Abstract
Alcohol is a known exogenous modulator of negative affect (anxiety, tension) in both animals and humans. It has been proposed that the anxiolytic effects of alcohol are mediated via the amygdala, an area critical to fear perception and responding. However, little is known about the acute effects of alcohol on amygdala reactivity to threatening information in humans. We used functional magnetic resonance imaging and a well validated task to probe amygdala responses to social signals of threat in 12 healthy, social drinkers after a double-blind crossover administration of alcohol or placebo. We found that alcohol significantly reduced amygdala reactivity to threat signals. The current findings fit well with the notion that alcohol may attenuate threat-based responding and provide a potential brain-based mechanism for the link between alcohol and anxiety, and/or social threat perception.
INTRODUCTION
People enjoy drinking alcohol because of its ability to enhance positive (e.g., elation, relaxation) and attenuate negative (e.g., tension, anxiety, stress) affective states (Battista et al.; Cooper et al., 1995; Kassel et al., 2000; Levenson et al., 1980; Sayette, 1999; Sher et al., 2007). Those who drink to cope with stress and/or relieve anxiety may be at increased risk for developing alcohol use disorders (AUD) (Cooper et al., 1995; Ray et al., 2009; Schroder and Perrine, 2007). Moreover, patients with anxiety disorders often also have problems with alcohol abuse and dependence (Kushner et al., 1999). An improved understanding of the subjective response to alcohol and motivation to continue its use could serve as an important endophenotype to better characterize genetic, personality and behavioral risk factors for AUD (Morean and Corbin, 2010). However, not much is known about the neural mechanisms that mediate the relationship between alcohol and anxiety/negative affect.
Animal studies have also shown that alcohol reduces negative affect and dampens anxiety behaviors (Blanchard et al., 1993; Spanagel et al., 1995), and have suggested that alcohol exerts this effect within the brain's limbic fear system (e.g., GABA pathway, amygdala) (Allan et al., 1987; McBride, 2002; Moller et al., 1997; Sommer et al., 2001). However, the acute effect of alcohol on fear-related brain function in humans is poorly understood. There is reason to expect that the locus of action for alcohol's anxiolytic effects in humans also involves the amygdala. In humans, similar to rodents, the amgydala is the key brain region for detection of signals of fear, and generation of anxiety responses (Adolphs et al., 1995; Davis et al., 2010; Davis and Whalen, 2001; Zald, 2003). Moreover, exaggerated amygdala reactivity to social signals of threat (i.e., fearful/angry faces) is a consistent and robust neuroanatomical finding in functional brain imaging studies of patients with anxiety disorders (Etkin and Wager, 2007; Shin and Liberzon, 2009). Interestingly, acute alcohol consumption has been shown to impair recognition of threatening/angry faces (Borrill et al., 1987), and reduce the attentional bias towards and negative perception of threatening faces, often observed in anxiety disorders (Stevens et al., 2008; Stevens et al., 2009). Few studies have examined how alcohol affects the brain's processing of socio-emotional cues.
Most relevant to the current study is a recent functional magnetic resonance imaging (fMRI) investigation of face emotion (fearful, neutral) processing following intravenous administration of alcohol by Gilman and colleagues (2008) who demonstrated that: 1) alcohol (>placebo) enhanced striatal reward circuits to neutral faces; and 2) amygdala reactivity to fearful (>neutral) faces observed during placebo was absent during the alcohol session (Gilman et al., 2008). The authors also observed that alcohol increased amygdala response to neutral faces, thereby attenuating any difference in amygdala activity between fearful and neutral faces, suggesting that the amygdala may be less able to serve as a detector of threatening information. Therefore, the exact effect of alcohol on amygdala function (attenuating its reactivity to signals of fear specifically vs. obscuring its ability to discriminate fearful vs. non-fearful/neutral signals) remains unclear. That said, this paper was the first to provide support for the notion that the anxiolytic effects of alcohol are mediated by the amgydala in humans.
To investigate the neural mechanism underlying the anxiolytic responses to alcohol in humans, we examined the effects of alcohol consumption on the threat-related amygdala activation. We used fMRI to study acute responses to alcohol ingestion (0.8g/kg; 16% volume alcohol) or placebo in healthy volunteers (n=12) and examined amygdala reactivity to social signals of threat (fearful and angry faces), in a double-blind, randomized, placebo-controlled design. The fMRI “activation” task was the Emotional Face Assessment Task (EFAT), a well validated probe of amygdala reactivity specific to social threat (i.e., fearful and angry vs. non-threatening happy faces) (Phan et al., 2008) that has proven useful as a bioassay of pharmacologic (Arce et al., 2008; Paulus et al., 2005; Phan et al., 2008) and genetic (Hariri et al., 2005; Hariri et al., 2009) modulation of fear responding in humans. Additionally, studies using similar probes have detected exaggerated amygdala reactivity to threat in patients with anxiety disorders (Etkin and Wager, 2007). Similar to the acute effects produced by pharmacologic agents often used in the treatment of excessive anxiety (Arce et al., 2008; Harmer et al., 2003; Paulus et al., 2005), we hypothesized that compared to placebo, alcohol would diminish the amygdala reactivity to social signals of threat. Importantly, the present study differed from Gilman et al (2008) in a number of respects including that the present study 1) used happy faces as the ‘control’ for the threat condition rather than neutral faces; 2) included a more clinically-relevant subpopulation, i.e., frequent binge social drinkers, rather than moderate drinkers; and 3) utilized a more ecologically valid route of alcohol administration, oral ingestion, rather than IV infusion.
MATERIALS AND METHODS
Participants
Twelve healthy, right-handed volunteers completed this study (see Table 1 for sample characteristics). Among study candidates who were screened by telephone, approximately 50% were eligible for in-laboratory screening, and all candidates attending the in-person screening were accepted into the study. There was one additional female subject who was initially enrolled in the study, but she withdrew prior to the imaging protocol in her first session.
Table 1.
Demographic and alcohol drinking behavior of participants
| Demographics | Mean±SD |
|---|---|
| N male/N female | 10/2 |
| Age (years) | 23.2 ± 1.8 |
| Education (years) | 15.7 ± 1.2 |
| BMI (kg.m-3) | 23.8 ± 2.9 |
| Race | |
| # Caucasian | 8 |
| # Asian | 3 |
| # African American | 1 |
| Alcohol Drinking Behavior1 | |
|---|---|
| Alcohol drinking days/month | 13.2 ± 7.1 |
| Drinks/drinking day | 6.7 ± 3.5 |
| Binges in last month2 | 7.8 ± 3.4 |
| Max drinks in last month3 | 13.5 ± 7.7 |
| Days since last use4 | 3.8 ± 1.7 |
All results reported as standard drinks (12 oz beer, 5 oz wine, or 1.5 oz of liquor)
Binge = 5+ drinks on one occasion (4+ for females)
Maximum number of drinks consumed on any occasion during the last month
Average number of days prior to each session since alcohol last consumed
Participants were selected to meet criteria for heavy social drinking consistent with our prior studies (King et al., 2009; King et al., 2010b; King et al., 2002), defined as consuming ≥ 10 standard alcoholic drinks per week with one to five weekly “binge” drinking episodes (+5 drinks per occasion for men; 4+ drinks for women) (SAMHSA, 2005). A standard alcoholic drink is 12 ounces of beer, 5 ounces of wine, or 1.5 ounces (one shot) of liquor (ICAP, 1998). We have shown that those who are classified as heavy drinkers (vs. light drinkers) report significantly greater subjective stimulation, liking and wanting alcohol, and fewer sedative-like effects after consuming an intoxicating alcohol dose compared with placebo (King et al., 2010b; King et al., 2002). The rationale for including this subgroup included both ethical concerns, i.e., to avoid adverse effects of alcohol consumption while in the fMRI scanner, and scientific considerations, i.e., heavy social drinkers do not experience alcohol withdrawal which may confound study measures, but they exhibit heightened alcohol reward without excessive sedative effects, and are at risk for future alcohol problems (King et al., 2010b; McCarty et al., 2004) .
