Abstract
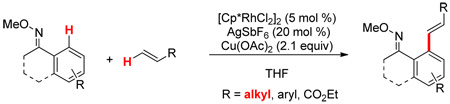
Oxime directed aromatic C–H bond activation and oxidative coupling to alkenes is reported using a cationic Rh(III) catalyst. Significantly, the method can be used to oxidatively couple unactivated, aliphatic alkenes.
Directed C–H bond activation and oxidative coupling with alkenes provide an atom economical alternative to traditional transformations such as the Heck reaction. Palladium,1 ruthenium,2 and rhodium3 have been reported to effect the former transformation. However, nearly all oxidative couplings have employed “activated” alkenes such as acrylates and styrenes.1e,f Herein we report a general method for the oxidative coupling of aryl O-methyl oximes with unactivated alkenes via C–H bond functionalization using a cationic Rh(III) catalyst.
We began our exploration with attempts to oxidatively couple 1-hexene with imine 1a. We initially employed conditions reported by Miura for the oxidative coupling of 1-phenylpyrazole with acrylates using [Cp*RhCl2]2 as the catalyst in the presence of Cu(OAc)2 as an oxidant and with DMF as the solvent (Table 1, entry 1).3f Only trace amounts of the coupled product were observed, and none of the desired product was obtained with other solvents (entries 2 and 3). Prompted by reports that noted a positive effect of halide abstractors for rhodium catalysis, AgSbF6 was added4 and resulted in improved yields (entries 4–7) with the highest yield being obtained with ethanol as the solvent (entry 6). The combination of rhodium with silver was next tested without copper acetate or using other oxidants (Ag2CO3, benzoquinone, PhI(OAc)2), bases (NaOAc, lutidine) or acids (AcOH), but all of these modifications resulted either in a reduced yield or no coupled product (not shown). A survey of other directing groups established that O-methyl oxime 1b (entries 8–10) is superior to the corresponding N-benzyl imine 1a, providing good yields of the trans alkene product in THF (entry 10). Trace amounts of the cis product could also be detected under these conditions (5–10%) and no migration of the double bond was observed. Of note, while the reaction is water sensitive, it is not air sensitive. The reaction mixture could be exposed to the atmosphere with no decrease in yield. No coupled product was observed for acetanilide which has been reported to direct the oxidative coupling of styrenes using the same [Cp*RhCl2]2/AgSbF6 system (entry 11).3e
Table 1.
Optimization of the Oxidative Coupling Reaction
 | ||||
|---|---|---|---|---|
| entry | substrate | additive | solvent | yielda |
| 1 | Y = Bn (1a) | - | DMF | Trace |
| 2 | Y = Bn (1a) | - | THF | 0 |
| 3 | Y = Bn (1a) | - | tAmOH | 0 |
| 4 | Y = Bn (1a) | AgSbF6 | DMF | 11% |
| 5 | Y = Bn (1a) | AgSbF6 | tAmOH | 27% |
| 6 | Y = Bn (1a) | AgSbF6 | EtOH | 45% |
| 7 | Y = Bn (1a) | AgSbF6 | THF | 10% |
| 8 | Y = OMe (1b) | AgSbF6 | tAmOH | 60% |
| 9 | Y = OMe (1b) | AgSbF6 | DMF | 30% |
| 10 | Y = OMe (1b) | AgSbF6 | THF | 85% |
| 11 | acetanilide | AgSbF6 | THF | 0% |
NMR yield relative to 2,6-dimethoxytoluene as an internal standard
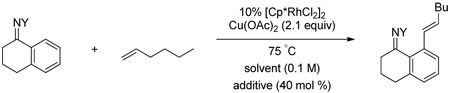
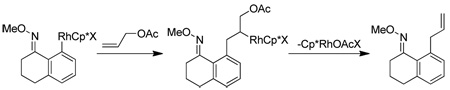
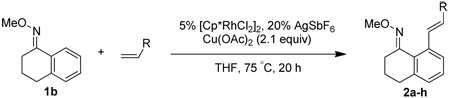
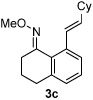
Alkene scope was next explored (Table 2). A β-branched alkene provided the coupled product in high yield (entry 2), while an α-branched alkene resulted in a somewhat lower yield (entry 3). The reaction is compatible with chloro and ester functionalities (entries 4 and 5). Notably, diene 2e preferentially couples at the terminal alkene position in preference to the more electronically activated α,β-unsaturated ester (entry 5). Reactions with activated alkenes such as styrene and ethyl acrylate proceed well to give good yields of the alkenylated products (entries 6 and 7). Interestingly, use of allyl acetate as the coupling partner lead to formation of the unconjugated terminal alkene 3h (entry 8). This allylated product presumably forms via a concerted elimination from a rhodium-acetate complex obtained upon insertion of the alkene in the initally generated rhodium-aryl species (eq 1).5 While this transformation is redox neutral, only trace amounts of product are obtained when Cu(OAc)2 is omitted. However, the reaction can be performed using substoichiometric amounts of Cu(OAc)2 (40 mol %) without a loss in yield.
 |
(1) |
Table 2.
