] [PubMed] [Google Scholar]
- 15.Zangari M, Esseltine D, Cavallo F, Neuwirth R, Elice F, Burns MJ, et al. Predictive value of alkaline phosphatase for response and time to progression in bortezomib-treated multiple myeloma patients. Am J Hematol. 2007;82(9):831–3. doi: 10.1002/ajh.20961. [DOI] [PubMed] [Google Scholar]
- 16.Terpos E, Heath DJ, Rahemtulla A, Zervas K, Chantry A, Anagnostopoulos A, et al. Bortezomib reduces serum dickkopf-1 and receptor activator of nuclear factor-kappa‚ ligand concentrations and normalises indices of bone remodeling in patients with relapsed multiple myeloma. Br J Haematol. 2006;135(5):688–92. doi: 10.1111/j.1365-2141.2006.06356.x. [DOI] [PubMed] [Google Scholar]
- 17.Giuliani N, Morandi F, Tagliaferri S, Lazzaretti M, Bonomini S, Crugnola M, et al. The proteasome inhibitor bortezomib affects osteoblast differentiation in vitro and in vivo in multiple myeloma patients. Blood. 2007;110(1):334–8. doi: 10.1182/blood-2006-11-059188. [DOI] [PubMed] [Google Scholar]
- 18.von Metzler I, Krebbel H, Hecht M, Manz RA, Fleissner C, Mieth M, et al. Bortezomib inhibits human osteoclastogenesis. Leukemia. 2007;21(9):2025–34. doi: 10.1038/sj.leu.2404806. [DOI] [PubMed] [Google Scholar]
- 19.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of Parathyroid Hormone (1–34) on Fractures and Bone Mineral Density in Postmenopausal Women With Osteoporosis. N Engl J Med. 2001;344(19):1434–41. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 20.Cosman F, Nieves J, Woelfert L, Gordon S, Shen V, Lindsay R. Parathyroid Responsivity in Postmenopausal Women with Osteoporosis During Treatment with Parathyroid Hormone. J Clin Endocrinol Metab. 1998;83(3):788–90. doi: 10.1210/jcem.83.3.4639. [DOI] [PubMed] [Google Scholar]
- 21.Lindsay R, Nieves J, Formica C, Henneman E, Woelfert L, Shen V, et al. Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet. 1997;350(9077):550–5. doi: 10.1016/S0140-6736(97)02342-8. [DOI] [PubMed] [Google Scholar]
- 22.Black DM, Bouxsein ML, Palermo L, McGowan JA, Newitt DC, Rosen E, et al. Randomized Trial of Once-Weekly Parathyroid Hormone (1–84) on Bone Mineral Density and Remodeling. J Clin Endocrinol Metab. 2008;93(6):2166–72. doi: 10.1210/jc.2007-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson PG, Sonneveld P, Schuster M, Irwin D, Stadtmauer E, Facon T, et al. Extended follow up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trial. Blood. 2007;110(10):3557–60. doi: 10.1182/blood-2006-08-036947. [DOI] [PubMed] [Google Scholar]