All screening participants underwent the Structured Clinical Interview for DSM-IV [SCID-IV-Patient Edition] (First et al., 1995), which revealed that 10 out of the 12 subjects had met lifetime criteria for alcohol abuse, but none had ever met criteria for alcohol dependence. The Time Line Follow-Back interview (Sobell and Sobell, 1995) of participants’ past month alcohol drinking behaviors revealed that all subjects were non-daily drinkers: any drinking occurred on an average of 47% of days (13.2 ±7.1 days) including binge drinking on 30% of days (7.83±3.35 days). The typical quantity of alcohol consumed was 6.7 (± 3.5) standard drinks per drinking day with an average maximum quantity of 13.5 (±7.7 SD) drinks consumed on the heaviest drinking day. The average last self-reported last alcohol use prior to the study sessions was 3.8 (± 1.7) days (Table 1). All baseline breathalyzer tests were negative for presence of alcohol, confirming compliance with study abstinence instructions on the testing days.
Participants were excluded if they met lifetime history of any major Axis I or II mental disorders including anxiety disorders, and alcohol and substance dependence. They were also excluded if they were currently taking medications, had a history of neurological or medical illness as confirmed by medical examination, or had liver enzyme tests for aspartate aminotransferase and alanine transaminase out of the normal range. Finally, during screening, subjects completed the Claustrophobia Questionnaire (Radomsky et al., 2001) and visited the fMRI scanning environment to ensure comfort with the confinement of this procedure. All participants gave written informed consent after explanation of the experimental protocol, as approved by the Institutional Review Board and upon completion of the study, they were compensated with a check for $150.00.
Protocol
This study used a two-session, double-blind, placebo-controlled, within-subject design. The two sessions were scheduled at least 48 hours (h) apart and were counterbalanced in order. The order of beverage (alcohol, placebo) was randomized by computer number generator. The study was double blind in that neither the participant nor the experimenter (PM) knew the contents of the beverage. A separate investigator (AK) randomized and prepared the beverages. Other investigators conducted the main imaging analyses (KLP, MA, CS). Prior to each session, participants were instructed to abstain from alcohol, recreational drugs, and any psychoactive medications for at least 48 hours, as well as caffeine, food, and cigarette smoking for 3 hours. Upon arrival, the subject underwent abstinence verification, consumed a low-fat snack (20% daily calories), and acclimated to the laboratory.
Approximately 45 minutes after arrival, the participant was served a beverage and a small, placebo gel capsule (containing dextrose). In order to reduce alcohol expectancies, the subject was told that both the beverage and gel-capsule might contain alcohol, a stimulant, a sedative, a placebo, or a combination of these substances. The beverage only actually contained either a high dose of alcohol (ALC, 0.8 g/kg; 16% volume alcohol) or placebo (PBO, 0.0 g/kg; 1% volume ethanol as a taste mask). This high dose of alcohol was chosen as it yields clinically significant BrACs (peak between 0.08-0.09%) (King et al., 2002; 2009, 2010 submitted) and produces significant stimulant and sedative subjective changes compared with placebo beverage (King et al., 2002; Rueger et al., 2009), as well as anxiolytic effects (for review, see Morean & Corbin, 2010). In past studies with heavy social drinkers, a manipulation check of this instructional set and double-dummy administration procedure (King et al., 2009) revealed that 62.1% of participants receiving the high alcohol dose thought they received a substance other than alcohol, and 61.5% of participants receiving the placebo beverage thought it contained an active substance (King, personal communication 2010).
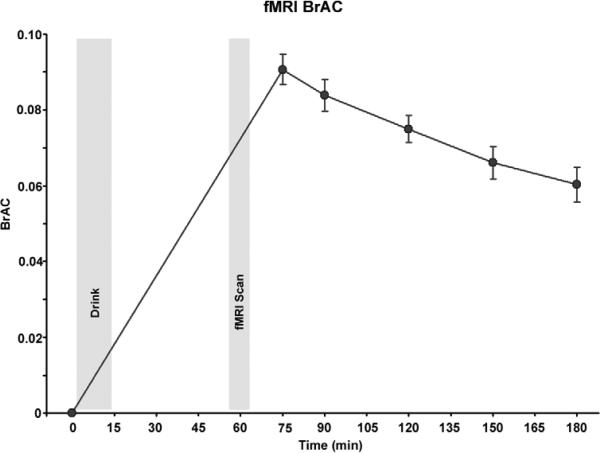
The beverages were prepared with Kool-Aid, water, Splenda®, and the appropriate dose of 190-proof ethanol based on body weight and were consumed through a straw in a lidded, opaque cup to conceal potential scent cues. Women received an 85% dose to adjust for total body water differences (Frezza et al., 1990; Sutker et al., 1983). Total beverage volumes for placebo and alcohol were identical (mean 472 mL; range 297-581mL) and based on subject's body weight. The subject consumed the beverage over a 13 minute interval, timed such that the upcoming fMRI task would concur with expected peak blood alcohol levels and subjective effects (Epstein et al., 2007; King et al., 2010b; King et al., 2002). Immediately following beverage consumption, the subject was escorted into the scanning room, underwent fMRI preparation (review of task instructions) and structural MRI prior to beginning the fMRI the EFAT task (described below) which commenced approximately 55 minutes after alcohol ingestion. Breath Alcohol Concentration (BrAC) levels for the session are depicted in Figure 1. Alcohol ingestion increased BrAC to (mean ± S.D.) 0.091 ± 0.014g % 75 minutes after initiation of the beverage with a slow elimination phase over the next few hours. Subjects were discharged when BrAC were <0.04 % and they showed no overt signs of intoxication (NIAAA, 2005). Prior to consumption of the alcohol or placebo beverage, subjects completed the Biphasic Alcohol Effects Scale (BAES, (Martin et al., 1993; Rueger et al., 2009)), which was repeated 75 minutes later i.e., immediately upon exiting of the scanner after completion of the EFAT fMRI task. Of note, the EFAT task followed a visual stimulus processing task (involving smoking/non-smoking images) as part another unrelated experiment, the results of which have been published (King et al., 2010a).
Figure 1.
Alcohol effects on breath alcohol levels (averaged over all subjects) throughout the session. “Drink” shade corresponds to the timing and duration of alcohol/placebo ingestion, and “fMRI Scan” corresponds to the timing and duration when brain activation was measured. Note, the linear increase from the first time point (prior to ingestion of the beverage) to the second time point (after fMRI scanning) is an estimation.
The EFAT task as employed in our pharmacological fMRI paradigm has been described previously (Phan et al., 2008), and similar to prior pharmacological fMRI studies, is known to reliably and robustly engage the amygdala (Hariri et al., 2002b; Tessitore et al., 2002; Kirsch et al., 2005; Paulus et al., 2005). In brief, participants viewed a trio of faces and selected one of the two faces (bottom) that expressed the same emotion as the target face (top). The identity of all three faces was always different, and an equal number of male and female faces were presented. The target and congruent probe face displayed one of three expressions (angry, fearful, or happy), and the other (incongruent) probe face always displayed a neutral/nonemotional expression. This design allowed us to isolate amygdala reactivity specifically to social threat (angry and fearful faces) relative to non-threat (happy faces), which have similar perceptual characteristics except for the threat/non-threat signal conveyed. The face photographs were selected from the validated stimulus set from Gur et al. (2002). Of note, the angry, fearful, and happy target faces were presented in separate blocks. Three blocks of each target expression were presented, and no target stimuli were repeated within or across blocks. To maintain attention and allow limbic brain responses to return to baseline, the face matching tasks were interspersed with a ‘control’ task, in which subjects matched simple geometric shapes (circles, rectangles, or triangles) similar to instructions above. The paradigm consisted of eighteen 20 s blocks: 9 blocks of matching emotional faces, interleaved with 9 blocks of matching shapes (blocks occurred back-to-back without intervening fixation), counterbalanced across two runs for total task time of 6 min. Each task block contained four sequential matching trials, 5 s each. Participants responded to tasks by pressing the left or right response buttons with their dominant hand. These responses also provided a measure of participants’ response accuracy and reaction time.
Brain Imaging
Functional MRI was performed on a 3T GE magnetic resonance scanner which acquired functional images [i.e., blood oxygenated level- dependent (BOLD)] from 30 axial, 5-mm-thick slices using a T2*-sensitive gradient echo reverse spiral acquisition sequences (repetition time, 2000 ms; echo time, 25 ms; 64 × 64 matrix; 24 cm field of view; flip angle, 77), optimized to minimize susceptibility artifacts in the amygdala (Stenger et al., 2000). This was followed by a high- resolution, T1-weighted volumetric anatomical scan (three-dimensional magnetization-prepared rapid gradient echo) for anatomical localization.