Alkene Scopea
 | |||
|---|---|---|---|
| entry | alkene | product | yieldb |
| 1 |  |
75% | |
| 2 | 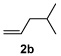 |
 |
84% |
| 3 | 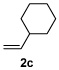 |
 |
53% |
| 4 | 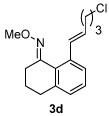 |
85% | |
| 5 | 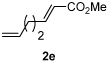 |
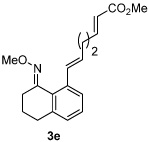 |
80% |
| 6 | 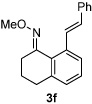 |
98% | |
| 7 | 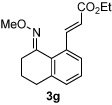 |
81% | |
| 8 | 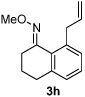 |
46% | |
All reactions were performed by heating the oxime (1 equiv), alkene (3 equiv), [Cp*RhCl2]2 (5 mol %), AgSbF6 (20 mol %), Cu(OAc)2 (2.1 equiv), and THF (0.1 M) in a sealed vial for 20 h at 75 °C.
Isolated yields after purification by chromatography are reported.
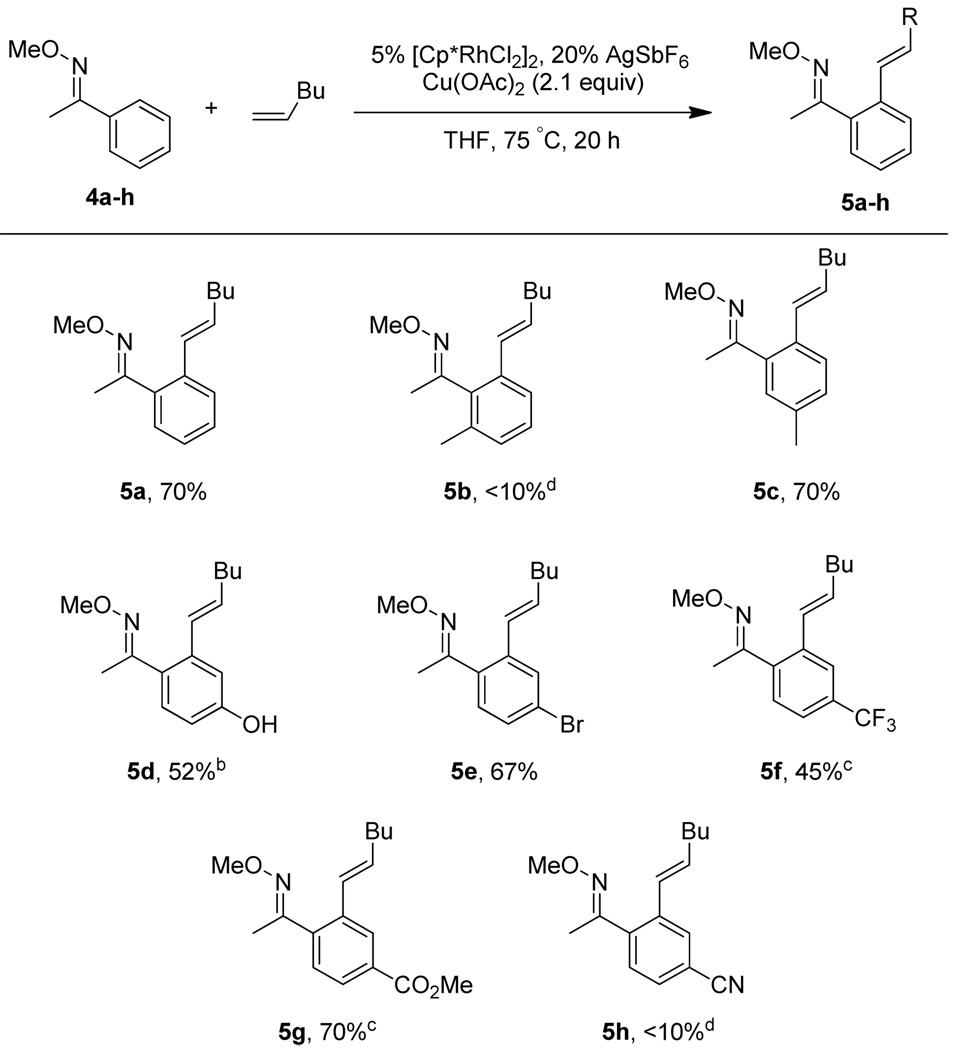
Under the optimized conditions, a variety of different aryl O-methyl oximes could be coupled with alkenes in good yield (Scheme 1). Substrates with para (4d–g) or meta (4c) substitution patterns and both electron withdrawing (4e–g) and releasing (4d) substituents were effective coupling partners. However, the electron poor substrates 4f and 4g required longer reaction times to achieve full conversion. The brominated analogue 4e not only coupled efficiently but also did not suffer any Heck coupling or proto-debromination. In contrast, the para-cyano aryl oxime 4h gave only a poor yield of coupled product (10% by NMR), probably due to coordination of the nitrile to the catalyst. A small amount of the bisalkenylated product, less than 10% by NMR, was observed for oximes 4a and 4e, and under the standard reaction conditions 4d resulted in 30% of the bisalkenylated product. However, this undesired product could be reduced to trace amounts using 1.5 equiv of the alkene. Ortho-methyl substituted aryl oxime 4b resulted in poor conversion, likely due to steric congestion between the directing group and the methyl substituent.3e
Scheme 1.
Aryl O-Methyl Oxime Scopea
a All reactions were performed by heating oxime (1 equiv), 1-hexene (3 equiv), [Cp*RhCl2]2 (5 mol %), AgSbF6 (20 mol %), Cu(OAc)2 (2.1 equiv), and THF (0.1 M) in a sealed vial for 20 h at 75 °C. Isolated yields after purification by chromatography are reported. b 1-hexene (1.5 equiv). c 36 h. d NMR yield relative to 2,6-dimethoxytoluene as an internal standard