Functional MRI Data Analysis
Data from all 12 subjects met criteria for high quality and scan stability with minimum motion correction (3 mm displacement in any one direction) and were subsequently included in fMRI analyses. The first four volumes from each run were discarded to allow for T1 equilibration effects. Functional data were analyzed using SPM2 (Welcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm) a similar approach is described in Phan et al. (2008). In brief, images were spatially realigned to correct for head motion, warped to an EPI template in Montreal Neurologic Institute (MNI) space, resampled to 2 mm3 voxels, and smoothed with an 8 mm3 kernel to minimize noise and residual differences in gyral anatomy. The general linear model was applied to the time series, convolved with the canonical hemodynamic response function (Friston et al., 1995) and with a 128 s high-pass filter. Condition effects were modeled with box-car regressors representing the occurrence of each block type (we did not model individual events within blocks). Effects were estimated at each voxel, and for each subject. Individual contrast maps [SPMs (statistical parametric maps)] were then analyzed at the second level in a random-effects statistical model (Holmes and Friston, 1998).
We assessed for significant differences in amygdala activation in the contrast of ‘Threat’ (fearful and anger) versus ‘Non-threat’ (happy faces) signals between drug conditions using one sample and paired sample t-tests. First, we examined the Threat and Non-threat contrast in the placebo condition to confirm that our task manipulation succeeded in activating amygdala threat-sensitive circuitry. Next, we contrasted the placebo condition against the alcohol condition to identify brain regions for which alcohol decreased or increased activation (PBO > ALC, ALC > PBO, respectively). We considered activations that survived p < 0.005 (uncorrected) for within- and between-session contrast t-maps. Third, to clarify the sources of differences in activation between PBO and ALC sessions in the Threat versus Non-threat contrast, we located 10mm spherical regions of interest (ROIs) at peak activations within amygdala. We extracted BOLD signal responses (parameter estimates, β weights [arbitrary units, a.u]) from these amygdala ROIs (i.e., signal responses averaged across all voxels within the ROIs) in, the Threat versus Non-threat contrast as well as the individual contrasts of each emotion face (fearful, angry, happy) contrasted with the control task (matching shapes). Fourth, given prior findings that alcohol has fairly broad effects in modulating BOLD responses, especially in posterior regions of the brain including visual cortex (Calhoun et al., 2004; Levin et al., 1998), cerebellum, and posterior ventral temporal lobe (i.e., fusiform gyrus) (Calhoun et al., 2004), in whole brain voxel-wise analysis, we examined task-related activations to emotion faces (collapsing across face types) in the ALC versus PBO sessions. Fifth, given prior findings of enhanced activation in ventral striatum due to alcohol (>placebo) to neutral faces (Gilman et al., 2008), we hypothesized that alcohol might exert a similar effect in response to non-threatening happy faces in the present study and thus examined brain responses to happy faces in the ALC (>PBO) condition.
Behavioral Data Analysis
We examined the subjective effects of ALC by comparing scores on the BAES stimulation and sedation subscales at baseline and at 75 minutes between the ALC and PBO sessions using repeated measure analysis of variance (ANOVA). The effects of ALC on emotion matching accuracy (percentage correct) and response times (in milliseconds) were assessed using a 2 × 2 ANOVA with beverage (ALC, PBO) by condition (Threat, Non-threat); significant main effects or drug by condition interactions were followed up with paired t tests (ALC vs PBO).
RESULTS
Behavioral results
All participants tolerated the beverages without complications. We observed the expected directional effects of alcohol on the BAES stimulation and sedation subscales but these changes were nonsignificant (stimulation: F(1,11) = 1.13, p = ns; sedation: F(1,11) = 0.52, p = ns) which may have been due to lack of power given the small sample size and the constraints of the scanning environment on subjective assessment. Behavioral results from the fMRI task (n = 7; missing button-press data for 5 subjects; see Table 2) show subjects performed the task accurately (accuracy range: 85 – 100%) and within the 5 s trial duration (reaction time range: 1042-1652msec). Subjects were more accurate and faster for the Non-threat compared with the Threat condition (accuracy: F(1,6) = 12.79, p = 0.012; response time: F(1,6) = 27.44, p = 0.002) but there were no differences in the alcohol compared with placebo sessions on either measure [(beverage: accuracy: F(1,6) = 2.54, p = 0.162), response time: F(1,6) = 0.65, p = 0.452) beverage × condition: accuracy: F(1,6) = 0.89, p = 0.383, response time: F(1,6) = 0.81, p = 0.403)].
Table 2.
Performance during Emotional Face Assessment Task
| Measure | Session | Condition | Mean | SD |
|---|---|---|---|---|
| Accuracy (%) | PBO | Threat | 85.7 | 13.4 |
| Non-threat | 89.3 | 17.8 | ||
| ALC | Threat | 92.9 | 2.0 | |
| Non-threat | 100.0 | 0.0 | ||
| Reaction Time (ms) | PBO | Threat | 1503.3 | 417.8 |
| Non-threat | 1042.2 | 162.5 | ||
| ALC | Threat | 1651.7 | 286.4 | |
| Non-threat | 1066.2 | 150.1 |
Neuroimaging results
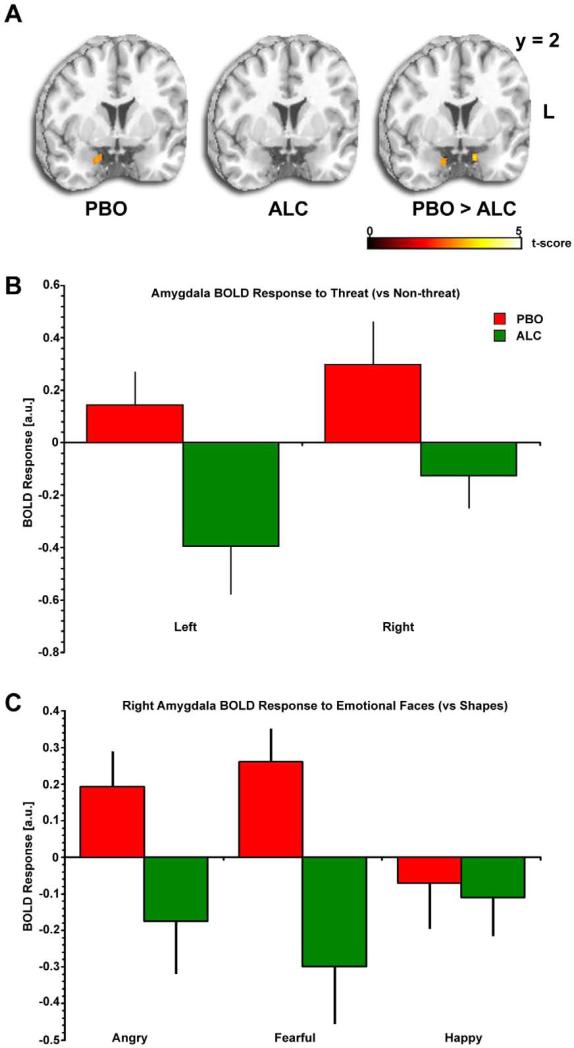
In whole brain voxel-wise analysis, we found that during the placebo session, amygdala activation was greater for Threat (fearful, angry) than Non-threat (happy) faces in the right lateral amygdala ([22, 8, –26]; Z = 2.91; p < 0.005, uncorrected) (Table 3; Figure 2A), confirming previous findings of amygdala reactivity to threat signals (Phan et al., 2002; Zald, 2003), and previously observed during the PBO condition of another study from our lab using the EFAT (Phan et al., 2008). Notably, however, this threat-related amygdala activation was absent during the ALC condition (Figure 2A), even at lowered significance thresholds (e.g., p < 0.05, uncorrected). Compared with placebo, ALC significantly attenuated right and left medial amygdala activation to Threat (Right: [14, -4, –26], Z =2.63; Left: [-12, 2, -26], Z = 2.86) (Table 3, Figure 2A). Alcohol enhanced activation in a number of brain regions including inferior and superior parietal cortex, right and left inferior frontal gyrus, and middle and superior occipital gyrus (Table 3). We did not have a priori hypotheses about these regions and report these results for future studies to further elucidate.