Recent reports have shown that Rh(III) complexes are efficient catalysts for the oxidative coupling of C–H bonds with acrylates and styrenes. However, the analogous transformation with non-activated olefins has not previously been demonstrated for Rh catalysts and is generally unknown. We have shown that given an appropriate directing group, the coupling of unactivated as well as functionalized terminal alkenes can be accomplished in moderate to good yields and under conditions that are compatible with commonly encountered functional groups.
Supplementary Material
Acknowledgment
This work was supported by the NIH grant GM069559 (to J.A.E) and by the Director, Office of Energy Research, Office of Basic Energy Sciences, Chemical Sciences Division, US Department of Energy under contract DE-AC02-05CH11231 (to R.G.B). M.B. acknowledges the 7th European Community Framework Programme, for a Marie Curie International Outgoing Fellowship, that supported this research. A.S.T is grateful for an Eli Lilly Fellowship.
Footnotes
Supporting Information Available: Experimental details, characterization data, and copies of 1H NMR and 13C NMR spectra of all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org
References
- 1.For reviews see: Chen X, Engle KM, Wang D-H, Yu J-Q. Angew. Chem. Int. Ed. 2009;48:5094–5115. doi: 10.1002/anie.200806273. Ferreira EM, Zhang H, Stoltz BM. Tetrahedron. 2008;64:5987–6001. doi: 10.1016/j.tet.2008.01.052. For selected recent individual reports see: Nishikata T, Lipshutz BH. Org. Lett. 2010;12:1972–1975. doi: 10.1021/ol100331h. Wasa M, Engle KM, Yu J-Q. J. Am. Chem. Soc. 2010;132:3680–3681. doi: 10.1021/ja1010866. Engle KM, Wang D-H, Yu J-Q. Angew. Chem. Int. Ed. 2010;49:6169–6173. doi: 10.1002/anie.201002077. Shi B-F, Zhang Y-H, Lam JK, Wang D-H, Yu J-Q. J. Am. Chem. Soc. 2010;132:460–461. doi: 10.1021/ja909571z. Wang D-H, Engle KM, Shi B-F, Yu J-Q. Science. 2010;327:315–319. doi: 10.1126/science.1182512. Rubia-Garcia A, Arrayas RG, Carretero JC. Angew Chem. Int. Ed. 2009;48:6511–6515. doi: 10.1002/anie.200902802. Zhang Y-H, Shi B-F, Yu J-Q. J. Am. Chem. Soc. 2009;131:5072–5074. doi: 10.1021/ja900327e. Cho SH, Hwang SJ, Chang S. J. Am. Chem. Soc. 2008;130:9254–9256. doi: 10.1021/ja8026295. Würtz S, Rakshit S, Neumann JJ, Dröge T, Glorius F. Angew. Chem. Int. Ed. 2008;47:7230–7233. doi: 10.1002/anie.200802482. Cai G, Fu Y, Li Y, Wan X, Shi Z. J. Am. Chem. Soc. 2007;129:7666–7673. doi: 10.1021/ja070588a. Grimster