Table 3.
Activation to Threat (> Non-threat) between placebo and alcohol sessions
| Volume (mm3) | Z-score | MNI Coordinates | ||||||
|---|---|---|---|---|---|---|---|---|
| Session | Brain Region | Laterality | p-value | x | y | Z | ||
| PBO | Supplementary Motor Area | R | 1184 | 3.82 | <0.001 | 14 | -12 | 52 |
| R | 72 | 2.75 | 0.003 | 18 | -30 | 52 | ||
| Middle Temporal Gyrus | R | 1440 | 3.28 | 0.001 | 48 | -50 | 6 | |
| Cerebellum | L | 280 | 3.14 | 0.001 | -4 | -68 | -14 | |
| Amygdala | R | 176 | 2.91 | 0.002 | 22 | 8 | -26 | |
| | ||||||||
| ALC | Inferior Frontal Gyrus | R | 176 | 3.65 | <0.001 | 48 | 46 | -16 |
| R | 1608 | 3.50 | <0.001 | 48 | 40 | -2 | ||
| L | 328 | 3.34 | <0.001 | -52 | 44 | -14 | ||
| L | 568 | 2.92 | 0.002 | -32 | 30 | 10 | ||
| L | 424 | 2.79 | 0.003 | -44 | 42 | 6 | ||
| R | 232 | 2.79 | 0.003 | 44 | 20 | 22 | ||
| L | 40 | 2.62 | 0.004 | -52 | 22 | 26 | ||
| Middle Frontal Gyrus | L | 728 | 3.61 | <0.001 | -40 | 46 | 32 | |
| R | 96 | 3.03 | 0.001 | 54 | 40 | 28 | ||
| Cerebellum | R | 240 | 3.36 | <0.001 | 42 | -44 | -30 | |
| Inferior Parietal Gyrus | L | 264 | 2.67 | 0.004 | -34 | -50 | 44 | |
| | ||||||||
| PBO > ALC | Mid Cingulate | L | 488 | 3.94 | <0.001 | -6 | -14 | 28 |
| R | 48 | 2.64 | 0.004 | 10 | 6 | 36 | ||
| Thalamus | L | 56 | 3.17 | 0.001 | -8 | -14 | 0 | |
| Pallidum | L | 56 | 2.89 | 0.002 | -16 | 4 | -6 | |
| Amygdala | L | 88 | 2.86 | 0.002 | -12 | 2 | -26 | |
| R | 32 | 2.63 | 0.004 | 14 | -4 | -26 | ||
| Putamen | L | 88 | 2.84 | 0.002 | -22 | -2 | -8 | |
| Cerebellum | R | 160 | 2.83 | 0.002 | 2 | -62 | -8 | |
| Cuneus | R | 64 | 2.77 | 0.003 | 2 | -94 | 22 | |
| | ||||||||
| ALC > PBO | Inferior Parietal Gyrus | L | 10416 | 4.06 | <0.001 | -36 | -74 | 44 |
| Angular Gyrus | R | 1520 | 3.99 | <0.001 | 40 | -64 | 54 | |
| Inferior Frontal Gyrus | L | 4984 | 3.93 | <0.001 | -50 | 42 | -14 | |
| R | 296 | 3.21 | 0.001 | 48 | 46 | -16 | ||
| L | 456 | 2.95 | 0.002 | -42 | 24 | 6 | ||
| L | 248 | 2.76 | 0.003 | -42 | 34 | -2 | ||
| L | 64 | 2.62 | 0.004 | -44 | 38 | 8 | ||
| Superior Parietal Gyrus | R | 128 | 3.28 | 0.001 | 24 | -72 | 56 | |
| Middle Frontal Gyrus | L | 1728 | 3.24 | 0.001 | -40 | 36 | 28 | |
| R | 144 | 3.04 | 0.001 | 58 | 44 | 12 | ||
| Superior Frontal Gyrus | L | 504 | 3.18 | 0.001 | -24 | 56 | 22 | |
| Middle Occipital Gyrus | L | 232 | 3.14 | 0.001 | -42 | -84 | 24 | |
| R | 88 | 2.85 | 0.002 | 34 | -88 | 34 | ||
| Superior Occipital Gyrus | R | 48 | 2.78 | 0.003 | 22 | -74 | 46 | |
| Inferior Temporal Gyrus | R | 80 | 2.75 | 0.003 | 64 | -52 | -18 | |
All activations are p < 0.005 (uncorrected); assessment of cluster volume was performed at p < 0.005 (uncorrected). ALC, alcohol; PBO, placebo; MNI, Montreal Neurologic Institute.
Figure 2.
Alcohol effects on amygdala activation to social signals of threat. A) Statistical t-maps overlaid on a canonical brain rendering (MNI coronal y-plane = 2) showing right lateral amygdala activation to Threat (> Non-threat) faces is present during the PBO session but absent during the ALC session (i.e., greater threat-related amygdala reactivity in the PBO relative to the ALC session); Statistical t-score scale is shown at the bottom of the brain rendering and for display purposes, t-maps are thresholded at p<0.01. L, Left. B) Mean BOLD Response (β weights ±SEM) extracted from amygdala ROIs showing activation to Threat (> Non-threat) faces in the PBO session (more evident in the right amgydala than the left, consistent with Figure 2A), but no activation during the ALC session. PBO, placebo; ALC, alcohol. C) Mean BOLD Response (β weights ±SEM) extracted from right amygdala ROI showing alcohol attenuates (PBO>ALC) activation to Threat (Angry , Fearful) faces but does not affect responses to Non-threat (Happy Faces).
Follow-up ROI analysis of extracted BOLD signal responses (parameter estimates, β weights [arbitrary units, a.u]) revealed that within the right amygdala ROI, there was significant activation to threat-related (angry/fearful > happy) faces during the PBO session, which was abolished during the ALC session (Figure 2B). In order to clarify the direction and specificity of alcohol's effects, follow-up contrasts of the individual emotion face types (fearful, angry, happy) versus the control task (matching shapes) were conducted using pair-wise t-tests (PBO versus ALC) and revealed a lateralized effect. In the left amygdala ROI, there were no differences between fearful, angry, or happy faces (versus control) in the PBO versus ALC condition (PBO>ALC: fearful p = 0.15; angry p = 0.35; happy p = 0.11). However, in the right amygdala ROI, alcohol attenuated activation to fearful and angry faces, but did not affect activation to happy, faces (PBO>ALC: fearful p = 0.06; angry p = 0.01; happy p = 0.71; Figure 2C).
In further whole brain voxel-wise analysis, we compared brain responses to Faces (collapsing across the three emotion face types) versus Shapes in the ALC compared to PBO conditions. Compared to placebo, alcohol was associated with diminished activation in multiple large regions of visual cortex encompassing calcarine and lingual gyri ([46, -82, -0], Z = 2.85) and bilateral fusiform gyrus (Right: [40, -44, –20], Z =2.72; Left: [-36, -62, -14], Z = 4.36). In the opposite contrast, alcohol was found to increase activation in multiple regions of superior frontal gyrus and superior, middle, and inferior temporal regions (see Table 4). We also compared brain activations to ALC>PBO in response to Happy faces. Activations were observed in supplementary motor cortex ([16, -4, 70], Z = 5.11) and posterior middle temporal gyrus ([48, -50, 14], Z = 4.62) However, we did not observe any activations in ventral striatum, caudate, or any other striatal regions.
Table 4.