NP, Cauntlett C, Godfrey CRA, Gauntx MJ. Angew. Chem. Int. Ed. 2005;44:3125–3129. doi: 10.1002/anie.200500468. Zhang H, Ferreira EM, Stoltz BM. Angew. Chem. Int. Ed. 2004;43:6144–6148. doi: 10.1002/anie.200461294. Ferreira EM, Stoltz BM. J. Am. Chem. Soc. 2003;125:9578–9579. doi: 10.1021/ja035054y. Yokaota T, Tani M, Sakaguchi S, Ishii Y. J. Am. Chem. Soc. 2003;125:1476–1477. doi: 10.1021/ja028903a. Dams M, De Vos DE, Celen S, Jacobs PA. Angew. Chem. Int. Ed. 2003;42:3512–3515. doi: 10.1002/anie.200351524. Boele MDK, van Strijdonck GPF, de Vries AHM, Kamer PCJ, de Vries JG, van Leeuwen PWNM. J. Am. Chem. Soc. 2002;124:1586–1587. doi: 10.1021/ja0176907. Jia C, Lu W, Kitamura T, Fujiwara Y. Org. Lett. 1999;1:2097–2100. doi: 10.1021/ol006156l. Miura M, Tsuda T, Satoh T, Pivsa-Art S, Nomura M. J. Org. Chem. 1998;63:5211–5215. Miura M, Tsuda T, Satoh T, Nomura M. Chem. Lett. 1997:1103–1104.
- 2.Weissman H, Song X, Milstein D. J. Am. Chem. Soc. 2001;123:337–338. doi: 10.1021/ja003361n. [DOI] [PubMed] [Google Scholar]
- 3.For a review see: Satoh T, Miura M. Chem. Eur. J. 2010;16:11212–11222. doi: 10.1002/chem.201001363. For recent selected individual reports see: Patureau FW, Besset T, Glorius F. Angew. Chem. Int. Ed. 2011 doi: 10.1002/anie.201006222. in press. Wang F, Song G, Li X. Org. Lett. 2010:5426–5429. doi: 10.1021/ol1022596. Chen J, Song G, Pan C-L, Li X. Org. Lett. 2010:5430–5433. doi: 10.1021/ol102241f. Patureau FW, Glorius F. J. Am. Chem. Soc. 2010;132:9982–9983. doi: 10.1021/ja103834b. Umeda N, Hirano K, Satoh T, Miura M. J. Org. Chem. 2009;74:7094–7099. doi: 10.1021/jo901485v. Ueura K, Satoh T, Miura M. Org. Lett. 2007;9:1407–1409. doi: 10.1021/ol070406h. Matsumoto T, Periana RA, Taube DJ, Yoshida H. J. Cat. 2002;206:272–280.
- 4.(a) Stuart DR, Bertrand-Laperle M, Burgess KMN, Fagnou K. J. Am. Chem. Soc. 2008;130:16474–16475. doi: 10.1021/ja806955s. [DOI] [PubMed] [Google Scholar]; (b) Youn SW, Pastine SJ, Sames D. Org. Lett. 2004;6:581–584. doi: 10.1021/ol036385i. [DOI] [PubMed] [Google Scholar]
- 5.(a) Song J, Shen Q, Xu F, Lu X. Tetrahedron. 2007;63:5148–5153. [Google Scholar]; (b) Williams BS, Leatherman MD, White PS, Brookhart M. J. Am. Chem. Soc. 2005;127:5132–5146. doi: 10.1021/ja045969s. [DOI] [PubMed] [Google Scholar]; (c) Zhang Q, Xu W, Lu X. J. Org. Chem. 2005;70:1505–1507. doi: 10.1021/jo048414o. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.