Activation to Face (> Shapes) between placebo and alcohol sessions
| Volume (mm3) | Z-score | MNI Coordinates | ||||||
|---|---|---|---|---|---|---|---|---|
| Session | Brain Region | Laterality | p-value | x | y | z | ||
| PBO > ALC | Fusiform Gyrus / Lingual / Calcarine Gyrus | R/L | 46632 | 4.36 | <0.001 | -36 | -62 | -14 |
| L | 608 | 3.04 | 0.001 | -30 | -4 | -38 | ||
| R | 56 | 2.73 | 0.003 | 28 | -4 | -42 | ||
| R | 88 | 2.72 | 0.003 | 40 | -44 | -20 | ||
| Subgenual Anterior Cingulate | R | 128 | 3.81 | <0.001 | 4 | 18 | -8 | |
| Mid Cingulate | R | 728 | 3.51 | <0.002 | 20 | -20 | 44 | |
| Superior Parietal Gyrus | L | 272 | 3.22 | 0.001 | -26 | -52 | 56 | |
| Cerebellum | R | 184 | 3.20 | 0.001 | 34 | -42 | -34 | |
| R | 208 | 3.14 | 0.001 | 50 | -58 | -34 | ||
| Cuneus | R | 1504 | 3.12 | 0.001 | 8 | -90 | 36 | |
| Amygdala | R | 80 | 3.04 | 0.001 | 14 | -4 | -22 | |
| Pallidum | L | 152 | 2.99 | 0.001 | -20 | 0 | -6 | |
| R | 40 | 2.81 | 0.002 | 20 | 0 | 0 | ||
| Mid Occipital Gyrus | R | 240 | 2.85 | 0.002 | 46 | -82 | 0 | |
| Precentral Gyrus | R | 144 | 2.83 | 0.002 | 56 | 4 | 50 | |
| L | 752 | 2.74 | 0.003 | -46 | -6 | 42 | ||
| Lingual Gyrus | R | 48 | 2.76 | 0.003 | 18 | -50 | -2 | |
| | ||||||||
| ALC > PBO | Inferior Temporal Gyrus | L | 280 | 3.38 | <0.001 | -56 | -58 | -6 |
| Superior Frontal Gyrus | R | 184 | 3.35 | <0.001 | 16 | 26 | 62 | |
| L | 760 | 3.26 | 0.001 | -24 | 64 | 16 | ||
| L | 288 | 3.08 | 0.001 | -8 | 42 | 32 | ||
| Mid Temporal Gyrus | R | 56 | 3.01 | 0.001 | 64 | -2 | -24 | |
| Superior Temporal Gyrus | R | 56 | 2.86 | 0.002 | 58 | -6 | -12 | |
| Mid Occipital Gyrus | L | 56 | 2.79 | 0.003 | -34 | -84 | 18 | |
All activations are p < 0.005 (uncorrected); assessment of cluster volume was performed at p < 0.005 (uncorrected). ALC, alcohol; PBO, placebo; MNI, Montreal Neurologic Institute.
Relationship between alcohol effects on amygdala activation and subjective effects
To explore potential links between alcohol-related changes in amygdala activation and subjective effects, we computed Pearson product moment correlations between activation from the right and left amygdala ROIs in the Threat versus Non-threat contrast with change scores (baseline versus post-scan) on the stimulation and sedation sub-scales of the BAES during the ALC session. We also correlated between-session differences (ALC versus PBO) in threat-related amygdala activation and BAES scores. No significant correlations were observed (all p values > 0.4).
DISCUSSION
Using a placebo-controlled design and a well-validated fMRI probe of threat processing, we show that during the interval corresponding to peak BrAC, acute administration of a high dose of alcohol significantly attenuated amygdala reactivity to social signals of threat in healthy non-dependent drinkers who have regular and heavy consumption of alcohol. Alcohol's attenuating effects in amygdala were relatively specific to fearful and angry faces, and relative to placebo, alcohol did not affect significantly affect amygdala activation to happy faces. Our finding of diminished threat-related amygdala reactivity during alcohol intoxication is consistent with our a priori hypothesis, and contributes to a growing body of evidence proposing that alcohol's acute effects on reducing negative affect (e.g., stress, tension, anxiety) are mediated by attenuation of fear and threat processing in the amygdala.
Our results are broadly consistent with and extend previous findings reported in the study by Gilman and colleagues (2008) who explored acute effects of alcohol using similar socio-emotional stimuli (fearful and neutral faces). These authors were the first to demonstrate that alcohol abolished the increased amygdala response to fearful faces, and concluded that alcohol may have attenuated the enhanced sensitivity of the amygdala to threatening information, which in part may explain the anxiolytic effects of alcohol. They also observed that alcohol enhanced activation of amygdala to neutral faces, thereby diminishing the difference in amygdala reactivity between fearful faces and neutral faces, suggesting that alcohol impaired the amygdala's ability to discriminate threat from non-threatening cues. We extend these findings by employing the EFAT paradigm, a reliable and selective probe of amygdala activation to social threat in both pharmacological (Arce et al., 2008; Paulus et al., 2005; Phan et al., 2008) and genetic (Hariri et al., 2005; Hariri et al., 2009) studies to demonstrate that alcohol attenuated activation of the amygdala, particularly the right amygdala, to social signals of threat (i.e., fearful and angry faces) with no effect on response to happy faces. Gilman and colleagues also found enhanced activation in reward regions of the brain including ventral striatum and other striatal regions to neutral faces due to alcohol. In the present study, we did not find enhanced activation in ventral striatum or other striatal regions in the Non-threat condition (i.e., happy faces) either contrasted against the Threat condition or contrasted against the control condition (matching shapes). This result suggests that alcohol's enhancement of brain responses in striatal regions identified by Gilman and colleagues may be specific to neutral faces and does not occur to happy faces. The difference between the two face types may perhaps be due the ambiguity of neutral faces compared to happy faces. The ambiguity of neutral faces relative to happy faces is evidenced by the validation study by Gur and colleagues for the face stimuli utilized in the current study, which showed that happy faces are more likely to classified correctly compared to neutral (as well as all other) face types (Gur et al., 2002) – a finding consistent with other studies using reaction time and accuracy measures (Hess et al., 1997; Horstmann et al., 2006; Leppanen and Hietanen, 2004). It is also noteworthy that Gilman and colleagues found increases in ‘elevation’ on the BAES and ‘liking’ on the Drug Effects Questionnaire (DEQ) due to alcohol, while in the present study differences in subjective effects as measured by the BAES were absent. It is possible that subjective effects mediate enhanced ventral striatal activity during alcohol intoxication, thus providing an alternative explanation for differences in responses in ventral striatum to face stimuli observed by Gilman and colleagues (2008) compared to the present study. It bears mentioning again that other differences between the two studies including different subject populations, i.e., frequent binge social drinkers in the present study rather than moderate drinkers, and different routes of alcohol administration, i.e., oral ingestion in the present study rather than IV infusion, may have also contributed to differences in results. To conclude, these results do replicate the finding of Gilman and colleagues (2008) that alcohol attenuates amygdala reactivity to threatening faces / fearful stimuli.
Several converging lines of evidence suggest that the amygdala is well-positioned as a brain target of alcohol's modulation of negative affect. The amygdala plays a critical role in threat perception and fear learning in animals and humans (LeDoux, 2000; Phan et al., 2002; Phelps, 2004; Zald, 2003). In particular, the amygdala is highly sensitive to social cues, conveyed by facial expressions that signal threat (i.e., fearful and angry faces). Acute alcohol consumption has been shown to diminish subjective anxiety (Levenson et al., 1980; Sher et al., 2007), impair recognition of threatening/angry faces (Borrill et al., 1987), and reduce the attentional bias towards and negative perception of threatening faces (Stevens et al., 2008; Stevens et al., 2009). Interestingly, it has been proposed that alcohol is used by patients with social phobia, who have consistently been shown to exhibit exaggerated amygdala reactivity to threatening faces (Etkin and Wager, 2007; Phan et al., 2006; Stein et al., 2002), as a coping strategy in order to dampen the effect of anxiety (Carrigan and Randall, 2003). The current study provides evidence in humans that alcohol's effects on fear/threat processing and anxiety may be mediated by its attenuation of reactivity of the amygdala to threat signals.
The neurochemical substrates by which alcohol exerts effects on the amygdala and/or anxiety are not fully understood, but convergent evidence suggests that alcohol enhances GABAergic synaptic transmission (Criswell and Breese, 2005; Kumar et al., 2009; Weiner and Valenzuela, 2006). Regions in which alcohol-induced pro-GABAergic effects have been found to occur include the central nucleus (Nie et al., 2004; Nie et al., 2009; Roberto et al., 2003; Roberto et al., 2004), and basolateral nucleus of the amygdala (Silberman et al., 2009; Silberman et al., 2008; Zhu and Lovinger, 2006), which are crucial for the expression of fear (LeDoux, 2000). It is noteworthy that a number of other neuroimaging studies in humans of pro-GABAergic agents such as the benzodiazepine lorazepam (Arce et al., 2006; Paulus et al., 2005) have produced attenuation in amygdala reactivity providing convergent evidence that GABAergic transmission may be a key neurobiological substrate by alcohol exerts effects on the amygdala. Interestingly, positron emission tomography (PET) studies using fluorodeoxyglucose have investigated alcohol's effects on regional cerebral metabolism in the brain, and have generally found increases in limbic regions including amygdala and other medial temporal regions (Volkow et al., 2008; Wang et al., 2000), which was found to parallel the effects of lorazepam (Wang et al., 2000). However, because these PET studies examine cerebral metabolism irrespective of an affective context (e.g., threat processing), it is difficult to make direct interpretations about their relevance to fear-related brain circuitry.
Another mechanism by which alcohol might affect processing of threat-related stimuli is that alcohol might modulate functioning of brain regions that process more basic features of visual stimuli, which would in turn impact ‘downstream’ regions such as amygdala that use this information as input. Consistent with this hypothesis, we observed diminished activation in the ALC (vs. PBO) condition to face stimuli (collapsing across the three face emotion types) in large regions of visual cortex and as well as bilateral fusiform gyrus, a region implicated in responding to human faces (Kanwisher et al., 1997; Kanwisher and Yovel, 2006). This result is consistent with prior studies that find that during visual stimulation tasks, alcohol diminishes activation in a visual perception network that encompasses large regions of visual cortex (Calhoun et al., 2004; Levin et al., 1998) as well as fusiform gyrus (Calhoun et al., 2004), though other studies have found a more complex pattern involving interactions between alcohol, brain region, and task (Van Horn et al., 2006). There are at least two pathways by which alcohol's effects on visual/face processing regions might diminish amygdala reactivity in response to threatening versus non-threatening faces. First, alcohol's effects on diminishing visual/face processing regions might be relatively specific to threatening faces, in turn resulting in diminished amygdala activation to threatening (more so than non-threatening) faces. An alternative hypothesis proposes that by diminishing activation in visual/face processing regions, alcohol diminishes the ability to discriminate between threatening versus non-threatening faces, thus diminishing amygdala reactivity in the contrast between the two conditions (this was the hypothesis that found support in Gilman et al (2008) discussed above). On a qualitative level, the observed effects here of alcohol on visual cortex appear to be more expansive than previously reported. Our results could neither support or refute the preceding hypotheses, due to the fact that since we failed to find amygdala activation to non-threatening faces at all, we were unable to assess the specificity of alcohol's effects on visual processing and amygdala processing separately to threatening faces versus non-threatening faces. Thus further study is required to assess the hypothesis that alcohol affects amygdala reactivity to threatening (versus non-threatening) faces by means of either or both of the aforementioned pathways involving effects on visual/face processing.
The current study, which identified amygdala attenuation to threatening social information by alcohol in non-dependent, heavy drinkers, could inform parallel studies that have shown that that individuals dependent on alcohol or vulnerable to alcohol dependence show deficits in socio-emotional processing and exhibit atypical amygdala responses to social cues of threat. For example, studies have found deficits in emotional facial recognition in patients with alcoholism (Clark et al., 2007; Foisy et al., 2007), which persist after prolonged abstinence (Kornreich et al., 2001). Using an emotion face task similar to the one used in the current study, Glahn and colleagues (2007) found reduced amygdala activation to fearful faces in adolescents with high measures of disinhibition and a family history of alcoholism. Moreover, men with a prior history of alcohol dependence now in sustained, long-term remission exhibit diminished amygdala reactivity to emotional faces (Marinkovic et al., 2009). Collectively, these findings raise intriguing questions about whether the acute effects of alcohol on amygdala processing of threat faces are related to these other abnormalities in amygdala processing of socio-emotional information that have been observed in these clinical and pre-clinical populations.
This study should be considered preliminary, with major limitations as well as several key related questions remain to be examined. First, it is known that alcohol has vasodilatory effects (Volkow et al., 1988), which would be predicted to reduce BOLD responses, given otherwise equal underlying patterns of neuronal activation (Ogawa et al., 1990). This prediction is consistent with diminished activation in visual (Calhoun et al., 2004; Levin et al., 1998) and auditory cortex (Seifritz et al., 2000) in previous fMRI studies of alcohol challenge. In addition, alcohol might impact the onset, amplitude, or other aspects of the shape of the hemodynamic response function (HRF). One recent study (Luchtmann et al., 2010) found a complex pattern of region-specific effects in which alcohol increased the temporal lag of the HRF in some regions (visual cortex, primary motor cortex), but also affected underlying patterns of neuronal activation in other regions (supplementary motor area). Given these considerations, the main findings of the present study, i.e., attenuation of amygdala reactivity to threatening faces due to alcohol, should be considered preliminary and should be interpreted in light of potential confounds that might arise due to alcohol's potentially complex effects on the BOLD response.
A second limitation of this study is that although sufficiently powered to detect changes in amygdala reactivity by pharmacologic challenges (Paulus et al., 2005), the small sample size likely reduced statistical power to detect changes in alcohol subjective responses. In addition, the physical constraints of the fMRI scanning environment may have dampened stimulation ratings of alcohol and increased sedation ratings during placebo which may have obscured measurement of these constructs compared with the effects often observed in ambulatory human laboratory settings (King et al., 2002, 2010 submitted) For example, ratings of items that comprise the stimulation scale, such as talkative, energized, and vigorous may have been attenuated due to the physical immobility and lack of personal interaction required in neuroimaging, and similarly, maintaining the prone position may have increased placebo session sedation ratings, which includes items such as inactive, sedated, sluggish, etc., usually unaffected after placebo beverage consumption (King et al., 2010b; King et al., 2002). Third, we administered a single dose of alcohol in this initial study, and thus were unable to determine whether there are dose-dependent or sub-threshold effects of alcohol at lower or higher doses on amygdala reactivity to social threat. Fourth, our study focused on healthy heavy drinkers who were not dependent on alcohol. As noted above, previous studies have identified a range of deficits in the amygdala in populations vulnerable to alcoholism or with long-term exposure to alcohol, or in moderate social drinkers. It is unclear if the present study findings would generalize to lighter social drinkers who show differential subjective responses to alcohol than their heavier drinking counterparts (King et al 2002; King et al. 2010 submitted). Future studies are needed to investigate effects of acute intoxicating doses of alcohol on amygdala processing in a wider range of drinkers, individuals with, or at risk for, alcohol dependence, and individuals with anxiety disorders (Carrigan and Randall, 2003).
In summary, using a placebo-controlled design and a well-validated fMRI probe of threat processing, we found acute administration of an intoxicating dose of alcohol produced significant decreases in amygdala reactivity to threatening faces in healthy participants with heavy social drinking patterns. Together with emerging evidence from animal studies, these findings suggest that attenuation of fear/threat detection circuitry within the amygdala may contribute to alcohol's well-known acute effects in diminishing negative affect.
Acknowledgements
Supported by a seed grant from the University of Chicago Brain Research Foundation (AK), #R03-DA024197 (KLP), #R01- AA013746 (AK), National Center for Research Resources Grant #UL1RR024999.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/ Conflict of Interest: All authors report no conflict of interest.
- Alcohol attenuates amygdala response to threat in healthy non-dependent drinkers
- Alcohol's effects in amygdala were relatively specific to fearful and angry faces
- Alcohol broadly diminished activation visual cortex reactivity of emotional faces
References Cited
- Adolphs R, Tranel D, Damasio H, Damasio AR. Fear and the human amygdala. J Neurosci. 1995;15:5879–5891. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan AM, Huidobro-Toro JP, Bleck V, Harris RA. Alcohol and the GABA receptor-chloride channel complex of brain. Alcohol Alcohol Suppl. 1987;1:643–646. [PubMed] [Google Scholar]
- Arce E, Miller DA, Feinstein JS, Stein MB, Paulus MP. Lorazepam dose-dependently decreases risk-taking related activation in limbic areas. Psychopharmacology (Berl) 2006;189:105–116. doi: 10.1007/s00213-006-0519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arce E, Simmons AN, Lovero KL, Stein MB, Paulus MP. Escitalopram effects on insula and amygdala BOLD activation during emotional processing. Psychopharmacology (Berl) 2008;196:661–672. doi: 10.1007/s00213-007-1004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battista SR, Stewart SH, Ham LS. A Critical Review of Laboratory-Based Studies Examining the Relationships of Social Anxiety and Alcohol Intake. Curr Drug Abuse Rev. doi: 10.2174/1874473711003010003. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Magee L, Veniegas R, Blanchard DC. Alcohol and anxiety: ethopharmacological approaches. Prog Neuropsychopharmacol Biol Psychiatry. 1993;17:171–182. doi: 10.1016/0278-5846(93)90041-p. [DOI] [PubMed] [Google Scholar]
- Borrill JA, Rosen BK, Summerfield AB. The influence of alcohol on judgement of facial expression of emotion. Br J Med Psychol. 1987;60(Pt 1):71–77. [PubMed] [Google Scholar]
- Calhoun V, Altschul D, McGinty V, Shih R, Scott D, Sears E, Pearlson G. Alcohol intoxication effects on visual perception: an fMRI study. Human brain mapping. 2004;21:15–26. doi: 10.1002/hbm.10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrigan MH, Randall CL. Self-medication in social phobia: a review of the alcohol literature. Addict Behav. 2003;28:269–284. doi: 10.1016/s0306-4603(01)00235-0. [DOI] [PubMed] [Google Scholar]
- Clark US, Oscar-Berman M, Shagrin B, Pencina M. Alcoholism and judgments of affective stimuli. Neuropsychology. 2007;21:346–362. doi: 10.1037/0894-4105.21.3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ML, Frone MR, Russell M, Mudar P. Drinking to regulate positive and negative emotions: a motivational model of alcohol use. J Pers Soc Psychol. 1995;69:990–1005. doi: 10.1037//0022-3514.69.5.990. [DOI] [PubMed] [Google Scholar]
- Criswell HE, Breese GR. A conceptualization of integrated actions of ethanol contributing to its GABAmimetic profile: a commentary. Neuropsychopharmacology. 2005;30:1407–1425. doi: 10.1038/sj.npp.1300750. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Epstein AM, Sher TG, Young MA, King AC. Tobacco chippers show robust increases in smoking urge after alcohol consumption. Psychopharmacology (Berl) 2007;190:321–329. doi: 10.1007/s00213-006-0438-8. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSMIV-Patient Edition (SCID-P) American Psychiatric Press; Washington, DC: 1995. [Google Scholar]
- Foisy ML, Kornreich C, Fobe A, D'Hondt L, Pelc I, Hanak C, Verbanck P, Philippot P. Impaired emotional facial expression recognition in alcohol dependence: do these deficits persist with midterm abstinence? Alcohol Clin Exp Res. 2007;31:404–410. doi: 10.1111/j.1530-0277.2006.00321.x. [DOI] [PubMed] [Google Scholar]
- Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med. 1990;322:95–99. doi: 10.1056/NEJM199001113220205. [DOI] [PubMed] [Google Scholar]
- Gilman J, Ramchandani V, Davis M, Bjork J, Hommer D. Why we like to drink: a functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. Journal of Neuroscience. 2008;28:4583–4591. doi: 10.1523/JNEUROSCI.0086-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Lovallo WR, Fox PT. Reduced amygdala activation in young adults at high risk of alcoholism: studies from the Oklahoma family health patterns project. Biol Psychiatry. 2007;61:1306–1309. doi: 10.1016/j.biopsych.2006.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Sara R, Hagendoorn M, Marom O, Hughett P, Macy L, Turner T, Bajcsy R, Posner A, Gur RE. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J Neurosci Methods. 2002;115:137–143. doi: 10.1016/s0165-0270(02)00006-7. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, Weinberger DR. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005;62:146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Gorka A, Hyde LW, Kimak M, Halder I, Ducci F, Ferrell RE, Goldman D, Manuck SB. Divergent effects of genetic variation in endocannabinoid signaling on human threat- and reward-related brain function. Biol Psychiatry. 2009;66:9–16. doi: 10.1016/j.biopsych.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer CJ, Bhagwagar Z, Perrett DI, Vollm BA, Cowen PJ, Goodwin GM. Acute SSRI administration affects the processing of social cues in healthy volunteers. Neuropsychopharmacology. 2003;28:148–152. doi: 10.1038/sj.npp.1300004. [DOI] [PubMed] [Google Scholar]
- Hess U, Blairy S, Kleck RE. The intensity of emotional facial expressions and decoding accuracy. Journal of Nonverbal Behavior. 1997;21 [Google Scholar]
- Horstmann G, Scharlau I, Ansorge U. More efficient rejection of happy than of angry face distractors in visual search. Psychon Bull Rev. 2006;13:1067–1073. doi: 10.3758/bf03213927. [DOI] [PubMed] [Google Scholar]
- ICAP . What Is a Standard Drink? International Center for Alcohol Policies Reports; Washington, DC: 1998. [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, Yovel G. The fusiform face area: a cortical region specialized for the perception of faces. Philos Trans R Soc Lond B Biol Sci. 2006;361:2109–2128. doi: 10.1098/rstb.2006.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassel JD, Jackson SI, Unrod M. Generalized expectancies for negative mood regulation and problem drinking among college students. J Stud Alcohol. 2000;61:332–340. doi: 10.15288/jsa.2000.61.332. [DOI] [PubMed] [Google Scholar]
- King A, McNamara P, Angstadt M, Phan KL. Neural substrates of alcohol-induced smoking urge in heavy drinking nondaily smokers. Neuropsychopharmacology. 2010a;35:692–701. doi: 10.1038/npp.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A, McNamara P, Conrad M, Cao D. Alcohol-induced increases in smoking behavior for nicotinized and denicotinized cigarettes in men and women. Psychopharmacology (Berl) 2009;207:107–117. doi: 10.1007/s00213-009-1638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, de Wit H, McNamara P, Cao D. Stimulant and sedative alcohol responses and relationship to future drinking. 2010b doi: 10.1001/archgenpsychiatry.2011.26. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Houle T, de Wit H, Holdstock L, Schuster A. Biphasic alcohol response differs in heavy versus light drinkers. Alcohol Clin Exp Res. 2002;26:827–835. [PubMed] [Google Scholar]
- Kornreich C, Blairy S, Philippot P, Hess U, Noel X, Streel E, Le Bon O, Dan B, Pelc I, Verbanck P. Deficits in recognition of emotional facial expression are still present in alcoholics after mid- to long-term abstinence. J Stud Alcohol. 2001;62:533–542. doi: 10.15288/jsa.2001.62.533. [DOI] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology (Berl) 2009;205:529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner MG, Sher KJ, Erickson DJ. Prospective analysis of the relation between DSM-III anxiety disorders and alcohol use disorders. Am J Psychiatry. 1999;156:723–732. doi: 10.1176/ajp.156.5.723. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Leppanen JM, Hietanen JK. Positive facial expressions are recognized faster than negative facial expressions, but why? Psychol Res. 2004;69:22–29. doi: 10.1007/s00426-003-0157-2. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Sher KJ, Grossman LM, Newman J, Newlin DB. Alcohol and stress response dampening: pharmacological effects, expectancy, and tension reduction. J Abnorm Psychol. 1980;89:528–538. doi: 10.1037//0021-843x.89.4.528. [DOI] [PubMed] [Google Scholar]
- Levin J, Ross M, Mendelson J, Kaufman M, Lange N, Maas L, Mello N, Cohen B, Renshaw P. Reduction in BOLD fMRI response to primary visual stimulation following alcohol ingestion. Psychiatry Research-Neuroimaging Section. 1998;82:135–146. doi: 10.1016/s0925-4927(98)00022-5. [DOI] [PubMed] [Google Scholar]
- Luchtmann M, Jachau K, Tempelmann C, Bernarding J. Alcohol induced region-dependent alterations of hemodynamic response: implications for the statistical interpretation of pharmacological fMRI studies. Exp Brain Res. 2010;204:1–10. doi: 10.1007/s00221-010-2277-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Oscar-Berman M, Urban T, O'Reilly CE, Howard JA, Sawyer K, Harris GJ. Alcoholism and dampened temporal limbic activation to emotional faces. Alcohol Clin Exp Res. 2009;33:1880–1892. doi: 10.1111/j.1530-0277.2009.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- McBride WJ. Central nucleus of the amygdala and the effects of alcohol and alcohol-drinking behavior in rodents. Pharmacol Biochem Behav. 2002;71:509–515. doi: 10.1016/s0091-3057(01)00680-3. [DOI] [PubMed] [Google Scholar]
- McCarty CA, Ebel BE, Garrison MM, DiGiuseppe DL, Christakis DA, Rivara FP. Continuity of binge and harmful drinking from late adolescence to early adulthood. Pediatrics. 2004;114:714–719. doi: 10.1542/peds.2003-0864-L. [DOI] [PubMed] [Google Scholar]
- Moller C, Wiklund L, Sommer W, Thorsell A, Heilig M. Decreased experimental anxiety and voluntary ethanol consumption in rats following central but not basolateral amygdala lesions. Brain Res. 1997;760:94–101. doi: 10.1016/s0006-8993(97)00308-9. [DOI] [PubMed] [Google Scholar]
- Morean ME, Corbin WR. Subjective response to alcohol: a critical review of the literature. Alcohol Clin Exp Res. 2010;34:385–395. doi: 10.1111/j.1530-0277.2009.01103.x. [DOI] [PubMed] [Google Scholar]
- NIAAA . Recommended Council Guidelines on Ethyl Alcohol Administration in Human Experimentation. National Advisory Council on Alcohol Abuse and Alcoholism; Bethesda, MD: 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Z, Schweitzer P, Roberts AJ, Madamba SG, Moore SD, Siggins GR. Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science. 2004;303:1512–1514. doi: 10.1126/science.1092550. [DOI] [PubMed] [Google Scholar]
- Nie Z, Zorrilla EP, Madamba SG, Rice KC, Roberto M, Siggins GR. Presynaptic CRF1 receptors mediate the ethanol enhancement of GABAergic transmission in the mouse central amygdala. ScientificWorldJournal. 2009;9:68–85. doi: 10.1100/tsw.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Castillo G, Simmons AN, Stein MB. Dose-dependent decrease of activation in bilateral amygdala and insula by lorazepam during emotion processing. Arch Gen Psychiatry. 2005;62:282–288. doi: 10.1001/archpsyc.62.3.282. [DOI] [PubMed] [Google Scholar]
- Phan KL, Angstadt M, Golden J, Onyewuenui I, Povpovska A, de Wit H. Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. J Neurosci. 2008;28 doi: 10.1523/JNEUROSCI.5603-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between Amygdala Hyperactivity to Harsh Faces and Severity of Social Anxiety in Generalized Social Phobia. Biol Psychiatry. 2006;59:424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Current Opinion Neurobiology. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Radomsky AS, Rachman S, Thordarson DS, McIsaac HK, Teachman BA. The Claustrophobia Questionnaire. J Anxiety Disord. 2001;15:287–297. doi: 10.1016/s0887-6185(01)00064-0. [DOI] [PubMed] [Google Scholar]
- Ray LA, MacKillop J, Leventhal A, Hutchison KE. Catching the alcohol buzz: an examination of the latent factor structure of subjective intoxication. Alcohol Clin Exp Res. 2009;33:2154–2161. doi: 10.1111/j.1530-0277.2009.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci U S A. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci. 2004;24:10159–10166. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueger SY, McNamara PJ, King AC. Expanding the utility of the Biphasic Alcohol Effects Scale (BAES) and initial psychometric support for the Brief-BAES (B-BAES). Alcohol Clin Exp Res. 2009;33:916–924. doi: 10.1111/j.1530-0277.2009.00914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA . National Survey on Drug Use and Health. Office of Applied Studies; Bethesda, MD: 2005. [Google Scholar]
- Sayette MA. Does drinking reduce stress? Alcohol Res Health. 1999;23:250–255. [PMC free article] [PubMed] [Google Scholar]
- Schroder KE, Perrine MW. Covariations of emotional states and alcohol consumption: evidence from 2 years of daily data collection. Soc Sci Med. 2007;65:2588–2602. doi: 10.1016/j.socscimed.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifritz E, Bilecen D, Hanggi D, Haselhorst R, Radu EW, Wetzel S, Seelig J, Scheffler K. Effect of ethanol on BOLD response to acoustic stimulation: implications for neuropharmacological fMRI. Psychiatry Res. 2000;99:1–13. doi: 10.1016/s0925-4927(00)00054-8. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Bartholow BD, Peuser K, Erickson DJ, Wood MD. Stress-response-dampening effects of alcohol: attention as a mediator and moderator. J Abnorm Psychol. 2007;116:362–377. doi: 10.1037/0021-843X.116.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The Neurocircuitry of Fear, Stress, and Anxiety Disorders. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Ariwodola OJ, Weiner JL. Differential effects of GABAB autoreceptor activation on ethanol potentiation of local and lateral paracapsular GABAergic synapses in the rat basolateral amygdala. Neuropharmacology. 2009;56:886–895. doi: 10.1016/j.neuropharm.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Shi L, Brunso-Bechtold JK, Weiner JL. Distinct mechanisms of ethanol potentiation of local and paracapsular GABAergic synapses in the rat basolateral amygdala. J Pharmacol Exp Ther. 2008;324:251–260. doi: 10.1124/jpet.107.128728. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Alcohol Timeline Follow-Back Users’ Manual. Addiction Research Foundation; Toronto, Canada: 1995. [Google Scholar]
- Sommer W, Moller C, Wiklund L, Thorsell A, Rimondini R, Nissbrandt H, Heilig M. Local 5,7-dihydroxytryptamine lesions of rat amygdala: release of punished drinking, unaffected plus-maze behavior and ethanol consumption. Neuropsychopharmacology. 2001;24:430–440. doi: 10.1016/S0893-133X(00)00210-4. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Montkowski A, Allingham K, Stohr T, Shoaib M, Holsboer F, Landgraf R. Anxiety: a potential predictor of vulnerability to the initiation of ethanol self-administration in rats. Psychopharmacology (Berl) 1995;122:369–373. doi: 10.1007/BF02246268. [DOI] [PubMed] [Google Scholar]
- Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry. 2002;59:1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- Stevens S, Gerlach AL, Rist F. Effects of alcohol on ratings of emotional facial expressions in social phobics. J Anxiety Disord. 2008;22:940–948. doi: 10.1016/j.janxdis.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Stevens S, Rist F, Gerlach AL. Influence of alcohol on the processing of emotional facial expressions in individuals with social phobia. Br J Clin Psychol. 2009;48:125–140. doi: 10.1348/014466508X368856. [DOI] [PubMed] [Google Scholar]
- Sutker PB, Tabakoff B, Goist KC, Jr., Randall CL. Acute alcohol intoxication, mood states and alcohol metabolism in women and men. Pharmacol Biochem Behav. 1983;18(Suppl 1):349–354. doi: 10.1016/0091-3057(83)90198-3. [DOI] [PubMed] [Google Scholar]
- Van Horn J, Yanos M, Schmitt P, Grafton S. Alcohol-induced suppression of BOLD activity during goal-directed visuomotor performance. Neuroimage. 2006;31:1209–1221. doi: 10.1016/j.neuroimage.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Ma Y, Zhu W, Fowler JS, Li J, Rao M, Mueller K, Pradhan K, Wong C, Wang GJ. Moderate doses of alcohol disrupt the functional organization of the human brain. Psychiatry Res. 2008;162:205–213. doi: 10.1016/j.pscychresns.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Mullani N, Gould L, Adler SS, Guynn RW, Overall JE, Dewey S. Effects of acute alcohol intoxication on cerebral blood flow measured with PET. Psychiatry Res. 1988;24:201–209. doi: 10.1016/0165-1781(88)90063-7. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Franceschi D, Fowler JS, Thanos PK, Scherbaum N, Pappas N, Wong CT, Hitzemann RJ, Felder CA. Regional brain metabolism during alcohol intoxication. Alcohol Clin Exp Res. 2000;24:822–829. [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol Ther. 2006;111:533–554. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Brain Res Rev. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
- Zhu PJ, Lovinger DM. Ethanol potentiates GABAergic synaptic transmission in a postsynaptic neuron/synaptic bouton preparation from basolateral amygdala. J Neurophysiol. 2006;96:433–441. doi: 10.1152/jn.01380.2005. [DOI] [PubMed] [Google Scholar]